Obstetric Results after Fertility-Sparing Management of Non-Epithelial Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
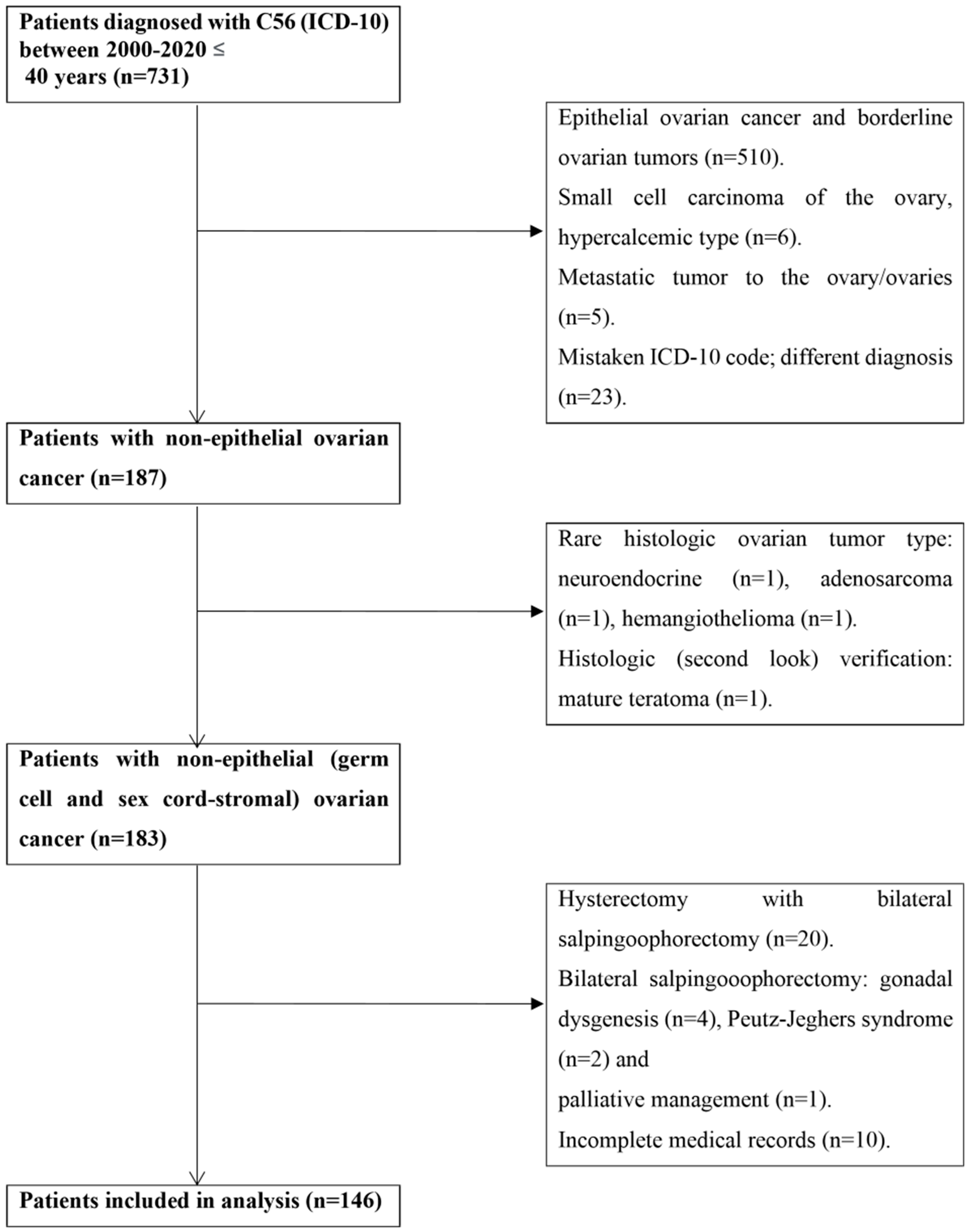
2. Materials and Methods
2.1. Ethics Approval
2.2. Statistics
3. Results
3.1. Surgical Management
3.2. Adjuvant Treatment
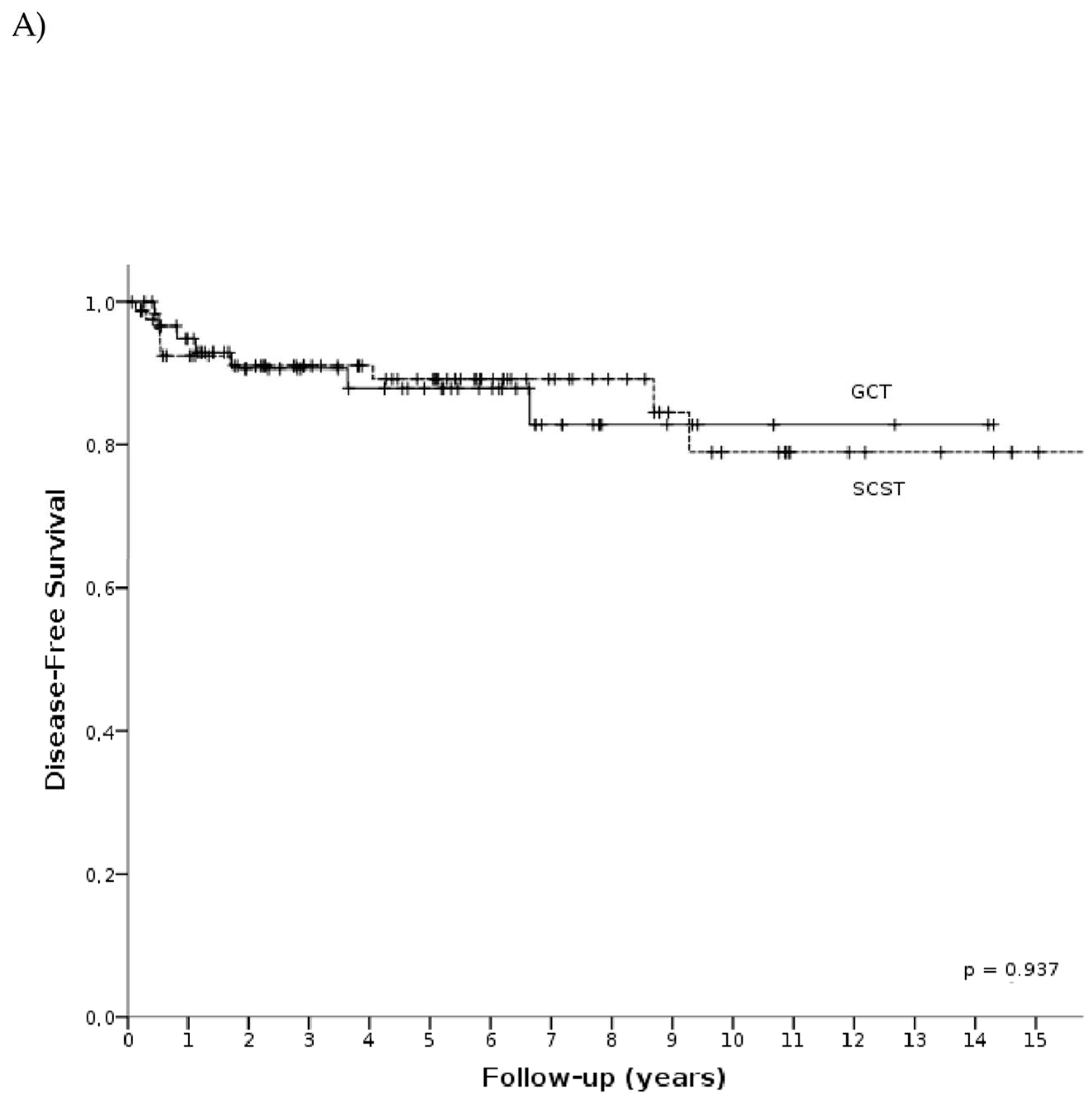
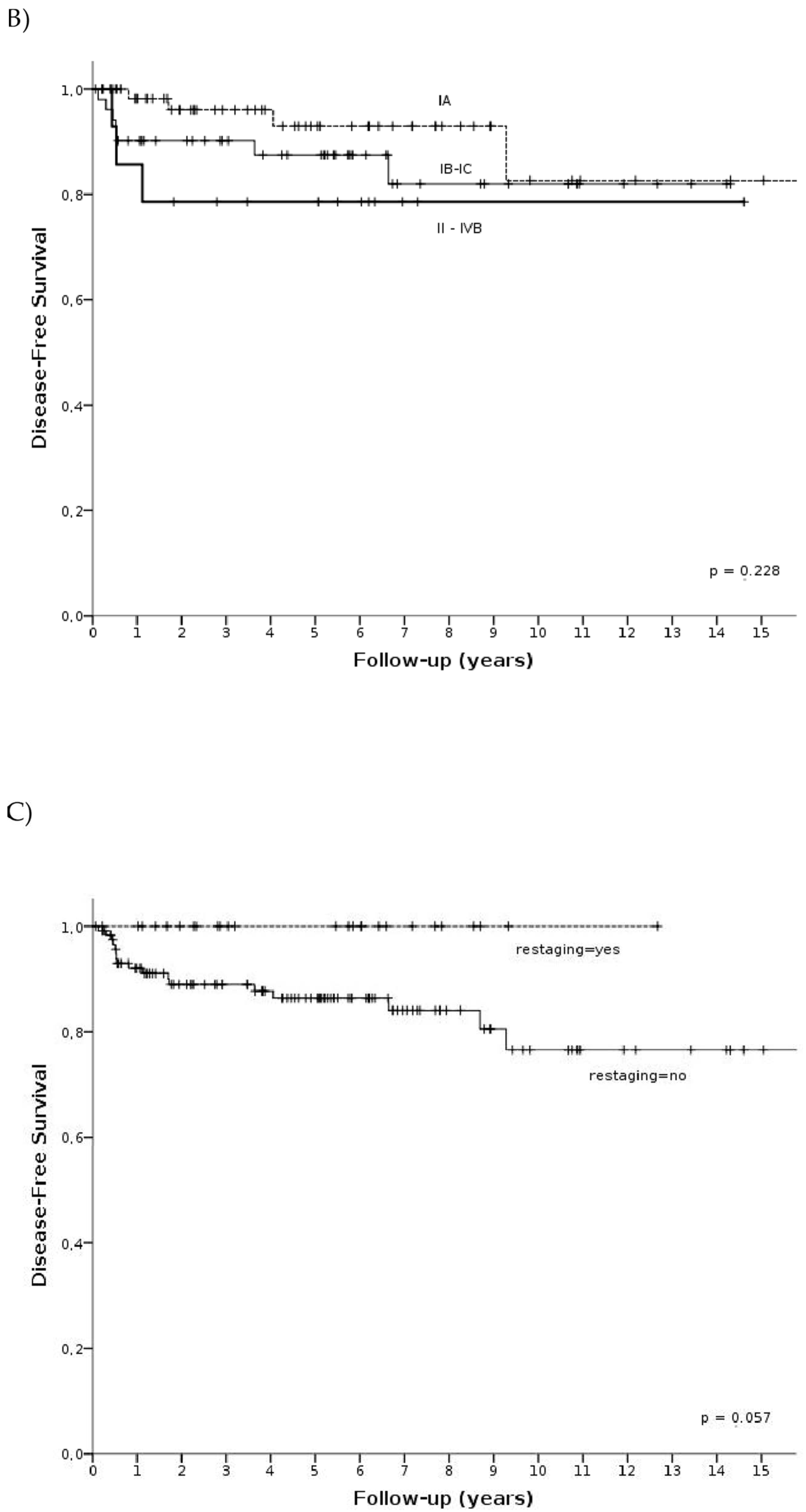
3.3. Survival Analysis
3.4. Obstetric Outcomes
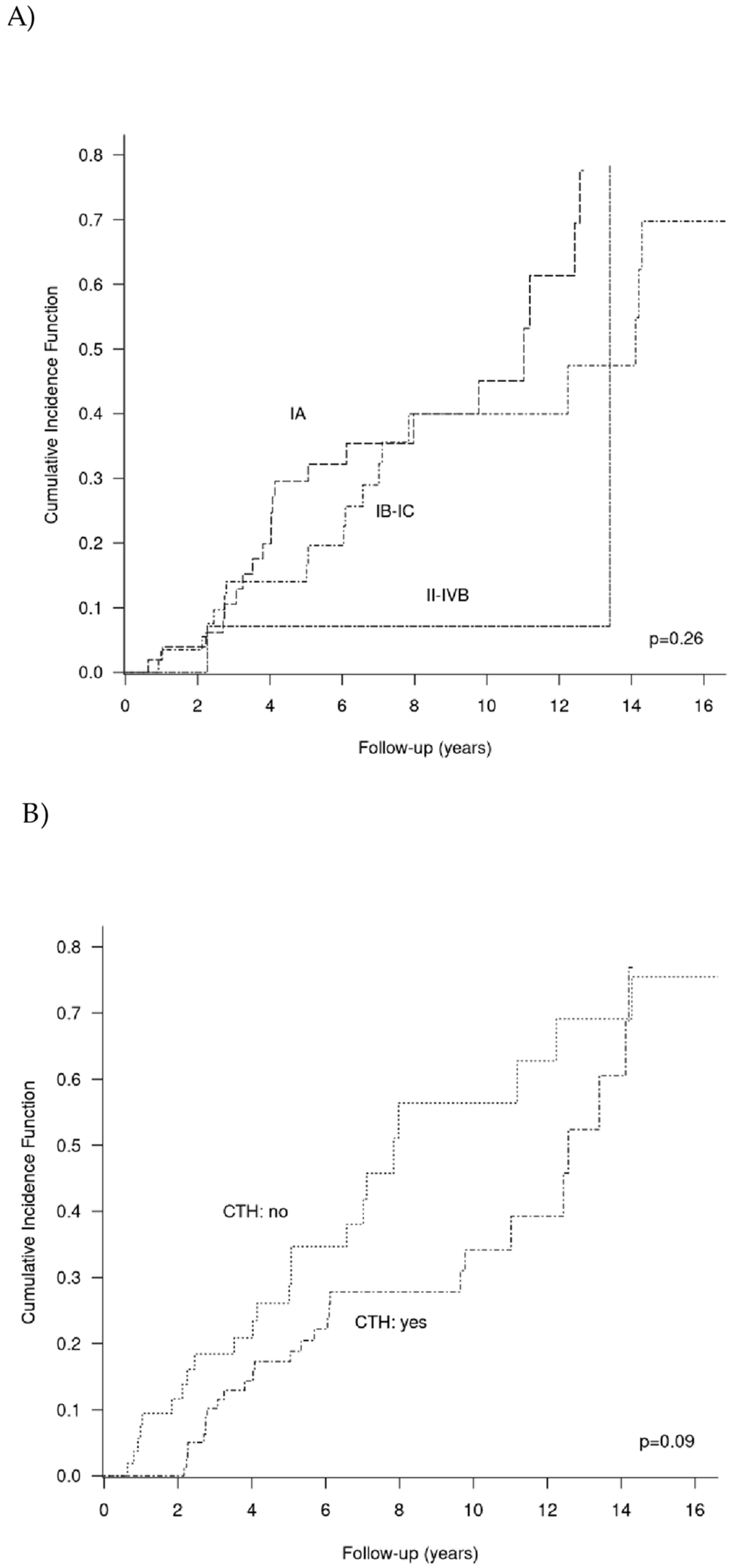
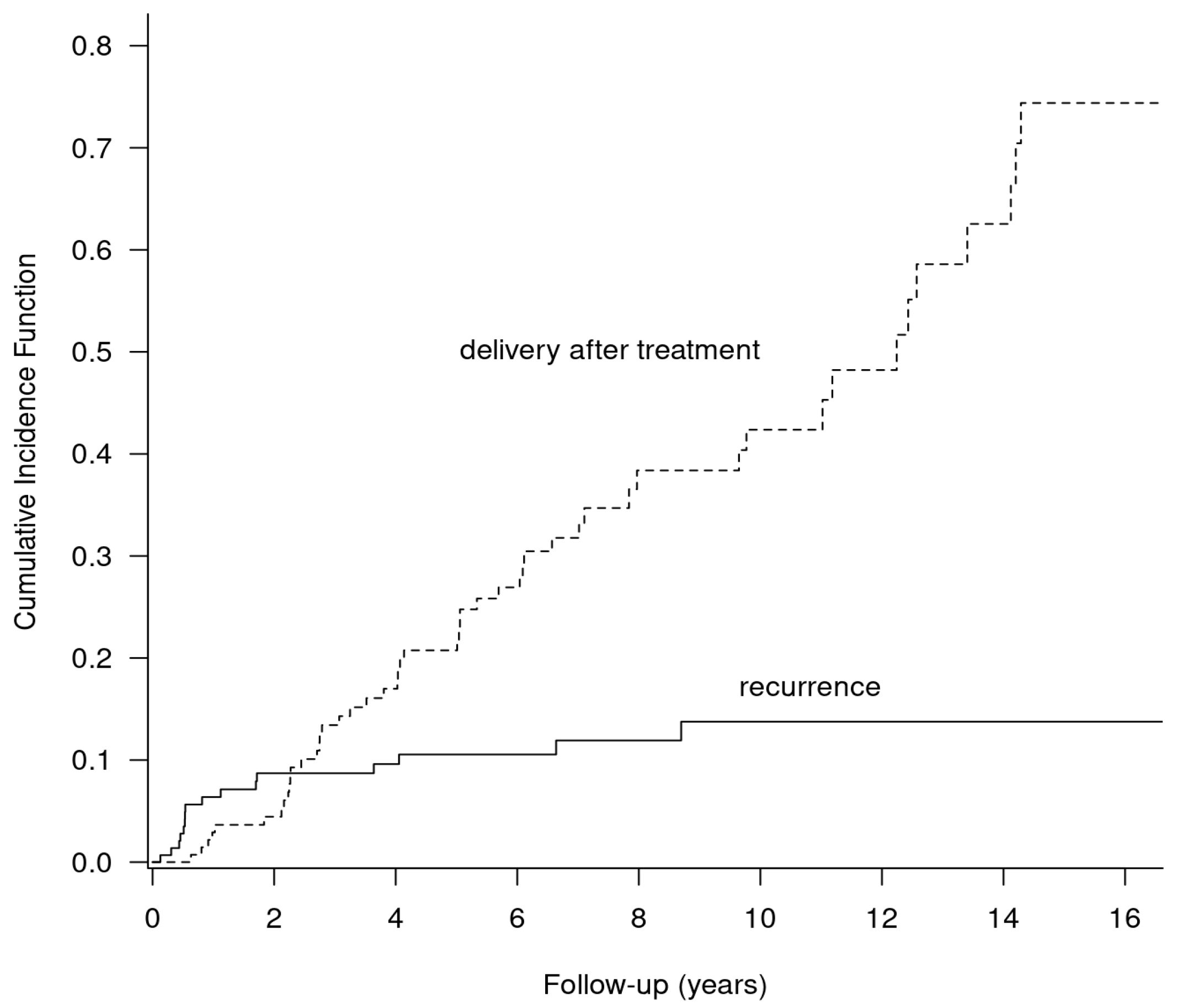
3.5. Competing Risks Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv1–iv18. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.O.; Berwick, M.; Verschraegen, C.F.; Wiggins, C.; Lansing, L.; Muller, C.Y.; Qualls, C.R. Incidence and Survival Rates for Female Malignant Germ Cell Tumors. Obstet. Gynecol. 2006, 107, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Haroon, S.; Zia, A.; Idrees, R.; Memon, A.; Fatima, S.; Kayani, N. Clinicopathological spectrum of ovarian sex cord-stromal tumors; 20 years’ retrospective study in a developing country. J. Ovarian Res. 2013, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Shah, S.; Parker, J.; Soor, P.; Limbu, A.; Sheriff, M.; Boussios, S. Non-Epithelial Ovarian Cancers: How Much Do We Really Know? Int. J. Environ. Res. Public Health 2022, 19, 1106. [Google Scholar] [CrossRef]
- Boussios, S.; Zarkavelis, G.; Seraj, E.; Zerdes, I.; Tatsi, K.; Pentheroudakis, G. Non-epithelial Ovarian Cancer: Elucidating Uncommon Gynaecological Malignancies. Anticancer Res. 2016, 36, 5031–5042. [Google Scholar] [CrossRef]
