Evaluation of Intratumoral Response Heterogeneity in Metastatic Colorectal Cancer and Its Impact on Patient Overall Survival: Findings from 10,551 Patients in the ARCAD Database
Abstract
Simple Summary
Abstract
1. Introduction
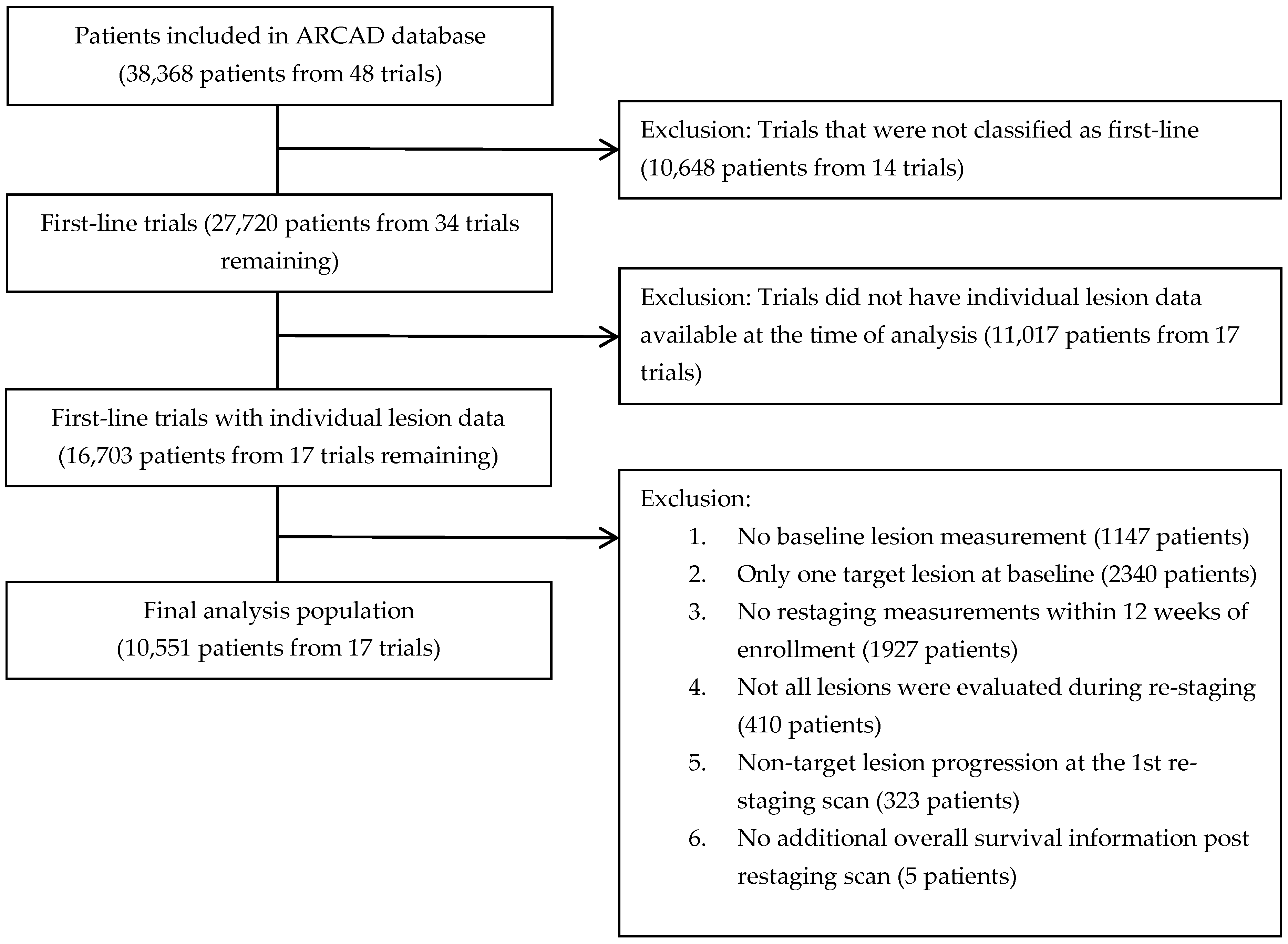
2. Patients and Methods
2.1. Population Analysis
2.2. Cycle-by-Cycle Tumor Measurements
- Only data from a maximum of five target lesions were used without considering non-measurable lesions, as we do not have numeric measurements associated with non-measurable lesions. In cases with more than five target lesions, the largest ones were selected to evaluate the response.
