Interdisciplinary Therapeutic Approaches to Atypical and Malignant Meningiomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Pathophysiology
3. Classification
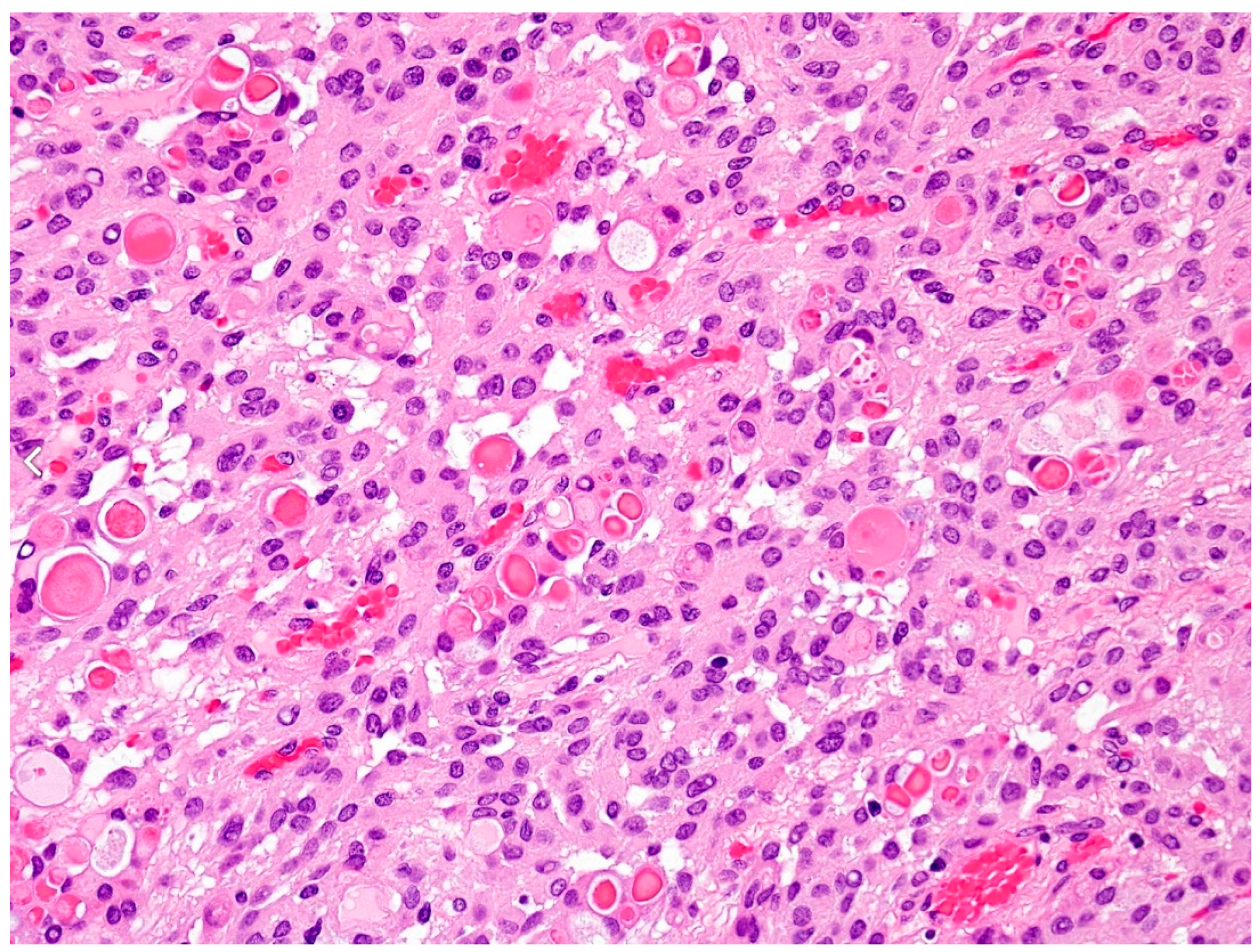
3.1. Malignant vs. Benign Meningiomas
3.2. Meningiomatosis
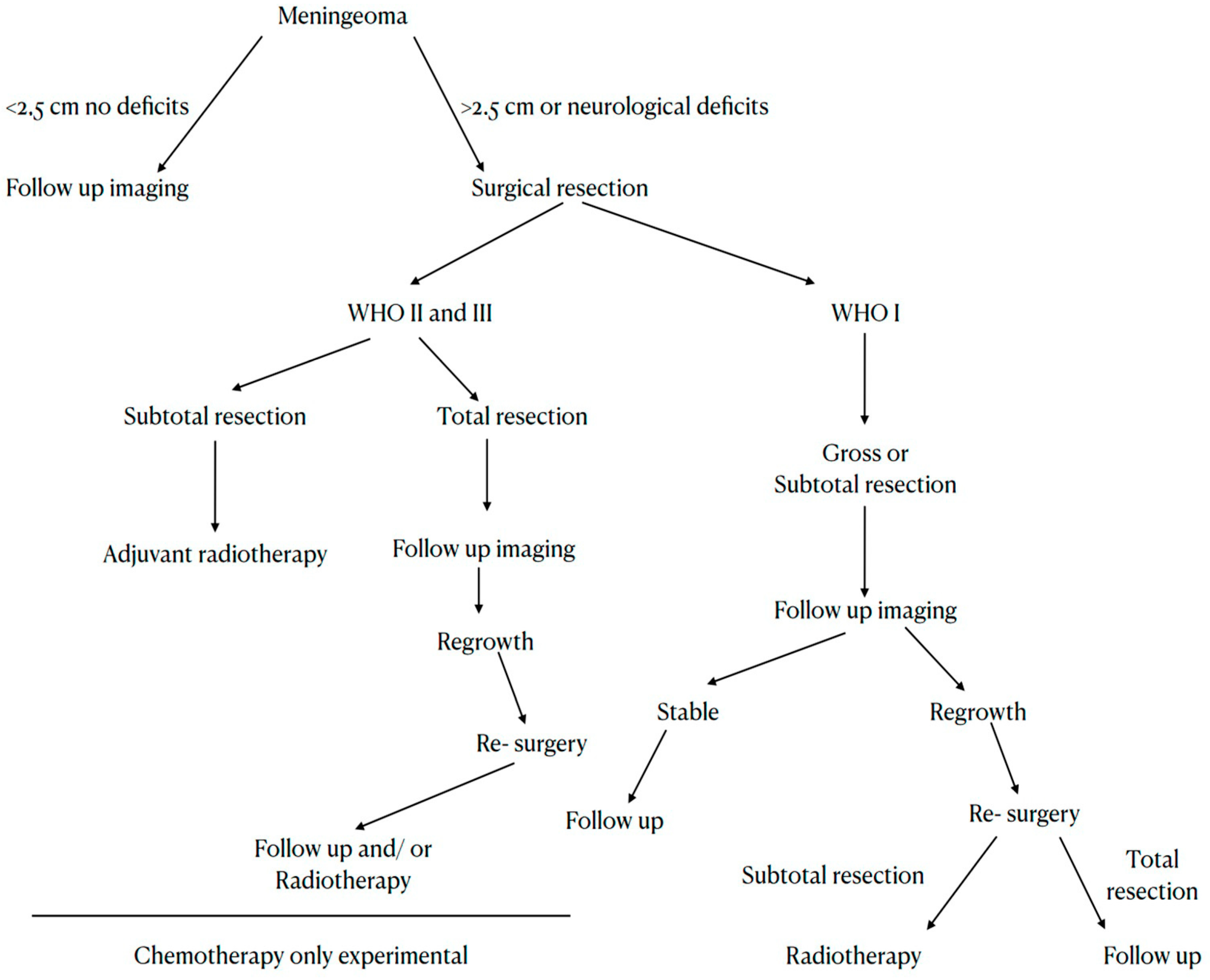
4. Therapeutical Options
4.1. Surgery
4.2. Adjuvant (Radio)Therapy
4.3. Pharmacological Therapy
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baldi, I.; Engelhardt, J.; Bonnet, C.; Bauchet, L.; Berteaud, E.; Grüber, A.; Loiseau, H. Epidemiology of Meningiomas. Neurochirurgie 2018, 64, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Spiegl-Kreinecker, S.; Lötsch, D.; Neumayer, K.; Kastler, L.; Gojo, J.; Pirker, C.; Pichler, J.; Weis, S.; Kumar, R.; Webersinke, G.; et al. TERT Promoter Mutations Are Associated with Poor Prognosis and Cell Immortalization in Meningioma. Neuro-Oncology 2018, 20, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Biczok, A.; Kraus, T.; Suchorska, B.; Terpolilli, N.A.; Thorsteinsdottir, J.; Giese, A.; Tonn, J.C.; Schichor, C. TERT Promoter Mutation Is Associated with Worse Prognosis in WHO Grade II and III Meningiomas. J. Neuro-Oncol. 2018, 139, 671–678. [Google Scholar] [CrossRef]
- Behling, F.; Hempel, J.M.; Schittenhelm, J. Brain Invasion in Meningioma—A Prognostic Potential Worth Exploring. Cancers 2021, 13, 3259. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.N.; Braun, Y.; Plate, K.H. Classification of Meningiomas—Advances and Controversies. Chin. Clin. Oncol. 2017, 6, S2. [Google Scholar] [CrossRef]
- Riemenschneider, M.J.; Perry, A.; Reifenberger, G. Histological Classification and Molecular Genetics of Meningiomas. Lancet Neurol. 2006, 5, 1045–1054. [Google Scholar] [CrossRef]
- Proctor, D.T.; Ramachandran, S.; Lama, S.; Sutherland, G.R. Towards Molecular Classification of Meningioma: Evolving Treatment and Diagnostic Paradigms. World Neurosurg. 2018, 119, 366–373. [Google Scholar] [CrossRef]
- Zaher, A.; Abdelbari Mattar, M.; Zayed, D.H.; Ellatif, R.A.; Ashamallah, S.A. Atypical Meningioma: A Study of Prognostic Factors. World Neurosurg. 2013, 80, 549–553. [Google Scholar] [CrossRef]
- Palma, L.; Celli, P.; Franco, C.; Cervoni, L.; Cantore, G. Long-Term Prognosis for Atypical and Malignant Meningiomas: A Study of 71 Surgical Cases. J. Neurosurg. 1997, 86, 793–800. [Google Scholar] [CrossRef]
- Spille, D.C.; Adeli, A.; Sporns, P.B.; Heß, K.; Streckert, E.M.S.; Brokinkel, C.; Mawrin, C.; Paulus, W.; Stummer, W.; Brokinkel, B. Predicting the Risk of Postoperative Recurrence and High-Grade Histology in Patients with Intracranial Meningiomas Using Routine Preoperative MRI. Neurosurg. Rev. 2021, 44, 1109. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System Tumours: A Practical Update on What Neurosurgeons Need to Know—A Minireview. Acta Neurochir. 2022, 164, 2453. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Javalkar, V.; Banerjee, A.D. Petroclival Meningiomas: Study on Outcomes, Complications and Recurrence Rates: Clinical Article. J. Neurosurg. 