- Johansen, G.; Dahm-Kähler, P.; Staf, C.; Rådestad, A.F.; Rodriguez-Wallberg, K.A. Fertility-sparing surgery for treatment of non-epithelial ovarian cancer: Oncological and reproductive outcomes in a prospective nationwide population-based cohort study. Gynecol. Oncol. 2019, 155, 287–293. [Google Scholar] [CrossRef]
- Piątek, S.; Szymusik, I.; Dańska-Bidzińska, A.; Ołtarzewski, M.; Trojan, G.; Bidziński, M. Fertility-Sparing Management May Be Considered in Young Women with Uterine Sarcoma. J. Clin. Med. 2022, 11, 4761. [Google Scholar] [CrossRef]
- Vasta, F.M.; Dellino, M.; Bergamini, A.; Gargano, G.; Paradiso, A.; Loizzi, V.; Bocciolone, L.; Silvestris, E.; Petrone, M.; Cormio, G.; et al. Reproductive Outcomes and Fertility Preservation Strategies in Women with Malignant Ovarian Germ Cell Tumors after Fertility Sparing Surgery. Biomedicines 2020, 8, 554. [Google Scholar] [CrossRef]
- Dellino, M.; Silvestris, E.; Loizzi, V.; Paradiso, A.; Loiacono, R.; Minoia, C.; Daniele, A.B.; Cormio, G. Germinal ovarian tumors in reproductive age women: Fertility-sparing and outcome. Medicine 2020, 99, e22146. [Google Scholar] [CrossRef]
- Wang, D.; Cang, W.; Zhu, S.; Jia, C.; Cao, D.; Yang, J.; Xiang, Y. Oncological and Reproductive Outcomes in Patients with Advanced-Stage Ovarian Immature Teratoma: Experience from a Tertiary Center. Front. Oncol. 2022, 12, 822341. [Google Scholar] [CrossRef]
- Negri, S.; Grassi, T.; Fruscio, R. Use of staging for sex cord stromal tumours. Curr. Opin. Oncol. 2022, 34, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Sood, A.K.; Deavers, M.T.; Milojevic, L.; Gershenson, D.M. Patterns of metastasis in sex cord-stromal tumors of the ovary: Can routine staging lymphadenectomy be omitted? Gynecol. Oncol. 