- We only utilized the measurement of the longest diameter per lesion since the current standard response criteria (RECIST 1.1) are based on unidimensional measurements.
- New lesion information was not used for this analysis because it was not consistently available across trials (it was only available in four trials).
- The image-based assessment schedule was slightly different across trials; therefore, we considered any assessments that occurred between baseline and 12 weeks from registration. If multiple assessments were available, we chose the assessment that was closest to 12 weeks and included the complete set of tumor measurements.
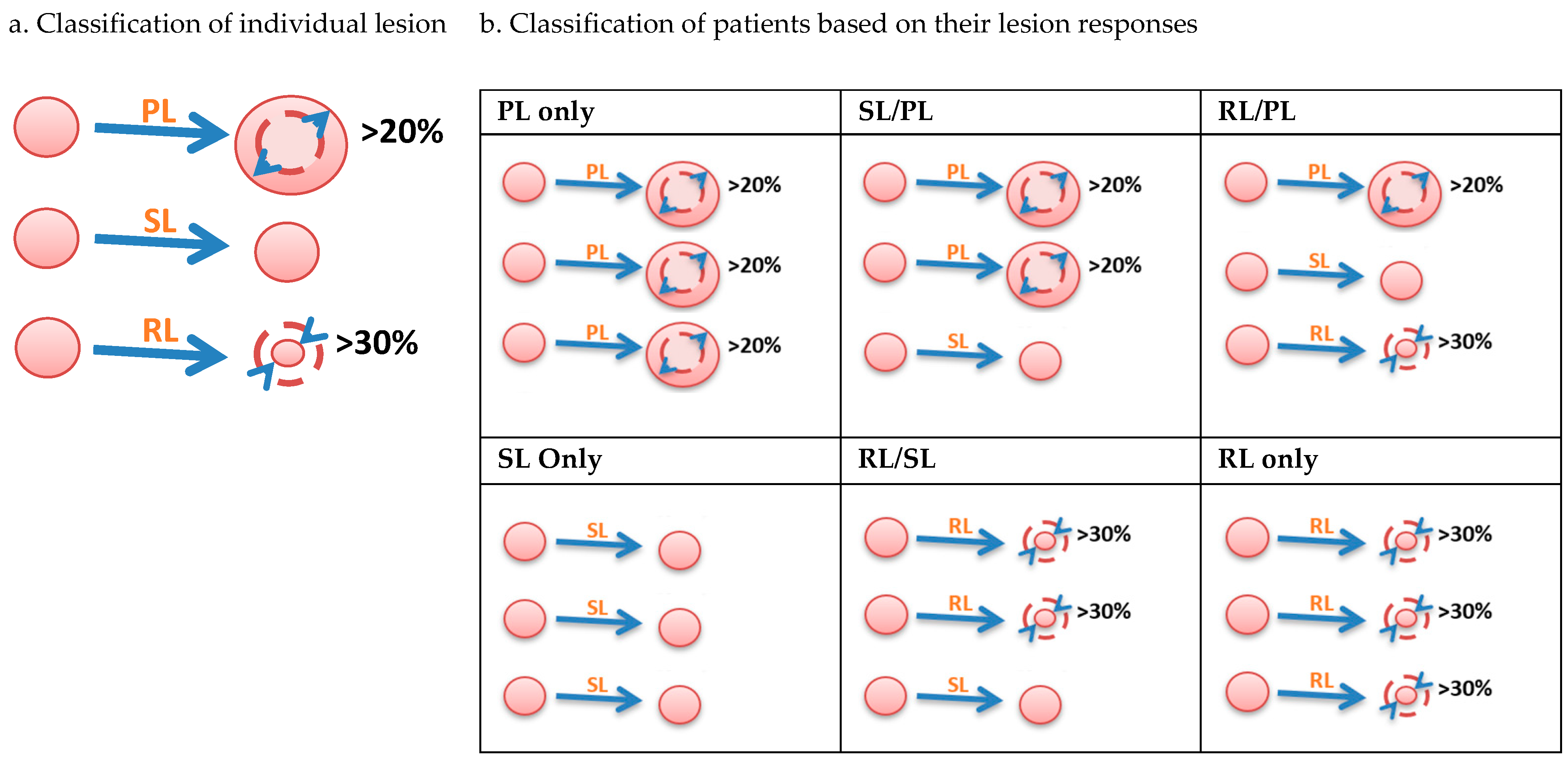
2.3. Definition of Lesion-Based Response Criteria at 12 Weeks (LBR12)
- Step 1: Classify individual lesions in each patient.