2011, 114, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.H.P.; de Tahara, A.; Almeida, A.N.; Simm, R.; Silva, A.N.; da Maldaun, M.V.C.; Panagopoulos, A.T.; Zicarelli, C.A.; Silva, P.G. Olfactory Groove Meningiomas: Approaches and Complications. J. Clin. Neurosci. 2009, 16, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; de Santana, P.A.; Calfat Maldaun, M.V.; Panagopoulos, A.T.; da Silva, A.N.; Zicarelli, C.A.; Pires de Aguiar, P.H. Petroclival Meningiomas: Surgical Management and Common Complications. J. Clin. Neurosci. 2009, 16, 655–659. [Google Scholar] [CrossRef]
- Sekhar, L.N.; Wright, D.C.; Richardson, R.; Monacci, W. Petroclival and Foramen Magnum Meningiomas: Surgical Approaches and Pitfalls. J. Neuro-Oncol. 1996, 29, 249–259. [Google Scholar] [CrossRef]
- Dincer, A.; Morales-Valero, S.F.; Robert, S.M.; Tabor, J.K.; O’Brien, J.; Yalcin, K.; Fulbright, R.K.; Erson-Omay, Z.; Dunn, I.F.; Moliterno, J. Surgical Strategies for Intracranial Meningioma in the Molecular Era. J. Neuro-Oncol. 2023, 162, 253. [Google Scholar] [CrossRef]
- Boviatsis, E.J.; Bouras, T.I.; Kouyialis, A.T.; Themistocleous, M.S.; Sakas, D.E. Impact of Age on Complications and Outcome in Meningioma Surgery. Surg. Neurol. 2007, 68, 407–411. [Google Scholar] [CrossRef]
- Ottenhausen, M.; Banu, M.A.; Placantonakis, D.G.; Tsiouris, A.J.; Khan, O.H.; Anand, V.K.; Schwartz, T.H. Endoscopic Endonasal Resection of Suprasellar Meningiomas: The Importance of Case Selection and Experience in Determining Extent of Resection, Visual Improvement, and Complications. World Neurosurg. 2014, 82, 442–449. [Google Scholar] [CrossRef]
- Simpson, D. The Recurrence of Intracranial Meningiomas after Surgical Treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22. [Google Scholar] [CrossRef]
- Sanai, N.; Sughrue, M.E.; Shangari, G.; Chung, K.; Berger, M.S.; McDermott, M.W. Risk Profile Associated with Convexity Meningioma Resection in the Modern Neurosurgical Era: Clinical Article. J. Neurosurg. 2010, 112, 913–919. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Kane, A.J.; Shangari, G.; Rutkowski, M.J.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. The Relevance of Simpson Grade I and II Resection in Modern Neurosurgical Treatment of World Health Organization Grade I Meningiomas: Clinical Article. J. Neurosurg. 2010, 113, 1029–1035. [Google Scholar] [CrossRef]
- Behling, F.; Fodi, C.; Hoffmann, E.; Renovanz, M.; Skardelly, M.; Tabatabai, G.; Schittenhelm, J.; Honegger, J.; Tatagiba, M. The Role of Simpson Grading in Meningiomas after Integration of the Updated WHO Classification and Adjuvant Radiotherapy. Neurosurg. Rev. 2021, 44, 2329–2336. [Google Scholar] [CrossRef]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An Overview of Meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Pathology Outlines—WHO Grading of Meningiomas. Available online: https://www.pathologyoutlines.com/topic/cnstumorwhomeningioma.html (accessed on 20 June 2023).