2009, 113, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Kanninen, T.T.; Holcomb, K.; Sisti, G.; Witkin, S.S. Prevalence of lymph node metastasis and prognostic significance of lymphadenectomy in apparent early-stage malignant ovarian sex cord-stromal tumors. Gynecol. Oncol. 2017, 145, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shah, J.P.; Bryant, C.S.; Imudia, A.N.; Cote, M.L.; Ali-Fehmi, R.; Malone, J.M.; Morris, R.T. The prevalence and prognostic impact of lymph node metastasis in malignant germ cell tumors of the ovary. Gynecol. Oncol. 2008, 110, 125–132. [Google Scholar] [CrossRef]
- Ertas, I.E.; Taskin, S.; Goklu, R.; Bilgin, M.; Goc, G.; Yildirim, Y.; Ortac, F. Long-term oncological and reproductive outcomes of fertility-sparing cytoreductive surgery in females aged 25 years and younger with malignant ovarian germ cell tumors. J. Obstet. Gynaecol. Res. 2014, 40, 797–805. [Google Scholar] [CrossRef]
- Billmire, D.; Vinocur, C.; Rescorla, F.; Cushing, B.; London, W.; Schlatter, M.; Davis, M.; Giller, R.; Lauer, S.; Olson, T. Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: An intergroup study. J. Pediatr. Surg. 2004, 39, 424–429. [Google Scholar] [CrossRef]
- Mahdi, H.; Swensen, R.E.; Hanna, R.; Kumar, S.; Ali-Fehmi, R.; Semaan, A.; Tamimi, H.; Morris, R.T.; Munkarah, A.R. Prognostic impact of lymphadenectomy in clinically early stage malignant germ cell tumour of the ovary. Br. J. Cancer 2011, 105, 493–497. [Google Scholar] [CrossRef]
- Kaur, B. Pathology of malignant ovarian germ cell tumours. Diagn. Histopathol. 2020, 26, 289–297. [Google Scholar] [CrossRef]
- Gershenson, D.M. Management of Ovarian Germ Cell Tumors. J. Clin. Oncol. 2007, 25, 2938–2943. [Google Scholar] [CrossRef]