- Step 2: Classify patients into six growth patterns.
2.4. Primary Endpoint
2.5. Statistical Analysis
3. Results
3.1. High Proportion of Patients Had Heterogeneous Tumor Responses
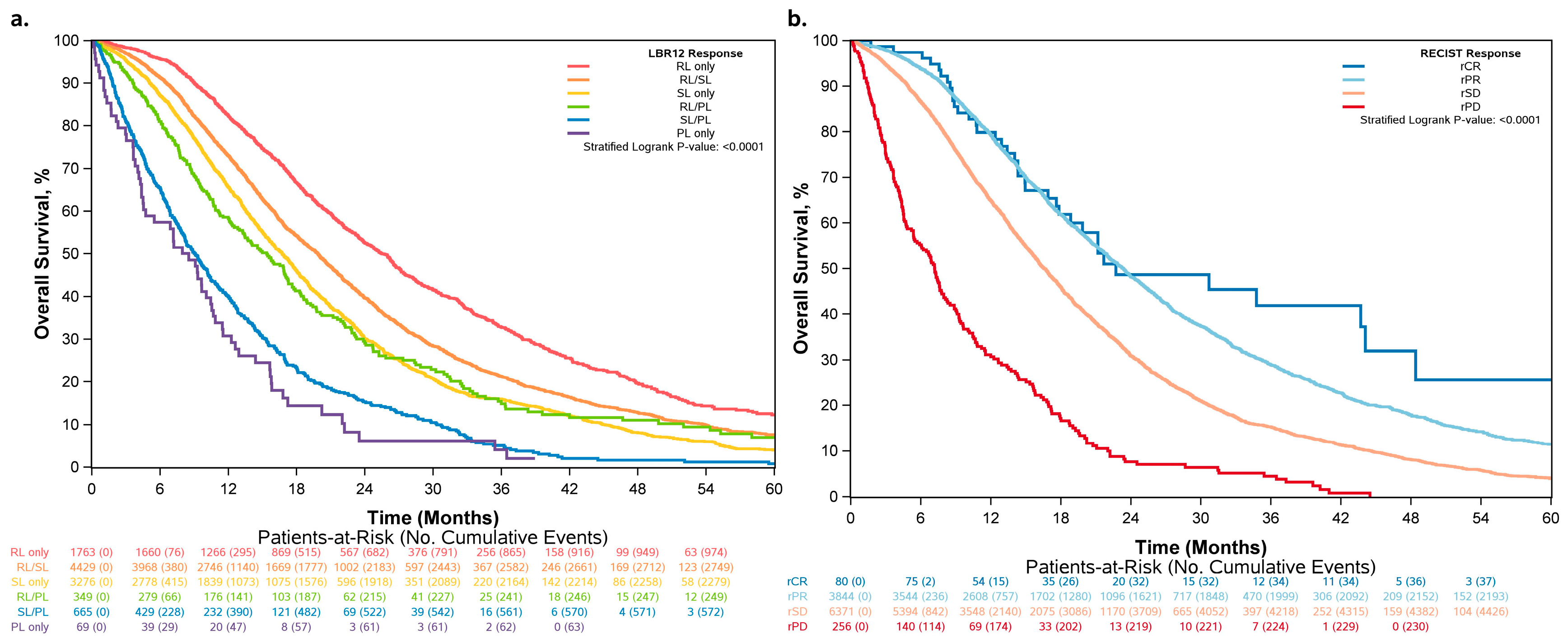
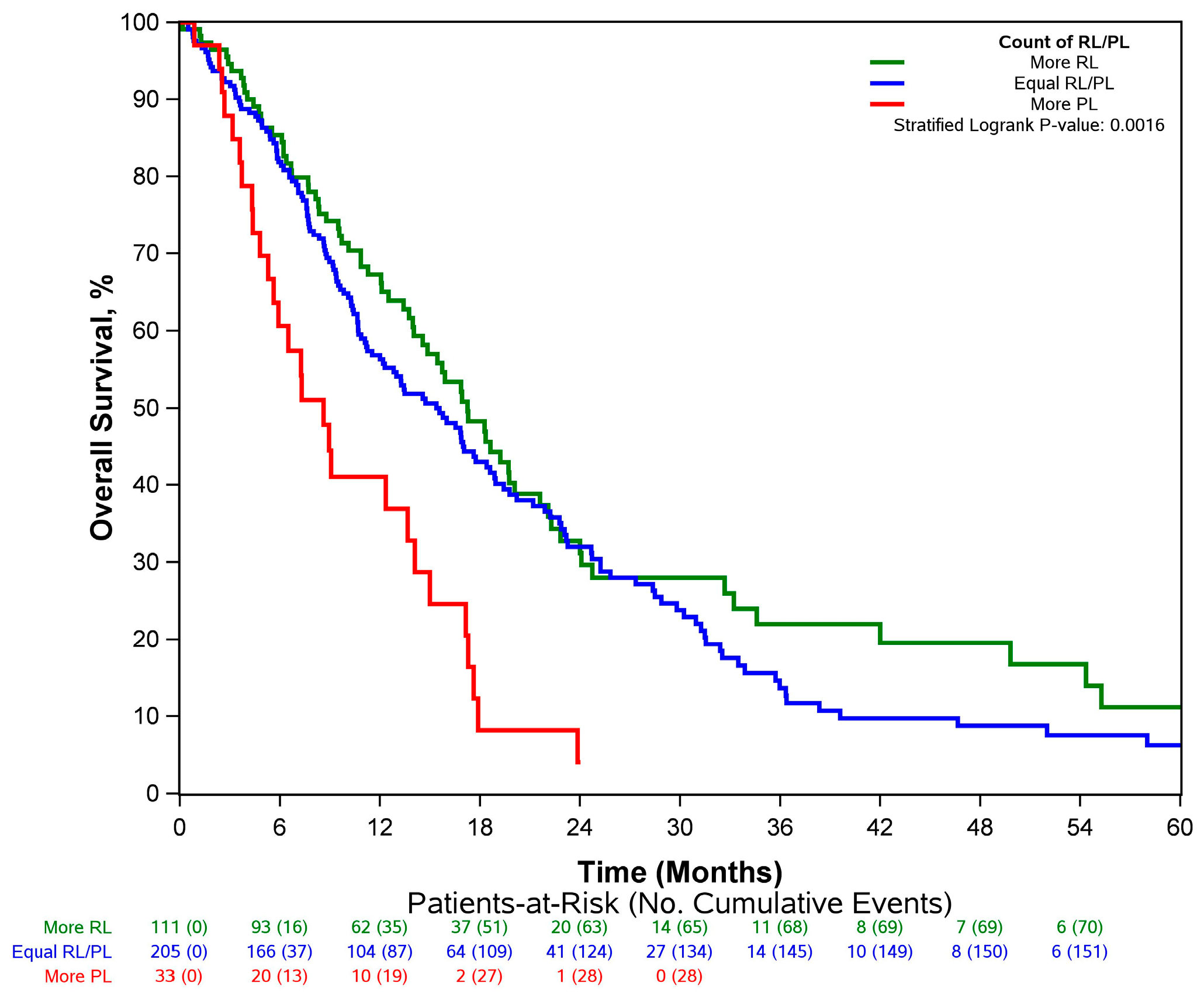
3.2. Overall Survival Increased across Patients Who Had More RLs and Fewer PLs
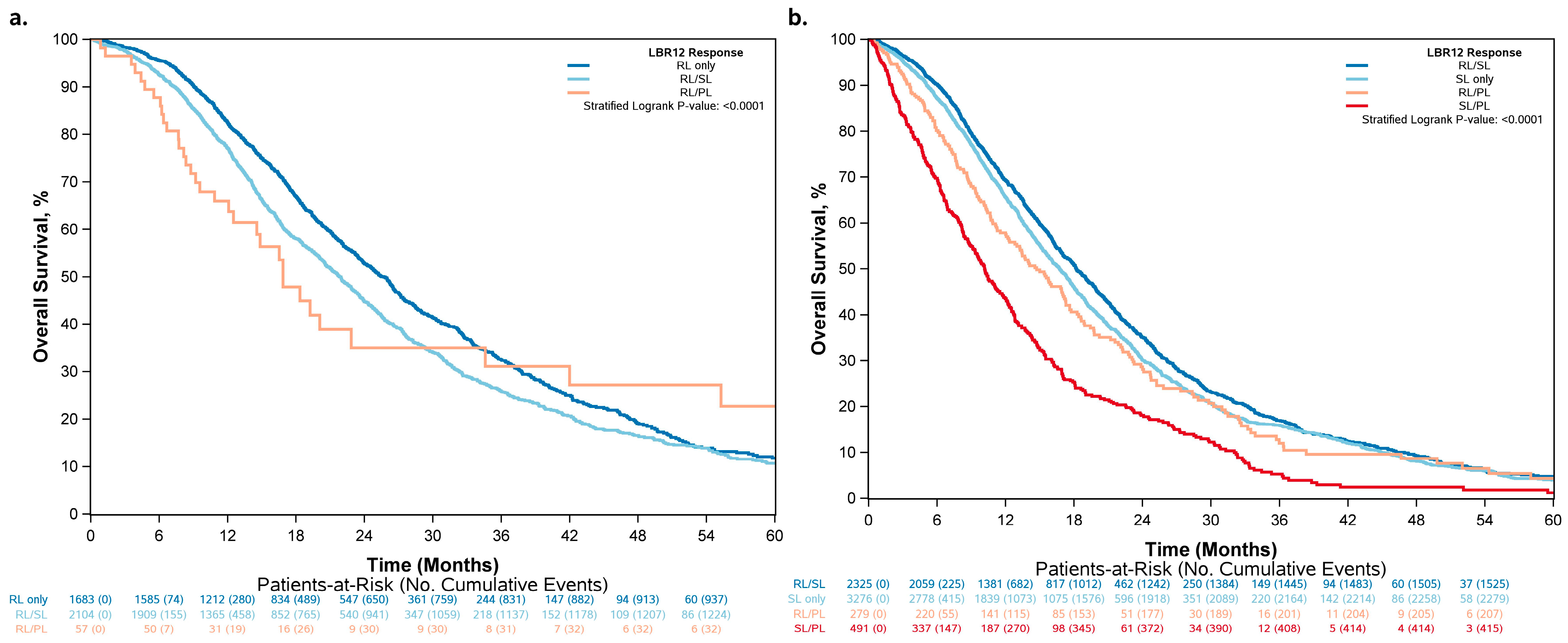
3.3. Differences in Classification by RECIST 1.1 vs. LBR12
3.4. LBR12 Produced a Higher Concordance Rate for Overall Survival Than RECIST at 12 Weeks
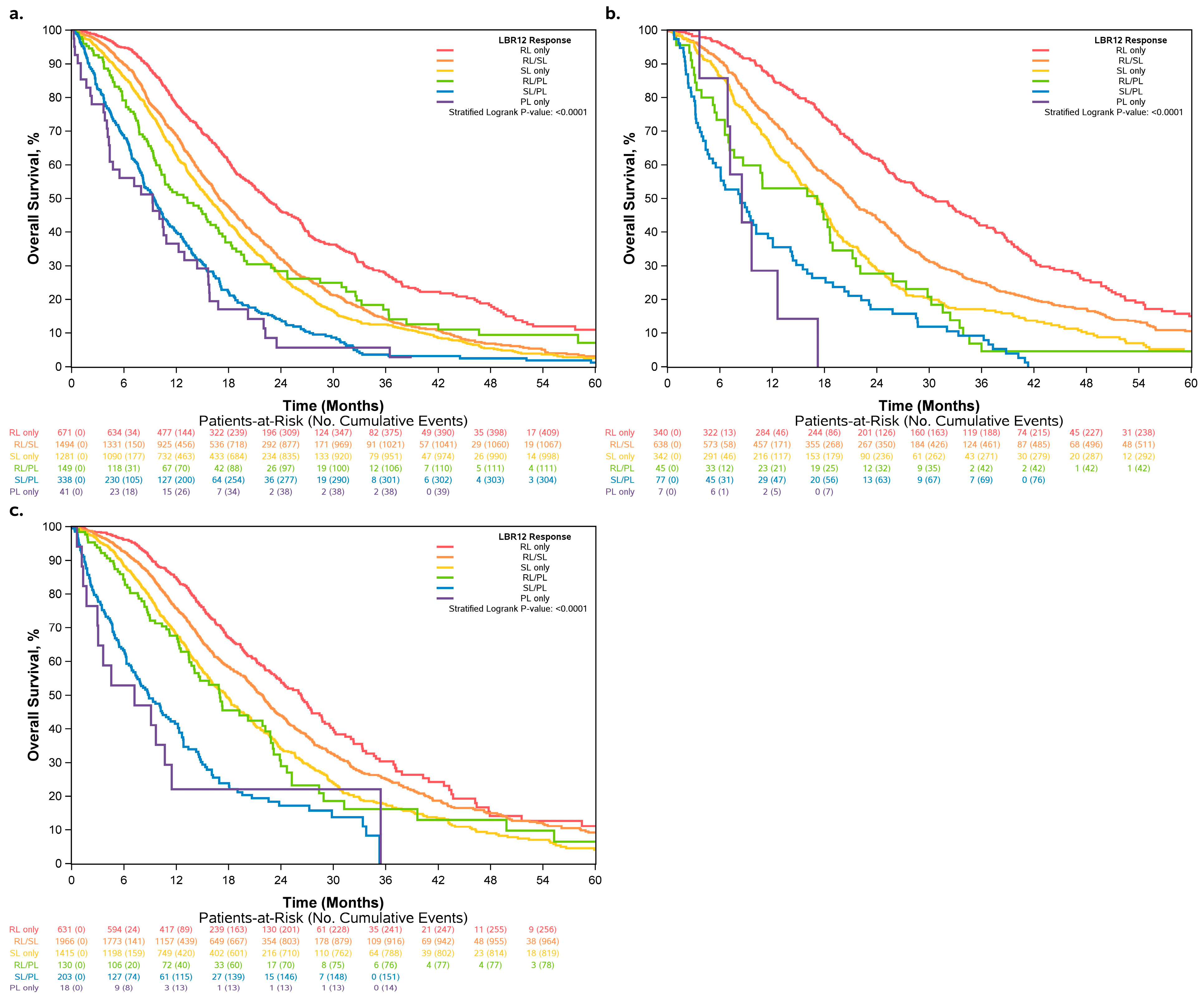
3.5. Sensitivity Analysis with Varying Definitions of LBR12
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics, Guidance for Industry. 2018. Available online: https://www.fda.gov/media/71195/download (accessed on 11 August 2023).
- European Medicines Agency. Evaluation of Anticancer Medicinal Products in Man—Scientific Guideline. 2023. Available online: https://www.ema.europa.eu/en/evaluation-anticancer-medicinal-products-man-scientific-guideline (accessed on 11 August 2023).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Di Bisceglie, A.M.; Bruix, J.; Kramer, B.S.; Lencioni, R.; Zhu, A.X.; Sherman, M.; Schwartz, M.; Lotze, M.; Talwalkar, J.; et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J. Natl. Cancer Inst. 2008, 100, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Giobbie-Hurder, A.; Gargano, M.; Suda, M.; Ramaiya, N.H.; Hodi, F.S. Developing a common language for tumor response to immunotherapy: Immune-related response criteria using unidimensional measurements. Clin. Cancer Res. 2013, 19, 3936–3943. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]