- Hortobágyi, T.; Bencze, J.; Murnyák, B.; Kouhsari, M.C.; Bognár, L.; Marko-Varga, G. Pathophysiology of Meningioma Growth in Pregnancy. Open Med. 2017, 12, 195–200. [Google Scholar] [CrossRef]
- Pendleton, C.; Olivi, A.; Brem, H.; Quiñones-Hinojosa, A. Harvey Cushing’s Early Treatment of Meningiomas: The Untold Story. World Neurosurg. 2013, 80, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Ragel, B.; Jensen, R.L. Pathophysiology of Meningiomas. Semin. Neurosurg. 2003, 14, 169–185. [Google Scholar]
- Boetto, J.; Peyre, M.; Kalamarides, M. Meningiomas from a Developmental Perspective: Exploring the Crossroads between Meningeal Embryology and Tumorigenesis. Acta Neurochir. 2021, 163, 57–66. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, A.I. Neurofibromatosis. Annu. Rev. Pathol. Mech. Dis. 2007, 2, 191–216. [Google Scholar] [CrossRef]
- Giangaspero, F.; Guiducci, A.; Lenz, F.A.; Mastronardi, L.; Burger, P.C. Meningioma with Meningioangiomatosis: A Condition Mimicking Invasive Meningiomas in Children and Young Adults: Report of Two Cases and Review of the Literature. Am. J. Surg. Pathol. 1999, 23, 872–875. [Google Scholar] [CrossRef]
- Umansky, F.; Shoshan, Y.; Rosenthal, G.; Fraifeld, S.; Spektor, S. Radiation-Induced Meningioma. Neurosurg. Focus. 2008, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A.B.; Shalit, M.N.; Cohen, M.L.; Zandbank, U.; Reichenthal, E. Radiation-Induced Cerebral Meningioma: A Recognizable Entity. J. Neurosurg. 1984, 61, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Cardis, E. Brain Tumour Risk in Relation to Mobile Telephone Use: Results of the INTERPHONE International Case–Control Study. Int. J. Epidemiol. 2010, 39, 675–694. [Google Scholar] [CrossRef]
- Phillips, L.E.; Koepsell, T.D.; van Belle, G.; Kukull, W.A.; Gehrels, J.A.; Longstreth, W.T. History of Head Trauma and Risk of Intracranial Meningioma: Population-Based Case-Control Study. Neurology 2002, 58, 1849–1852. [Google Scholar] [CrossRef]
- Galeone, C.; Malerba, S.; Rota, M.; Bagnardi, V.; Negri, E.; Scotti, L.; Bellocco, R.; Corrao, G.; Boffetta, P.; la Vecchia, C.; et al. A Meta-Analysis of Alcohol Consumption and the Risk of Brain Tumours. Ann. Oncol. 2013, 24, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Little, J.; Xu, T.; Zhao, X.; Guo, L.; Jia, X.; Huang, G.; Bi, D.; Liu, R. Risk Factors For Meningioma In Adults: A Case-Control Study In Northeast China. J. Cancer 1999, 83, 299–304. [Google Scholar] [CrossRef]
- Blamey, R.; Collins, J.; Crosignani, P.G.; Diczfalusy, E.; Heinemann, L.A.J.; la Vecchia, C.; Reeves, G.; Smith, I.E.; Trichopoulos, D.; Arisi, E.; et al. Hormones and Breast Cancer. Hum. Reprod. Update 2004, 10, 281–293. [Google Scholar] [CrossRef]
- Wahab, M.; Al-Azzawi, F. Meningioma and Hormonal Influences. Climacteric 2003, 6, 285–292. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Kruchko, C. Meningiomas: Causes and Risk Factors. Neurosurg. Focus. 2007, 23, E2. [Google Scholar] [CrossRef]
- Custer, B.; Longstreth, J.T.; Phillips, L.E.; Koepsell, T.D.; van Belle, G. Hormonal Exposures and the Risk of Intracranial Meningioma in Women: A Population-Based Case-Control Study. BMC Cancer 2006, 6, 152. [Google Scholar] [CrossRef]
- Claus, E.B.; Black, P.M.; Bondy, M.L.; Calvocoressi, L.; Schildkraut, J.M.; Wiemels, J.L.; Wrensch, M. Exogenous Hormone Use and Meningioma Risk. Cancer 2007, 110, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Wiemels, J.; Wrensch, M.; Claus, E.B. Epidemiology and Etiology of Meningioma. J. Neuro-Oncol. 2010, 99, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Jhawar, B.S.; Fuchs, C.S.; Colditz, G.A.; Stampfer, M.J. Sex Steroid Hormone Exposures and Risk for Meningioma. J. Neurosurg. 2003, 99, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Wigertz, A.; Lö Nn, S.; Mathiesen, T.; Ahlbom, A.; Feychting, M.; Interphone, S.; Group, S. Original Contribution Risk of Brain Tumors Associated with Exposure to Exogenous Female Sex Hormones. Am. J. Epidemiol. 2006, 164, 629–636. [Google Scholar] [CrossRef]
- Cea-Soriano, L.; Blenk, T.; Wallander, M.A.; Rodríguez, L.A.G. Hormonal Therapies and Meningioma: Is There a Link? Cancer Epidemiol. 2012, 36, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Quiñones-Hinojosa, A.; Harmon-Smith, M.; Bollen, A.W.; Mcdermott, M.W. Sex Steroid and Growth Factor Profile of a Meningioma Associated with Pregnancy. Can. J. Neurol. Sci. 2021, 32, 122–127. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.; Zhao, W.; Hou, Y.; Wen, C.; Wang, J.; Wu, P.; Guo, Z. An Overview of Managements in Meningiomas. Front. Oncol. 2020, 10, 564305. [Google Scholar] [CrossRef]
- Graillon, T.; Sanson, M.; Campello, C.; Idbaih, A.; Peyre, M.; Peyriere, H.; Basset, N.; Autran, D.; Roche, C.; Kalamarides, M.; et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clin. Cancer Res. 2020, 26, 552–557. [Google Scholar] [CrossRef]
- Holleczek, B.; Zampella, D.; Urbschat, S.; Sahm, F.; von Deimling, A.; Oertel, J.; Ketter, R. Incidence, Mortality and Outcome of Meningiomas: A Population-Based Study from Germany. Cancer Epidemiol. 2019, 62, 101562. [Google Scholar] [CrossRef]
- Dalle, C.L.; Magill, S.T.; Yen, A.J.; Shahin, M.N.; Lee, D.S.; Lucas, C.H.G.; Chen, W.C.; Viner, J.A.; Aghi, M.K.; Theodosopoulos, P.V.; et al. Meningioma Metastases: Incidence and Proposed Screening Paradigm. J. Neurosurg. 2019, 132, 1447–1455. [Google Scholar] [CrossRef]
- Pathology Outlines—Meningioma. Available online: https://www.pathologyoutlines.com/topic/cnstumormeningiomageneral.html (accessed on 20 June 2023).
- Jääskeläinen, J. Seemingly Complete Removal of Histologically Benign Intracranial Meningioma: Late Recurrence Rate and Factors Predicting Recurrence in 657 Patients. A Multivariate Analysis. Surg. Neurol. 1986, 26, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Goldman, C.K.; Bharara, S.; Palmer, C.A.; Vitek, J.; Tsai, J.C.; Weiss, H.L.; Gillespie, G.Y. Brain Edema in Meningiomas Is Associated with Increased Vascular Endothelial Growth Factor Expression. Neurosurgery 1997, 40, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Wan, Y.W.; Al-Ouran, R.; Revelli, J.P.; Cardenas, M.F.; Oneissi, M.; Xi, L.; Jalali, A.; Magnotti, J.F.; Muzny, D.M.; et al. Molecular Profiling Predicts Meningioma Recurrence and Reveals Loss of DREAM Complex Repression in Aggressive Tumors. Proc. Natl. Acad. Sci. USA 2019, 116, 21715–21726. [Google Scholar] [CrossRef]
- Khan, A.B.; English, C.W.; Chen, W.C.; Athukuri, P.; Bayley, J.C.; Brandt, V.L.; Shetty, A.; Hadley, C.C.; Choudhury, A.; Lu, H.C.; et al. Even Heterozygous Loss of CDKN2A/B Greatly Accelerates Recurrence in Aggressive Meningioma. Acta Neuropathol. 2023, 145, 501–503. [Google Scholar] [CrossRef]