- Chan, J.K.; Tewari, K.S.; Waller, S.; Cheung, M.K.; Shin, J.Y.; Osann, K.; Kapp, D.S. The influence of conservative surgical practices for malignant ovarian germ cell tumors. J. Surg. Oncol. 2008, 98, 111–116. [Google Scholar] [CrossRef]
- Di Tucci, C.; Casorelli, A.; Morrocchi, E.; Palaia, I.; Muzii, L.; Panici, P.B. Fertility management for malignant ovarian germ cell tumors patients. Crit. Rev. Oncol. 2017, 120, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Thomakos, N.; Malakasis, A.; Machairiotis, N.; Zarogoulidis, P.; Rodolakis, A. Fertility Sparing Management in Non-Epithelial Ovarian Cancer. Which Patients, What Procedure and What Outcome? J. Cancer 2018, 9, 4659–4664. [Google Scholar] [CrossRef] [PubMed]
- Presti, A.L.; Ruvolo, G.; Gancitano, R.; Cittadini, E. Ovarian function following radiation and chemotherapy for cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Raafat, F.; Robinson, K.; Imeson, J.; Gornall, P.; Sokal, M.; Gray, E.; McKeever, P.; Hale, J.; Bailey, S.; et al. The United Kingdom Children’s Cancer Study Group’s Second Germ Cell Tumor Study: Carboplatin, Etoposide, and Bleomycin Are Effective Treatment for Children with Malignant Extracranial Germ Cell Tumors, with Acceptable Toxicity. J. Clin. Oncol. 2000, 18, 3809–3818. [Google Scholar] [CrossRef] [PubMed]
- Tangir, J.; Zelterman, D.; Ma, W.; Schwartz, P.E. Reproductive function after conservative surgery and chemotherapy for malignant germ celltumors of the ovary. Obstet. Gynecol. 2003, 101, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zamani, N.; Poor, M.R.; Dizajmehr, S.G.; Alizadeh, S.; Gilani, M.M. Fertility sparing surgery in malignant ovarian Germ cell tumor (MOGCT): 15 years experiences. BMC Women’s Health 2021, 21, 282. [Google Scholar] [CrossRef]