- Fleuren, G.J.; Gorter, A.; Kuppen, P.J.K.; Litvinov, S.; Warnaar, S.O. Tumor heterogeneity and immunotherapy of cancer. Immunol. Rev. 1995, 145, 91–122. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.I.; Limburg, P.J.; Baron, J.A.; Burnett-Hartman, A.N.; Weisenberger, D.J.; Laird, P.W.; Sinicrope, F.A.; Rosty, C.; Buchanan, D.D.; Potter, J.D.; et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology 2015, 148, 77–87. [Google Scholar] [CrossRef]
- Kogita, A.; Yoshioka, Y.; Sakai, K.; Togashi, Y.; Sogabe, S.; Nakai, T.; Okuno, K.; Nishio, K. Inter- and intra-tumor profiling of multi-regional colon cancer and metastasis. Biochem. Biophys. Res. Commun. 2015, 458, 52–56. [Google Scholar] [CrossRef]
- Lips, E.H.; van Eijk, R.; de Graaf, E.J.; Doornebosch, P.G.; de Miranda, N.F.; Oosting, J.; Karsten, T.; Eilers, P.H.; Tollenaar, R.A.; van Wezel, T.; et al. Progression and tumor heterogeneity analysis in early rectal cancer. Clin. Cancer Res. 2008, 14, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Davnall, F.; Yip, C.S.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.J.; Goh, V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Farhangfar, C.J.; Meric-Bernstam, F.; Mendelsohn, J.; Mills, G.B.; Lucio-Eterovic, A.K. The impact of tumor heterogeneity on patient treatment decisions. Clin. Chem. 2013, 59, 38–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Kessel, C.; Samim, M.; Koopman, M.; Bosch, M.v.D.; Rinkes, I.B.; Punt, C.; van