- Baumgarten, P.; Gessler, F.; Schittenhelm, J.; Skardelly, M.; Tews, D.S.; Senft, C.; Dunst, M.; Imoehl, L.; Plate, K.H.; Wagner, M.; et al. Brain Invasion in Otherwise Benign Meningiomas Does Not Predict Tumor Recurrence. Acta Neuropathol. 2016, 132, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Spille, D.C.; Heß, K.; Sauerland, C.; Sanai, N.; Stummer, W.; Paulus, W.; Brokinkel, B. Brain Invasion in Meningiomas: Incidence and Correlations with Clinical Variables and Prognosis. World Neurosurg. 2016, 93, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Gousias, K.; Trakolis, L.; Simon, M. Meningiomas with CNS Invasion. Front. Neurosci. 2023, 17. [Google Scholar] [CrossRef]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B Homozygous Deletion Is Associated with Early Recurrence in Meningiomas. Acta Neuropathol. 2020, 140, 409. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. JNCI J. Natl. Cancer Inst. 2016, 108, 377. [Google Scholar] [CrossRef]
- Mirian, C.; Duun-Henriksen, A.K.; Juratli, T.; Sahm, F.; Spiegl-Kreinecker, S.; Peyre, M.; Biczok, A.; Tonn, J.C.; Goutagny, S.; Bertero, L.; et al. Poor Prognosis Associated with TERT Gene Alterations in Meningioma Is Independent of the WHO Classification: An Individual Patient Data Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 378–387. [Google Scholar] [CrossRef]
- Goutagny, S.; Nault, J.C.; Mallet, M.; Henin, D.; Rossi, J.Z.; Kalamarides, M. High Incidence of Activating TERT Promoter Mutations in Meningiomas Undergoing Malignant Progression. Brain Pathol. 2014, 24, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Corniola, M.V.; Meling, T.R. Management of Recurrent Meningiomas: State of the Art and Perspectives. Cancers 2022, 14, 3995. [Google Scholar] [CrossRef] [PubMed]
- Voß, K.M.; Spille, D.C.; Sauerland, C.; Suero Molina, E.; Brokinkel, C.; Paulus, W.; Stummer, W.; Holling, M.; Jeibmann, A.; Brokinkel, B. The Simpson Grading in Meningioma Surgery: Does the Tumor Location Influence the Prognostic Value? J. Neuro-Oncol. 2017, 133, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Przybylowski, C.J.; Hendricks, B.K.; Frisoli, F.A.; Zhao, X.; Cavallo, C.; Moreira, L.B.; Gandhi, S.; Sanai, N.; Almefty, K.K.; Lawton, M.T.; et al. Prognostic Value of the Simpson Grading Scale in Modern Meningioma Surgery: Barrow Neurological Institute Experience. J. Neurosurg. 2020, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, G.; Maiuri, F.; de Divitiis, E.; Bonavolontà, G.; Tranfa, F.; Iuliano, A.; Strianese, D. Lateral Orbitotomy for Removal of Sphenoid Wing Meningiomas Invading the Orbit. Oper. Neurosurg. 2010, 66, ons287–ons292. [Google Scholar] [CrossRef]
- Abdel Aziz, K.M.; Sanan, A.; van Loveren, H.R.; Tew, J.; Keller, J.T.; Pensak, M.L.; Diaz Day, J. Petroclival Meningiomas: Predictive Parameters for Transpetrosal Approaches. Neurosurgery 2000, 47, 139–152. [Google Scholar] [CrossRef]
- Petridis, A.K.; Thissen, J.; Brassel, F.; Meila, D.; Scholz, M. Perspectives in Meningioma Treatment. J. Neurol. Disord. 2015, 3. [Google Scholar] [CrossRef]
- Santacroce, A.; Walier, M.; Régis, J.; Liščák, R.; Motti, E.; Lindquist, C.; Kemeny, A.; Kitz, K.; Lippitz, B.; Álvarez, R.M.; et al. Long-Term Tumor Control of Benign Intracranial Meningiomas After Radiosurgery in a Series of 4565 Patients. Neurosurgery 2012, 70, 32–39. [Google Scholar] [CrossRef]
- Maguire, P.D.; Clough, R.; Friedman, A.H.; Halperin, E.C. Fractionated External-Beam Radiation Therapy for Meningiomas of the Cavernous Sinus. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 75–79. [Google Scholar] [CrossRef]
- Pollock, B.E.; Stafford, S.L.; Link, M.J.; Brown, P.D.; Garces, Y.I.; Foote, R.L. Single-Fraction Radiosurgery of Benign Intracranial Meningiomas. Neurosurgery 2012, 71, 604–613. [Google Scholar] [CrossRef]
- Gousias, K.; Schramm, J.; Simon, M. The Simpson Grading Revisited: Aggressive Surgery and Its Place in Modern Meningioma Management. J. Neurosurg. 2016, 125, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.; Box, G.; Galvin, A.; Brotchie, P.; Trost, N.; Sutherland, T. Magnetic Resonance Imaging of Meningiomas: A Pictorial Review. Insights Imaging 2014, 5, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulos, A.D.; Alexiou, G.A.; Goussia, A.; Papadopoulos, A.; Kyritsis, A.P.; Polyzoidis, K.S.; Voulgaris, S.; Tsiouris, S. (99m)Tc-Tetrofosmin Brain SPECT in the Assessment of Meningiomas-Correlation with Histological Grade and Proliferation Index. J. Neuro-Oncol. 2008, 89, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.H.; McDermott, M.W. The Simpson Grade: Abandon the Scale but Preserve the Message. J. Neurosurg. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Park, S.; Cha, Y.J.; Suh, S.H.; Lee, I.J.; Lee, K.S.; Hong, C.K.; Kim, J.W. Risk Group-Adapted Adjuvant Radiotherapy for WHO Grade I and II Skull Base Meningioma. J. Cancer Res. Clin. Oncol. 2019, 145, 1351–1360. [Google Scholar] [CrossRef]
- Nanda, A.; Vannemreddy, P. Recurrence and Outcome in Skull Base Meningiomas: Do They Differ from Other Intracranial Meningiomas? Skull Base 2008, 18, 243. [Google Scholar] [CrossRef]
- Parteki, S.; Raval, M.; Kumari, R.; Singhal, S.; Gupta, N. Multiple Cranial Nerve Schwannomas with Multifocal Cystic Meningomatosis in a Case of Neurofibromatosis Type 2. Eur. J. Radiol. Extra 2010, 75, e87–e91. [Google Scholar] [CrossRef]
- Black, P.; Morokoff, A.; Zauberman, J.; Claus, E.; Carroll, R. Meningiomas: Science and Surgery. Clin. Neurosurg. 2007, 54, 91. [Google Scholar]
- Chaurasia, B.; Hossain, M.; Kanti Barua, K.; Islam Rokib, R.; Biswas, P.; Sardar, M.; Raut, V.; Samadder, S.; Chaurasiya, R.K.; Chowdhury, D.; et al. Meningiomatosis in Non–Neurofibromatosis Patient: How We Dealt with 8 Tumors. J. Neurol. Stroke 2018, 8, 307–310. [Google Scholar] [CrossRef]
- Stangl, A.P.; Wellenreuther, R.; Lenartz, D.; Kraus, J.A.; Menon, A.G.; Schramm, J.; Wiestler, O.D.; Von Deimling, A. Clonality of Multiple Meningiomas. J. Neurosurg. 1997, 86, 853–858. [Google Scholar] [CrossRef]
- Heinrich, B.; Hartmann, C.; Stemmer-Rachamimov, A.O.; Louis, D.N.; Maccollin, M. Multiple Meningiomas: Investigating the Molecular Basis of Sporadic and Familial Forms. Int. J. Cancer 2002, 103, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Maruyama, T.; Jacoby, L.B.; Herman, J.G.; Gusella, J.F.; Black, P.M.L.; Wu, J.K. Clonal Analysis of a Case of Multiple Meningiomas Using Multiple Molecular Genetic Approaches: Pathology Case Report. Neurosurgery 1999, 45, 409–416. [Google Scholar] [CrossRef]
- Petrella, R.; Levine, S.; Wilmot, P.L.; Ashar, K.D.; Casamassima, A.C.; Shapiro, L.R. Multiple Meningiomas in a Patient with Constitutional Ring Chromosome 22. Am. J. Med. Genet. 1993, 47, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Lomas, J.; Bello, M.J.; Alonso, M.E.; Gonzalez-Gomez, P.; Arjona, D.; Kusak, M.E.; De Campos, J.M.; Sarasa, J.L.; Rey, J.A. Loss of Chromosome 22 and Absence of NF2 Gene Mutation in a Case of Multiple Meningiomas. Hum. Pathol. 2002, 33, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.J.; Tew, J.M.; Simon, M.; Menon, A.G. Evidence for Clonal Spread in the Development of Multiple Meningiomas. J. Neurosurg. 1995, 83, 705–709. [Google Scholar] [CrossRef]
- Multiple Meningiomas in Different Neuraxial Compartments. Report of Two Cases—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/10817390/ (accessed on 17 July 2023).