- Turan, V.; Lambertini, M.; Lee, D.-Y.; Wang, E.; Clatot, F.; Karlan, B.Y.; Demeestere, I.; Bang, H.; Oktay, K. Association of Germline BRCA Pathogenic Variants with Diminished Ovarian Reserve: A Meta-Analysis of Individual Patient-Level Data. J. Clin. Oncol. 2021, 39, 2016–2024. [Google Scholar] [CrossRef]
- Zanetta, G.; Bonazzi, C.; Cantù, M.G.; Bini, S.; Locatelli, A.; Bratina, G.; Mangioni, C. Survival and Reproductive Function After Treatment of Malignant Germ Cell Ovarian Tumors. J. Clin. Oncol. 2001, 19, 1015–1020. [Google Scholar] [CrossRef]
- Yang, B.; Yu, Y.; Chen, J.; Zhang, Y.; Yin, Y.; Yu, N.; Chen, G.; Zhu, S.; Huang, H.; Yuan, Y.; et al. Possibility of women treated with fertility-sparing surgery for non-epithelial ovarian tumors to safely and successfully become pregnant—A Chinese retrospective cohort study among 148 cases. Front. Med. 2018, 12, 509–517. [Google Scholar] [CrossRef]
- Piątek, S.; Szymusik, I.; Bidziński, M. Reproductive Results in Cancer Survivors after Fertility Sparing Management: The Need for the Standardization of Definitions. Cancers 2023, 15, 3569. [Google Scholar] [CrossRef] [PubMed]

| Variable | n = 146 (100%) | GCT (n = 84; 100%) | SCST (n = 62, 100%) |
|---|---|---|---|
| Histology | Dysgerminoma (n = 26, 30.95%) | Granulosa cell tumor (n = 46, 74.19%) | |
| Immature teratoma (n = 25, 29.76%) | Sertoli-Leydig cell tumor (n = 13, 20.97%) | ||
| Mixed GCT (n = 19, 22.62%) | Mixed SCST (n = 1, 1.61%) | ||
| Yolk sac tumor (n = 11, 13.1%) | Other (n = 2, 3.23%) | ||
| Other (n = 3, 3.57%) | |||
| Age at diagnosis (years) | |||
| <25 | 64 (43.84%) | 46 (54.76%) | 18 (29.03%) |
| 25–30 | 44 (30.14%) | 29 (34.53%) | 15 (24.19%) |
| 31–35 | 26 (17.81%) | 5 (5.95%) | 21 (33.87%) |