Hillegersberg, R. Radiological heterogeneity in response to chemotherapy is associated with poor survival in patients with colorectal liver metastases. Eur. J. Cancer 2013, 49, 2486–2493. [Google Scholar] [CrossRef] [PubMed]
- De Gramont, A.; Haller, D.G.; Sargent, D.J.; Tabernero, J.; Matheson, A.; Schilsky, R.L. Toward efficient trials in colorectal cancer: The ARCAD Clinical Trials Program. J. Clin. Oncol. 2010, 28, 527–530. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Cochran, W.G. Test of Goodness of Fit. Ann. Math. Stat. 1952, 23, 315–345. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; Wiley Series in Probability and Mathematical Statistics; Wiley: New York, NY, USA, 1980; p. xi. 321p. [Google Scholar]
- Cox, D. Regression Models and Life Tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.S.; Zalcberg, J.R.; Tu, D.; Au, H.-J.; Berry, S.R.; Krahn, M.; Price, T.; Simes, R.J.; et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Sobrero, A.F.; Maurel, J.; Fehrenbacher, L.; Scheithauer, W.; Abubakr, Y.A.; Lutz, M.P.; Vega-Villegas, M.E.; Eng, C.; Steinhauer, E.U.; Prausova, J.; et al. EPIC: Phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta 2010, 1805, 105–117. [Google Scholar] [CrossRef]
- Misale, S.; Yaeger, R.; Hobor, S.; Scala, E.; Janakiraman, M.; Liska, D.; Valtorta, E.; Schiavo, R.; Buscarino, M.; Siravegna, G.; et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012, 486, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Yonesaka, K.; Zejnullahu, K.; Okamoto, I.; Satoh, T.; Cappuzzo, F.; Souglakos, J.; Ercan, D.; Rogers, A.; Roncalli, M.; Takeda, M.; et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci. Transl. Med. 2011, 3, 99ra86. [Google Scholar] [CrossRef]