- Butti, G.; Assietti, R.; Casalone, R.; Paoletti, P. Multiple Meningiomas: A Clinical, Surgical, and Cytogenetic Analysis. Surg. Neurol. 1989, 31, 255–260. [Google Scholar] [CrossRef]
- Kacemi, I.E.; Miloudi, G. Multiple Meningiomatosis. Pan Afr. Med. J. 2021, 40. [Google Scholar] [CrossRef]
- Chen, S.Y.; Hsiao, C.Y.; Tsai, M.A. Multiple Intracranial and Spinal Meningiomas: Management of This Rare Condition. Fu-Jen J. Med. 2019. [Google Scholar] [CrossRef]
- Ohla, V.; Scheiwe, C. Meningiomatosis Restricted to the Left Cerebral Hemisphere with Acute Clinical Deterioration: Case Presentation and Discussion of Treatment Options. Surg. Neurol. Int. 2015, 6. [Google Scholar] [CrossRef]
- Torres, M.; Jackson, N.; O’Farrell, C.; Maher, O.; Niazi, T.; Raghed, J.; Khatib, Z. NFB-07. Meningiomatosis in an Adolescent with TRAF-7 Mutation Related Syndrome. Neuro-Oncology 2022, 24, i129. [Google Scholar] [CrossRef]
- Aldakhil, S.; Mathieu, D. Abscopal Effect Leading to Complete Disappearance of Extensive Meningiomatosis after Gamma Knife Radiosurgery: Case Report. Front. Surg. 2022, 9, 908645. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Lombardi, G.; Farina, P.; Kalamarides, M.; Sanson, M. Successful Treatment of Multiple Intracranial Meningiomas with the Antiprogesterone Receptor Agent Mifepristone (RU486). Acta Neurochir. 2014, 156, 1831–1835. [Google Scholar] [CrossRef]
- Figueroa, B.E.; Quint, D.J.; McKeever, P.E.; Chandler, W.F. Extracranial Metastatic Meningioma. Br. J. Radiol. 2014, 72, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Gottschling, S.; Bolz, J.; Kornhuber, M.; Alfieri, A.; Holzhausen, H.J.; Abbas, J.; Kösling, S. Distant Metastases in Meningioma: An Underestimated Problem. J. Neuro-Oncol. 2013, 112, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Ather Enam, S.; Abdulrauf, S.; Mehta, B.; Malik, G.M.; Mahmood, A. Metastasis in Meningioma. Acta Neurochir. 1996, 138, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Fountain, D.M.; Young, A.M.H.; Santarius, T. Malignant Meningiomas. Handb. Clin. Neurol. 2020, 170, 245–250. [Google Scholar] [CrossRef]
- Dziuk, T.W.; Woo, S.; Butler, E.B.; Thornby, J.; Grossman, R.; Dennis, W.S.; Lu, H.; Carpenter, L.S.; Chiu, J.K. Malignant Meningioma: An Indication for Initial Aggressive Surgery and Adjuvant Radiotherapy. J. Neuro-Oncol. 1998, 37, 177–188. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zheng, C.H.; Li, T.; Su, X.Y.; Lu, G.H.; Zhang, C.Y.; Xiao, S.W.; Tan, Y.F. Intracranial Meningioma Surgery in the Elderly (over 65 Years): Prognostic Factors and Outcome. Acta Neurochir. 2015, 157, 1549–1557. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, J.H.; Park, E.S.; Kim, J.H. “Wait-and-See” Strategies for Newly Diagnosed Intracranial Meningiomas Based on the Risk of Future Observation Failure. World Neurosurg. 2017, 107, 604–611. [Google Scholar] [CrossRef]
- Prabhu, V.C.; Melian, E.; Germanwala, A.v.; Solanki, A.A.; Borys, E.; Barton, K.; Anderson, D.E. Cranial Base Meningiomas. World Neurosurg. 2018, 109, 258–262. [Google Scholar] [CrossRef]
- Starke, R.M.; Przybylowski, C.J.; Sugoto, M.; Fezeu, F.; Awad, A.J.; Ding, D.; Nguyen, J.H.; Sheehan, J.P. Gamma Knife Radiosurgery of Large Skull Base Meningiomas. J. Neurosurg. 2015, 122, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Patibandla, M.R.; Lee, C.C.; Sheehan, J. Stereotactic Radiosurgery of Central Skull Base Meningiomas—Volumetric Evaluation and Long-Term Outcomes. World Neurosurg. 2017, 108, 176–184. [Google Scholar] [CrossRef]
- Sicking, J.; Voß, K.M.; Spille, D.C.; Schipmann, S.; Holling, M.; Paulus, W.; Hess, K.; Steinbicker, A.U.; Stummer, W.; Grauer, O.; et al. The Evolution of Cranial Meningioma Surgery—A Single-Center 25-Year Experience. Acta Neurochir. 2018, 160, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Apra, C.; Peyre, M.; Kalamarides, M. Current Treatment Options for Meningioma. Expert. Rev. Neurother. 2018, 18, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Wolf, M.B.; Kratochwil, C.; Giesel, F.L.; Combs, S.E.; Dimitrakopoulou-Strauss, A.; Gnirs, R.; Roethke, M.C.; Schlemmer, H.P.; Haberkorn, U. Comparison of 68Ga-DOTATOC-PET/CT and PET/MRI Hybrid Systems in Patients with Cranial Meningioma: Initial Results. Neuro-Oncology 2015, 17, 312–319. [Google Scholar] [CrossRef]
- Galldiks, N.; Albert, N.L.; Sommerauer, M.; Grosu, A.L.; Ganswindt, U.; Law, I.; Preusser, M.; Rhun, E.L.; Vogelbaum, M.A.; Zadeh, G.; et al. PET Imaging in Patients with Meningioma—Report of the RANO/PET Group. Neuro-Oncology 2017, 19, 1576. [Google Scholar] [CrossRef]
- Ivanidze, J.; Roytman, M.; Lin, E.; Magge, R.S.; Pisapia, D.J.; Liechty, B.; Karakatsanis, N.; Ramakrishna, R.; Knisely, J.; Schwartz, T.H.; et al. Gallium-68 DOTATATE PET in the Evaluation of Intracranial Meningiomas. J. Neuroimaging 2019, 29, 650–656. [Google Scholar] [CrossRef]
- Galldiks, N.; Lohmann, P.; Albert, N.L.; Tonn, J.C.; Langen, K.J. Current Status of PET Imaging in Neuro-Oncology. Neuro-Oncol. Adv. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Kunz, W.G.; Jungblut, L.M.; Kazmierczak, P.M.; Vettermann, F.J.; Bollenbacher, A.; Tonn, J.C.; Schichor, C.; Rominger, A.; Albert, N.L.; Bartenstein, P.; et al. Improved Detection of Transosseous Meningiomas Using 68Ga-DOTATATE PET/CT Compared with Contrast-Enhanced MRI. J. Nucl. Med. 2017, 58, 1580–1587. [Google Scholar] [CrossRef]
- Magill, S.T.; Dalle Ore, C.L.; Diaz, M.A.; Jalili, D.D.; Raleigh, D.R.; Aghi, M.K.; Theodosopoulos, P.V.; McDermott, M.W. Surgical Outcomes after Reoperation for Recurrent Non-Skull Base Meningiomas. J. Neurosurg. 2018, 131, 1179–1187. [Google Scholar] [CrossRef]
- Magill, S.T.; Lee, D.S.; Yen, A.J.; Lucas, C.H.G.; Raleigh, D.R.; Aghi, M.K.; Theodosopoulos, P.V.; McDermott, M.W. Surgical Outcomes after Reoperation for Recurrent Skull Base Meningiomas. J. Neurosurg. 2018, 130, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.E.; Gillespie, C.S.; Mustafa, M.A.; Taweel, B.A.; Bakhsh, A.; Kumar, S.; Keshwara, S.M.; Ali, T.; John, B.; Brodbelt, A.R.; et al. Clinical Outcomes Following Re-Operations for Intracranial Meningioma. Cancers 2021, 13, 4792. [Google Scholar] [CrossRef] [PubMed]
- Lemée, J.M.; Corniola, M.V.; Meling, T.R. Benefits of Re-Do Surgery for Recurrent Intracranial Meningiomas. Sci. Rep. 2020, 10, 303. [Google Scholar] [CrossRef]
- Huntoon, K.; Toland, A.M.S.; Dahiya, S. Meningioma: A Review of Clinicopathological and Molecular Aspects. Front. Oncol. 2020, 10, 579599. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.L.; Won, M.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Fogh, S.E.; Youssef, E.; et al. High-Risk Meningioma: Initial Outcomes From NRG Oncology/RTOG 0539. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 790–799. [Google Scholar] [CrossRef]