| 36–40 | 12 (8.21%) | 4 (4.76%) | 8 (12.91%) |
| FIGO stage | |||
| I | 133 (91.09%) | 74 (88.09%) | 59 (95.16%) |
| IA | 65 (44.52%) | 38 (45.24%) | 27 (43.55%) |
| IB | 1 (0.68%) | 1 (1.19%) | 0 |
| IC IC1 IC2 IC3 IC (not specified) | 51 (34.93%) 20 (13.69%) 10 (6.85%) 5 (3.42%) 16 (10.96%) | 28 (33.33%) 5 (5.95%) 7 (8.33%) 3 (3.57%) 13 (15.48%) | 23 (37.09%) 15 (24.19%) 3 (4.84%) 2 (3.22%) 3 (4.84%) |
| I (not specified) | 16 (10.96%) | 5 (5.95%) | 9 (14.51%) |
| II IIA IIB | 3 (2.05%) 1 (0.68%) 2 (1.37%) | 3 (3.57%) 1 (1.19%) 2 (2.38%) | 0 |
| III IIIA IIIB IIIC III (not specified) | 9 (6.16%) 3 (2.05%) 3 (2.05%) 2 (1.37%) 1 (0.68%) | 7 (8.33%) 3 (3.57%) 2 (2.38%) 1 (1.19%) 1 (1.19%) | 2 (3.22%) 0 1 (1.61%) 1 (1.61%) 0 |
| IVB | 1 (0.68%) | 1 (1.19%) | 0 |
| Primary surgical approach | |||
| Laparotomy | 96 (65.75%) | 71 (84.52%) | 25 (40.32%) |
| Laparoscopy | 39 (26.71%) | 12 (14.29%) | 27 (43.55%) |
| Unknown | 11 (7.53%) | 1 (1.19%) | 10 (16.13%) |
| Type of primary surgery | |||
| Cystectomy/tumorectomy | 15 (10.27%) | 13 (15.48%) | 2 (3.22%) |
| Adnexectomy | 120 (82.2%) | 63 (75%) | 57 (91.94%) |
| Adnexectomy with contralateral cystectomy | 11 (7.53%) | 8 (9.52%) | 3 (4.84%) |
| Restaging surgery | 26 (17.81%) | 13 (15.48%) | 13 (20.97%) |
| Pelvic lymphadenectomy * | 33 (22.6%) | 22 (26.19%) | 11 (17.74%) |
| Mean/Number (Range/%) | |
|---|---|
| Age | 27.62 (17–38) |
| Days between first and restaging surgery | 63 (34–83) |
| FIGO stage primary/after restaging | |
| IA => IA | 12 (46.15%) |
| IA => IC | 2 (7.69%) |
| IC => IC | 9 (34.62%) |
| IC => IIB | 1 (3.85%) |
| I unspecified => I unspecified | 2 (7.69%) |
| Histology | |
| Granulosa cell tumor | 9 (34.62%) |