- Van Emburgh, B.O.; Arena, S.; Siravegna, G.; Lazzari, L.; Crisafulli, G.; Corti, G.; Mussolin, B.; Baldi, F.; Buscarino, M.; Bartolini, A.; et al. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat. Commun. 2016, 7, 13665. [Google Scholar] [CrossRef]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef]
- Al-Shamsi, H.O.; Fahmawi, Y.; Dahbour, I.; Tabash, A.; Rogers, J.E.; Mares, J.E.; Blum, M.A.; Estrella, J.; Matamoros, A.; Sagebiel, T.; et al. Continuation of trastuzumab beyond disease progression in HER2-positive metastatic gastric cancer: The MD Anderson experience. J. Gastrointest. Oncol. 2016, 7, 499–505. [Google Scholar] [CrossRef]
- Baldeo, C.; Kaleem, T.; Paz-Fumagalli, R.; Copland, J.A.; Menefee, M.E. Mixed response to immunotherapy in lung cancer. J. Clin. Oncol. 2019, 37, 122. [Google Scholar] [CrossRef]
- Chatwal, M.S.; Creelan, B.C.; Gray, J.E.; Tanvetyanon, T. Initial tumor flare or mixed tumor response following single PD-1 vs. dual PD-1/CTLA-4 blockade in non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2018, 36, e21037. [Google Scholar] [CrossRef]
- Tabatabai, R.; Natale, R. Immunotherapy and Mixed Radiographic Response in Non-Small Cell Lung Cancer. J. Cancer Clin. 2018, 1, 1005. [Google Scholar]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.M.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE-177 Study. J. Clin. Oncol. 2020, 38, LBA4. [Google Scholar] [CrossRef]