- Rogers, L.; Zhang, P.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Brachman, D.; Jenrette, J.M.; et al. Intermediate-Risk Meningioma: Initial Outcomes from NRG Oncology RTOG 0539. J. Neurosurg. 2017, 129, 35–47. [Google Scholar] [CrossRef]
- Soni, P.; Davison, M.A.; Shao, J.; Momin, A.; Lopez, D.; Angelov, L.; Barnett, G.H.; Lee, J.H.; Mohammadi, A.M.; Kshettry, V.R.; et al. Extent of Resection and Survival Outcomes in World Health Organization Grade II Meningiomas. J. Neuro-Oncol. 2021, 151, 173–179. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, R.; Khosla, D.; Salunke, P.S.; Gupta, S.K.; Radotra, B.D. Survival and Failure Patterns in Atypical and Anaplastic Meningiomas: A Single-Center Experience of Surgery and Postoperative Radiotherapy. J. Cancer Res. Ther. 2015, 11, 735–739. [Google Scholar] [CrossRef]
- Weber, D.C.; Ares, C.; Villa, S.; Peerdeman, S.M.; Renard, L.; Baumert, B.G.; Lucas, A.; Veninga, T.; Pica, A.; Jefferies, S.; et al. Adjuvant Postoperative High-Dose Radiotherapy for Atypical and Malignant Meningioma: A Phase-II Parallel Non-Randomized and Observation Study (EORTC 22042-26042). Radiother. Oncol. 2018, 128, 260–265. [Google Scholar] [CrossRef]
- Noël, G.; Renard, A.; Valéry, C.; Mokhtari, K.; Mazeron, J.J. Role of Radiotherapy in the Treatment of Cerebral Meningiomas. Cancer Radiother. 2001, 5, 217–236. [Google Scholar] [CrossRef]
- Pearson, B.E.; Markert, J.M.; Fisher, W.S.; Guthrie, B.L.; Fiveash, J.B.; Palmer, C.A.; Riley, K. Hitting a Moving Target: Evolution of a Treatment Paradigm for Atypical Meningiomas amid Changing Diagnostic Criteria. Neurosurg. Focus. 2008, 24, E3. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Labrousse, F.; Jouvet, A.; Bauchet, L.; Kalamaridès, M.; Menei, P.; Deruty, R.; Moreau, J.J.; Fèvre-Montange, M.; Guyotat, J. WHO Grade II and III Meningiomas: A Study of Prognostic Factors. J. Neuro-Oncol. 2009, 95, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Farzin, M.; Boehmer, J.; Oehlke, O.; Molls, M.; Debus, J.; Grosu, A.L. Clinical Outcome after High-Precision Radiotherapy for Skull Base Meningiomas: Pooled Data from Three Large German Centers for Radiation Oncology. Radiother. Oncol. 2018, 127, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Rydzewski, N.R.; Lesniak, M.S.; Chandler, J.P.; Kalapurakal, J.A.; Pollom, E.; Tate, M.C.; Bloch, O.; Kruser, T.; Dalal, P.; Sachdev, S. Gross Total Resection and Adjuvant Radiotherapy Most Significant Predictors of Improved Survival in Patients with Atypical Meningioma. Cancer 2018, 124, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Boskos, C.; Feuvret, L.; Noel, G.; Habrand, J.L.; Pommier, P.; Alapetite, C.; Mammar, H.; Ferrand, R.; Boisserie, G.; Mazeron, J.J. Combined Proton and Photon Conformal Radiotherapy for Intracranial Atypical and Malignant Meningioma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 399–406. [Google Scholar] [CrossRef]
- Park, H.J.; Kang, H.C.; Kim, I.H.; Park, S.H.; Kim, D.G.; Park, C.K.; Paek, S.H.; Jung, H.W. The Role of Adjuvant Radiotherapy in Atypical Meningioma. J. Neuro-Oncol. 2013, 115, 241–247. [Google Scholar] [CrossRef]
- Song, D.; Xu, D.; Han, H.; Gao, Q.; Zhang, M.; Wang, F.; Wang, G.; Guo, F. Postoperative Adjuvant Radiotherapy in Atypical Meningioma Patients: A Meta-Analysis Study. Front. Oncol. 2021, 11, 787962. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, W.L.; Aizer, A.; Hua, L.; Tian, M.; Den, J.; Tang, H.; Chen, H.; Wang, Y.; Mao, Y.; et al. Efficacy of Adjuvant Radiotherapy for Atypical and Anaplastic Meningioma. Cancer Med. 2019, 8, 13–20. [Google Scholar] [CrossRef]
- Pollock, B.E.; Stafford, S.L.; Link, M.J.; Garces, Y.I.; Foote, R.L. Stereotactic Radiosurgery of World Health Organization Grade II and III Intracranial Meningiomas: Treatment Results on the Basis of a 22-Year Experience. Cancer 2012, 118, 1048–1054. [Google Scholar] [CrossRef]
- Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-Induced Meningiomas: An Exhaustive Review of the Literature. World Neurosurg. 2017, 97, 635–644.e8. [Google Scholar] [CrossRef]
- Chen, W.C.; Perlow, H.K.; Choudhury, A.; Nguyen, M.P.; Mirchia, K.; Youngblood, M.W.; Lucas, C.H.G.; Palmer, J.D.; Magill, S.T.; Raleigh, D.R. Radiotherapy for Meningiomas. J. Neuro-Oncol. 2022, 160, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Bir, S.C.; Patra, D.P.; Maiti, T.K.; Bollam, P.; Minagar, A.; Nanda, A. Direct Comparison of Gamma Knife Radiosurgery and Microsurgery for Small Size Meningiomas. World Neurosurg. 2017, 101, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, A.A.; Wagle, N.; Zada, G. Recent Developments in Chemotherapy for Meningiomas: A Review. Neurosurg. Focus. 2013, 35, E18. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Hydroxyurea for Recurrent Surgery and Radiation Refractory High-Grade Meningioma. J. Neuro-Oncol. 2012, 107, 315–321. [Google Scholar] [CrossRef]
- Weston, G.J.; Martin, A.J.; Mufti, G.J.; Strong, A.J.; Gleeson, M.J. Hydroxyurea Treatment of Meningiomas: A Pilot Study. Skull Base 2006, 16, 157. [Google Scholar] [CrossRef]
- Raizer, J.J.; Grimm, S.A.; Rademaker, A.; Chandler, J.P.; Muro, K.; Helenowski, I.; Rice, L.; McCarthy, K.; Johnston, S.K.; Mrugala, M.M.; et al. A Phase II Trial of PTK787/ZK 222584 in Recurrent or Progressive Radiation and Surgery Refractory Meningiomas. J. Neuro-oncol. 2014, 117, 93–101. [Google Scholar] [CrossRef]
- Mair, M.J.; Berghoff, A.S.; Brastianos, P.K.; Preusser, M. Emerging Systemic Treatment Options in Meningioma. J. Neuro-Oncol. 2023, 161, 245–258. [Google Scholar] [CrossRef]
- Pachow, D.; Andrae, N.; Kliese, N.; Angenstein, F.; Stork, O.; Wilisch-Neumann, A.; Kirches, E.; Mawrin, C. MTORC1 Inhibitors Suppress Meningioma Growth in Mouse Models. Clin. Cancer Res. 2013, 19, 1180–1189. [Google Scholar] [CrossRef]
- Bertolini, F.; Pecchi, A.; Stefani, A.; Fontana, A.; Rossi, G. Everolimus Effectively Blocks Pulmonary Metastases from Meningioma. Neuro-Oncology 2015, 17, 1301–1302. [Google Scholar] [CrossRef][Green Version]
- Cardona, A.F.; Ruiz-Patiño, A.; Zatarain-Barrón, Z.L.; Hakim, F.; Jiménez, E.; Mejía, J.A.; Ramón, J.F.; Useche, N.; Bermúdez, S.; Pineda, D.; et al. Systemic Management of Malignant Meningiomas: A Comparative Survival and Molecular Marker Analysis between Octreotide in Combination with Everolimus and Sunitinib. PLoS ONE 2019, 14, e0217340. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Glantz, M.J. Interferon-Alpha for Recurrent World Health Organization Grade 1 Intracranial Meningiomas. Cancer 2008, 113, 2146–2151. [Google Scholar] [CrossRef]
- Meningioma Treated with Interferon-α, Evaluated with [11C]-l-Methionine Positron Emission Tomography1|Clinical Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/clincancerres/article/7/8/2269/200031/Meningioma-Treated-with-Interferon-Evaluated-with (accessed on 19 June 2023).