| Sertoli Leydig cell tumor | 4 (15.38%) |
| Mixed SCST | 2 (7.69%) |
| Dysgerminoma | 5 (19.23%) |
| Immature teratoma | 3 (11.54%) |
| Mixed GCT | 3 (11.54%) |
| Stage Histology | Number of Patients with Adjuvant Chemotherapy (Yes/No) | ||||||
|---|---|---|---|---|---|---|---|
| IA | IB | IC | I (Not Specified) | ≥II | Total | ||
| Germ cell tumor | Dysgerminoma | 2/8 | 1/- | 8/1 | -/- | 6/- | 17/9 |
| Immature teratoma | 9/5 | -/- | 6/- | 2/- | 3/- | 20/5 | |
| Mixed GCT | 4/2 | 8/1 | 3/- | 1/- | 16/3 | ||
| Yolk sac tumor | 6/1 | -/- | 3/- | -/- | 1/- | 10/1 | |
| Other GCT | -/2 | -/- | 1/- | -/- | -/- | 1/2 | |
| Total | 21/18 | 1/- | 26/2 | 5/- | 11/- | 64/20 | |
| Sex cord-stromal tumor | Granulosa cell tumor | -/20 | -/- | 9/10 | 3/2 | 2/- | 14/32 |
| Sertoli-Leydig | 3/3 | -/- | -/3 | 3/1 | -/- | 6/7 | |
| Mixed SCST | 1/- | -/- | -/- | -/- | -/- | 1/- | |
| Other SCST | -/1 | -/- | 1/- | -/- | -/- | 1/1 | |
| Total | 4/24 | -/- | 10/13 | 6/3 | 2/- | 22/40 | |
| Histology | Type of Treatment | Number of Patients | Number of Patients Who Gave at Least One Birth after Treatment (Birth Rate) | Number of Patients Who Gave at Least Two Births after Treatment | Number of Successful Pregnancies (Ending in Childbirth) | Mean Age (Years) |
|---|---|---|---|---|---|---|
| Germ cell tumor | FSS | 20 | 10 (50%) | 4 (20%) | 14 | 26.8 |
| FSS + CTH | 64 | 26 (40.63%) | 12 (18.75%) | 40 | 23.5 | |
| Sex cord- stromal tumor | FSS | 40 | 23 (57.5%) | 7 (17.5%) | 32 | 29.2 |
| FSS + CTH | 22 | 7 (31.81%) | 2 (9.1%) | 9 | 26 |
| Variable | Children Born after Treatment (n = 96) * | Children Conceived before Diagnosis (n = 10) | |
|---|---|---|---|
| Diagnosis during pregnancy | Diagnosis and delivery at the same time | ||