| RL Only (N = 1763) | RL/SL (N = 4429) | SL Only (N = 3276) | RL/PL (N = 349) | SL/PL (N = 665) | PL Only (N = 69) | Total (N = 10,551) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Age at Enrollment | <0.0001 1 | |||||||
| Missing | 1 | 0 | 1 | 0 | 0 | 0 | 2 | |
| Mean (STD) | 59 (11.3) | 60 (10.9) | 60 (11.1) | 59 (10.9) | 59 (11.6) | 58 (11.2) | 60 (11.1) | |
| Median (IQR) | 60 (52, 67) | 60 (53, 68) | 61 (54, 68) | 60 (51, 68) | 59 (51, 68) | 59 (53, 66) | 60 (53, 68) | |
| Range | 20, 84 | 18, 89 | 18, 88 | 26, 83 | 19, 83 | 24, 79 | 18, 89 | |
| Gender, n (%) | 0.0064 2 | |||||||
| Female | 712 (40.4%) | 1744 (39.4%) | 1288 (39.3%) | 162 (46.4%) | 299 (45.0%) | 22 (31.9%) | 4227 (40.1%) | |
| Male | 1050 (59.6%) | 2685 (60.6%) | 1988 (60.7%) | 187 (53.6%) | 366 (55.0%) | 47 (68.1%) | 6323 (59.9%) | |
| Missing | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Performance Status, n (%) | < 0.0001 2 | |||||||
| 0 | 1089 (62.3%) | 2480 (56.2%) | 1764 (54.4%) | 165 (47.4%) | 355 (54.1%) | 35 (51.5%) | 5888 (56.2%) | |
| 1 | 642 (36.7%) | 1871 (42.4%) | 1420 (43.8%) | 172 (49.4%) | 283 (43.1%) | 30 (44.1%) | 4418 (42.2%) | |
| 2 | 17 (1.0%) | 60 (1.4%) | 60 (1.8%) | 11 (3.2%) | 18 (2.7%) | 3 (4.4%) | 169 (1.6%) | |
| Missing | 15 | 18 | 32 | 1 | 9 | 1 | 76 | |
| Treatment Regimen, n (%) | < 0.0001 2 | |||||||
| Chemotherapy alone | 671 (38.1%) | 1494 (33.7%) | 1281 (39.1%) | 149 (42.7%) | 338 (50.8%) | 41 (59.4%) | 3974 (37.7%) | |