- Han, S.J.; Reis, G.; Kohanbash, G.; Shrivastav, S.; Magill, S.T.; Molinaro, A.M.; McDermott, M.W.; Theodosopoulos, P.V.; Aghi, M.K.; Berger, M.S.; et al. Expression and Prognostic Impact of Immune Modulatory Molecule PD-L1 in Meningioma. J. Neuro-Oncol. 2016, 130, 543–552. [Google Scholar] [CrossRef]
- Li, Y.D.; Veliceasa, D.; Lamano, J.B.; Lamano, J.B.; Kaur, G.; Biyashev, D.; Horbinski, C.M.; Kruser, T.J.; Bloch, O. Systemic and Local Immunosuppression in Patients with High-Grade Meningiomas. Cancer Immunol. Immunother. 2019, 68, 999–1009. [Google Scholar] [CrossRef]
- Garzon-Muvdi, T.; Bailey, D.D.; Pernik, M.N.; Pan, E. Basis for Immunotherapy for Treatment of Meningiomas. Front. Neurol. 2020, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Kim, A.E.; Giobbie-Hurder, A.; Lee, E.Q.; Wang, N.; Eichler, A.F.; Chukwueke, U.; Forst, D.A.; Arrillaga-Romany, I.C.; Dietrich, J.; et al. Phase 2 Study of Pembrolizumab in Patients with Recurrent and Residual High-Grade Meningiomas. Nat. Commun. 2022, 13, 1325. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Al-Khadairi, G.; Roelands, J.; Hendrickx, W.; Dermime, S.; Bedognetti, D.; Decock, J. NY-ESO-1 Based Immunotherapy of Cancer: Current Perspectives. Front. Immunol. 2018, 9, 354963. [Google Scholar] [CrossRef] [PubMed]
- Flem-Karlsen, K.; Fodstad, Ø.; Tan, M.; Nunes-Xavier, C.E. B7-H3 in Cancer—Beyond Immune Regulation. Trends Cancer 2018, 4, 401–404. [Google Scholar] [CrossRef]
- Proctor, D.T.; Patel, Z.; Lama, S.; Resch, L.; van Marle, G.; Sutherland, G.R. Identification of PD-L2, B7-H3 and CTLA-4 Immune Checkpoint Proteins in Genetic Subtypes of Meningioma. Oncoimmunology 2018, 8, e1512943. [Google Scholar] [CrossRef]
- Kaley, T.J.; Wen, P.; Schiff, D.; Ligon, K.; Haidar, S.; Karimi, S.; Lassman, A.B.; Nolan, C.P.; De Angelis, L.M.; Gavrilovic, I.; et al. Phase II Trial of Sunitinib for Recurrent and Progressive Atypical and Anaplastic Meningioma. Neuro-Oncology 2015, 17, 116. [Google Scholar] [CrossRef]
- Lou, E.; Sumrall, A.L.; Turner, S.; Peters, K.B.; Desjardins, A.; Vredenburgh, J.J.; McLendon, R.E.; Herndon, J.E.; McSherry, F.; Norfleet, J.; et al. Bevacizumab Therapy for Adults with Recurrent/Progressive Meningioma: A Retrospective Series. J. Neuro-Oncol. 2012, 109, 63. [Google Scholar] [CrossRef]
- Nayak, L.; Iwamoto, F.M.; Rudnick, J.D.; Norden, A.D.; Lee, E.Q.; Drappatz, J.; Omuro, A.; Kaley, T.J. Atypical and Anaplastic Meningiomas Treated with Bevacizumab. J. Neuro-Oncol. 2012, 109, 187–193. [Google Scholar] [CrossRef]
- Pei, J.; Li, P.; Gao, Y.H.; Tian, B.G.; Wang, D.Y.; Zheng, Y.; Liu, L.Y.; Zhang, Z.Y.; Huang, S.S.; Wen, M.; et al. Type IV Collagen-Derived Angiogenesis Inhibitor: Canstatin Low Expressing in Brain-Invasive Meningiomas Using Liquid Chromatography-Mass Spectrometry (LC-MS/MS). J. Neuro-Oncol. 2023, 161, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.J.; Skelton, W.P.; Woody, L.E.; Bregy, A.; Shah, A.H.; Vakharia, K.; Komotar, R.J. Role of Bevacizumab for Treatment-Refractory Meningiomas: A Systematic Analysis and Literature Review. Surg. Neurol. Int. 2018, 9. [Google Scholar] [CrossRef]
- Alexander, A.Y.; Onyedimma, C.; Bhandarkar, A.R.; Yolcu, Y.U.; Michalopoulos, G.D.; Bydon, M.; Link, M.J. The Role of Bevacizumab for Treatment-Refractory Intracranial Meningiomas: A Single Institution’s Experience and a Systematic Review of the Literature. Acta Neurochir. 2022, 164, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J. How Useful Is Chemotherapy for Atypical and Anaplastic Meningiomas? Expert. Opin. Pharmacother. 2022, 23, 1559–1561. [Google Scholar] [CrossRef]
- Wang, J.Z.; Patil, V.; Liu, J.; Dogan, H.; Tabatabai, G.; Yefet, L.S.; Behling, F.; Hoffman, E.; Bunda, S.; Yakubov, R.; et al. Increased MRNA Expression of CDKN2A Is a Transcriptomic Marker of Clinically Aggressive Meningiomas. Acta Neuropathol. 2023, 146, 145–162. [Google Scholar] [CrossRef]
- Bondy, M.; Lee Ligon, B. Epidemiology and Etiology of Intracranial Meningiomas: A Review. J. Neuro-Oncol. 1996, 29, 197–205. [Google Scholar] [CrossRef]
- Treatment of Human Malignant Meningiomas by G207, a Replication-Competent Multimutated Herpes Simplex Virus 11|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/55/21/4752/501834/Treatment-of-Human-Malignant-Meningiomas-by-G207-a (accessed on 19 June 2023).