| Sex | Male = 54 Female = 42 | Male = 1 Female = 2 | Male = 2 Female = 5 |
| Weight: Mean Standard deviation Range | 3469 g 498.5 g 2160–4950 g | 3520 g 533.3 g 2620–3640 g | 2787 g 795.9 g 1600–3620 g |
| Delivery | |||
| Preterm | 6 (6.25%) | 1 (33.33%) | 4 (57.14%) |
| 32 Hbd 34 Hbd 35 Hbd 36 Hbd | 1 1 1 3 | - - 1 - | 3 - - 1 |
| Full-term | 90 (93.75%) | 2 (66.67%) | 3 (42.86%) |
| Apgar score (points) | |||
| 8–10 | 90 (94.75%) | 2 (66.67%) | 7 (100%) |
| 4–7 | 1 (0.1%) | - | - |
| 0–3 | - | - | - |
| Unknown | 5 (5.2%) | 1 (33.33%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piątek, S.; Szymusik, I.; Sobiczewski, P.; Michalski, W.; Kowalska, M.; Ołtarzewski, M.; Bidziński, M. Obstetric Results after Fertility-Sparing Management of Non-Epithelial Ovarian Cancer. Cancers 2023, 15, 4170. https://doi.org/10.3390/cancers15164170
Piątek S, Szymusik I, Sobiczewski P, Michalski W, Kowalska M, Ołtarzewski M, Bidziński M. Obstetric Results after Fertility-Sparing Management of Non-Epithelial Ovarian Cancer. Cancers. 2023; 15(16):4170. https://doi.org/10.3390/cancers15164170
Chicago/Turabian StylePiątek, Szymon, Iwona Szymusik, Piotr Sobiczewski, Wojciech Michalski, Magdalena Kowalska, Mariusz Ołtarzewski, and Mariusz Bidziński. 2023. "Obstetric Results after Fertility-Sparing Management of Non-Epithelial Ovarian Cancer" Cancers 15, no. 16: 4170. https://doi.org/10.3390/cancers15164170
APA StylePiątek, S., Szymusik, I., Sobiczewski, P., Michalski, W., Kowalska, M., Ołtarzewski, M., & Bidziński, M. (2023). Obstetric Results after Fertility-Sparing Management of Non-Epithelial Ovarian Cancer. Cancers, 15(16), 4170. https://doi.org/10.3390/cancers15164170