- Jungwirth, G.; Yu, T.; Liu, F.; Cao, J.; Alaa Eddine, M.; Moustafa, M.; Abdollahi, A.; Warta, R.; Unterberg, A.; Herold-Mende, C. Pharmacological Landscape of FDA-Approved Anticancer Drugs Reveals Sensitivities to Ixabepilone, Romidepsin, Omacetaxine, and Carfilzomib in Aggressive Meningiomas. Clin. Cancer Res. 2023, 29, 233–243. [Google Scholar] [CrossRef]
- Fountzilas, G.; Kotoula, V.; Pectasides, D.; Kouvatseas, G.; Timotheadou, E.; Bobos, M.; Mavropoulou, X.; Papadimitriou, C.; Vrettou, E.; Raptou, G.; et al. Ixabepilone Administered Weekly or Every Three Weeks in HER2-Negative Metastatic Breast Cancer Patients; a Randomized Non-Comparative Phase II Trial. PLoS ONE 2013, 8, e69256. [Google Scholar] [CrossRef]
- Ibrahim, N.K. Ixabepilone: Overview of Effectiveness, Safety, and Tolerability in Metastatic Breast Cancer. Front. Oncol. 2021, 11, 617874. [Google Scholar] [CrossRef]
- Egerton, N. Ixabepilone (Ixempra), a Therapeutic Option for Locally Advanced or Metastatic Breast Cancer. Pharm. Ther. 2008, 33, 523. [Google Scholar]
- Brastianos, P.K.; Twohy, E.L.; Gerstner, E.R.; Kaufmann, T.J.; Iafrate, A.J.; Lennerz, J.; Jeyapalan, S.; Piccioni, D.E.; Monga, V.; Fadul, C.E.; et al. Alliance A071401: Phase II Trial of Focal Adhesion Kinase Inhibition in Meningiomas with Somatic NF2 Mutations. J. Clin. Oncol. 2023, 41, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Shahbandi, A.; Shah, D.S.; Hadley, C.C.; Patel, A.J. The Role of Pharmacotherapy in Treatment of Meningioma: A Systematic Review. Cancers 2023, 15, 483. [Google Scholar] [CrossRef] [PubMed]
- Pellerino, A.; Bruno, F.; Palmiero, R.; Pronello, E.; Bertero, L.; Soffietti, R.; Rudà, R. Clinical Significance of Molecular Alterations and Systemic Therapy for Meningiomas: Where Do We Stand? Cancers 2022, 14, 2256. [Google Scholar] [CrossRef]
- Dobran, M.; Marini, A.; Splavski, B.; Rotim, K.; Liverotti, V.; Nasi, D.; Iacoangeli, M. Surgical Treatment and Predictive Factors for Atypical Meningiomas: A Multicentric Experience. World Neurosurg. 2020, 144, e1–e8. [Google Scholar] [CrossRef]
- Schneider, M.; Schuss, P.; Güresir, Á.; Wach, J.; Hamed, M.; Vatter, H.; Güresir, E. Cranial Nerve Outcomes After Surgery for Frontal Skull Base Meningiomas: The Eternal Quest of the Maximum-Safe Resection with the Lowest Morbidity. World Neurosurg. 2019, 125, e790–e796. [Google Scholar] [CrossRef]
- Schneider, M.; Schuss, P.; Güresir, Á.; Borger, V.; Vatter, H.; Güresir, E. Surgery for Posterior Fossa Meningioma: Elevated Postoperative Cranial Nerve Morbidity Discards Aggressive Tumor Resection Policy. Neurosurg. Rev. 2021, 44, 953–959. [Google Scholar] [CrossRef]
- Giammattei, L.; Starnoni, D.; Levivier, M.; Messerer, M.; Daniel, R.T. Surgery for Clinoidal Meningiomas: Case Series and Meta-Analysis of Outcomes and Complications. World Neurosurg. 2019, 129, e700–e717. [Google Scholar] [CrossRef]
- Giordan, E.; Sorenson, T.J.; Lanzino, G. Optimal Surgical Strategy for Meningiomas Involving the Superior Sagittal Sinus: A Systematic Review. Neurosurg. Rev. 2020, 43, 525–535. [Google Scholar] [CrossRef]
- Oya, S.; Ikawa, F.; Ichihara, N.; Wanibuchi, M.; Akiyama, Y.; Nakatomi, H.; Mikuni, N.; Narita, Y. Nation-Wide Brain Tumor Registry-Based Study of Intracranial Meningioma in Japan: Analysis of Surgery-Related Risks. Neurol. Med.-Chir. 2020, 61, 98–106. [Google Scholar] [CrossRef]
- Williams, B.J.; Nguyen, J. Gamma Knife Radiosurgery for Parasellar Meningiomas: Long-Term Results Including Complications, Predictive Factors and Progression Free Survival. Artic. J. Neurosurg. 2011, 114, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Heald, J.B.; Carroll, T.A.; Mair, R.J. Simpson Grade: An Opportunity to Reassess the Need for Complete Resection of Meningiomas. Acta Neurochir. 2014, 156, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Samadian, M.; Sharifi, G.; Mousavinejad, S.A.; Amin, A.A.; Ebrahimzadeh, K.; Tavassol, H.H.; Borghei-Razavi, H.; Rezaei, O. Surgical Outcomes of Sphenoorbital En Plaque Meningioma: A 10-Year Experience in 57 Consecutive Cases. World Neurosurg. 2020, 144, e576–e581. [Google Scholar] [CrossRef]
- Savardekar, A.R.; Patra, D.P.; Bir, S.; Thakur, J.D.; Mohammed, N.; Bollam, P.; Georgescu, M.M.; Nanda, A. Differential Tumor Progression Patterns in Skull Base Versus Non–Skull Base Meningiomas: A Critical Analysis from a Long-Term Follow-Up Study and Review of Literature. World Neurosurg. 2018, 112, e74–e83. [Google Scholar] [CrossRef] [PubMed]
- da Broi, M.; Borrelli, P.; Meling, T.R. Predictors of Survival in Subtotally Resected WHO Grade I Skull Base Meningiomas. Cancers 2021, 13, 1451. [Google Scholar] [CrossRef]
- Keric, N.; Kalasauskas, D.; Freyschlag, C.F.; Gempt, J.; Misch, M.; Poplawski, A.; Lange, N.; Ayyad, A.; Thomé, C.; Vajkoczy, P.; et al. Impact of Postoperative Radiotherapy on Recurrence of Primary Intracranial Atypical Meningiomas. J. Neuro-Oncol. 2020, 146, 347–355. [Google Scholar] [CrossRef]
- Li, D.; Jiang, P.; Xu, S.; Li, C.; Xi, S.; Zhang, J.; Chen, Y.; Jiang, X.; Zhang, X.; Sai, K.; et al. Survival Impacts of Extent of Resection and Adjuvant Radiotherapy for the Modern Management of High-Grade Meningiomas. J. Neuro-Oncol. 2019, 145, 125–134. [Google Scholar] [CrossRef]
- Chun, S.W.; Kim, K.M.; Kim, M.S.; Kang, H.; Dho, Y.S.; Seo, Y.; Kim, J.W.; Kim, Y.H.; Park, C.K. Adjuvant Radiotherapy versus Observation Following Gross Total Resection for Atypical Meningioma: A Systematic Review and Meta-Analysis. Radiat. Oncol. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Abrar Choudhury, A.; Cady, M.A.; Lucas, C.H.G.; Najem, H.; Phillips, J.J.; Palikuqi, B.; Zakimi, N.; Joseph, T.; Ochoa Birrueta, J.; Chen, W.C.; et al. NOTCH3 Drives Meningioma Tumorigenesis and Resistance to Radiotherapy. bioRxiv 2023. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO Guideline on the Diagnosis and Management of Meningiomas. Neuro-Oncology 2021, 23, 1821. [Google Scholar] [CrossRef]

| WHO Grade | Description |
|---|---|
| Grade 1 | Low mitotic rate, <4 per 10 HPFs No brain invasion 9 histologic subtypes |
| Grade 2 (atypical-aggressive) | Mitotic rate 4–19 per 10 HPF or Brain invasion or ≥3 or 5 specific histologies: • Spontaneous or geographic necrosis; • Patternless sheet-like growth; • Prominent nucleoli; • High cellularity; • Small cells with high n:c ratio. |
| Grade 3 (anaplastic-aggressive and malignant) | Mitotic rate > 20 per 10 HPF (HPF: high-power field) or Specific histologies: papillary or rhabdoid (since WHO 2021 graded on the basis of the same criteria for atypia and anaplasia as for other meningioma variants). Since WHO 2021: frank anaplasia (melanoma-, sarcoma- or carcinoma-like histology) CDKN2A and/or CDKN2B homozygous deletion TERT promoter mutation |
| Grade 1 | Macroscopically complete tumor removal with affected dura and underlined bone. |
| Grade 2 | Macroscopically complete tumor removal with coagulation of affected dura only. |
| Grade 3 | Macroscopically complete tumor removal without removal of affected dura or underlying bone |
| Grade 4 | Subtotal tumor resection |
| Grade 5 | Tumor decompression with or without biopsy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trakolis, L.; Petridis, A.K. Interdisciplinary Therapeutic Approaches to Atypical and Malignant Meningiomas. Cancers 2023, 15, 4251. https://doi.org/10.3390/cancers15174251
Trakolis L, Petridis AK. Interdisciplinary Therapeutic Approaches to Atypical and Malignant Meningiomas. Cancers. 2023; 15(17):4251. https://doi.org/10.3390/cancers15174251
Chicago/Turabian StyleTrakolis, Leonidas, and Athanasios K. Petridis. 2023. "Interdisciplinary Therapeutic Approaches to Atypical and Malignant Meningiomas" Cancers 15, no. 17: 4251. https://doi.org/10.3390/cancers15174251
APA StyleTrakolis, L., & Petridis, A. K. (2023). Interdisciplinary Therapeutic Approaches to Atypical and Malignant Meningiomas. Cancers, 15(17), 4251. https://doi.org/10.3390/cancers15174251