| VEGFi | 631 (35.8%) | 1966 (44.4%) | 1415 (43.2%) | 130 (37.2%) | 203 (30.5%) | 18 (26.1%) | 4363 (41.4%) | |
| EGFRi | 340 (19.3%) | 638 (14.4%) | 342 (10.4%) | 45 (12.9%) | 77 (11.6%) | 7 (10.1%) | 1449 (13.7%) | |
| VEGFi and EGFRi | 121 (6.9%) | 331 (7.5%) | 238 (7.3%) | 25 (7.2%) | 47 (7.1%) | 3 (4.3%) | 765 (7.3%) | |
| Liver Affected, n (%) | 0.0038 2 | |||||||
| No | 220 (17.0%) | 567 (16.9%) | 520 (20.6%) | 47 (17.0%) | 81 (15.6%) | 9 (16.7%) | 1444 (18.0%) | |
| Yes | 1071 (83.0%) | 2780 (83.1%) | 2000 (79.4%) | 229 (83.0%) | 438 (84.4%) | 45 (83.3%) | 6563 (82.0%) | |
| Missing | 472 | 1082 | 756 | 73 | 146 | 15 | 2544 | |
| Lung Affected, n (%) | <0.0001 2 | |||||||
| No | 909 (70.6%) | 1964 (58.8%) | 1508 (60.2%) | 170 (62.3%) | 344 (67.1%) | 40 (76.9%) | 4935 (61.9%) | |
| Yes | 379 (29.4%) | 1374 (41.2%) | 997 (39.8%) | 103 (37.7%) | 169 (32.9%) | 12 (23.1%) | 3034 (38.1%) | |
| Missing | 475 | 1091 | 771 | 76 | 152 | 17 | 2582 | |
| N of Metastatic Sites, n (%) | <0.0001 2 | |||||||
| 0 | 3 (0.2%) | 18 (0.5%) | 8 (0.3%) | 1 (0.4%) | 0 (0.0%) | 1 (1.9%) | 31 (0.4%) | |
| 1 | 687 (53.1%) | 1288 (38.5%) | 1047 (41.6%) | 91 (33.0%) | 184 (35.5%) | 27 (50.9%) | 3324 (41.5%) | |
| 2+ | 603 (46.6%) | 2041 (61.0%) | 1463 (58.1%) | 184 (66.7%) | 334 (64.5%) | 25 (47.2%) | 4650 (58.1%) | |
| Missing | 470 | 1082 | 758 | 73 | 147 | 16 | 2546 | |
| Number of Lesions at Baseline | <0.0001 1 | |||||||
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean (STD) | 3.3 (1.2) | 3.8 (1.2) | 3.2 (1.2) | 4.1 (1.0) | 3.8 (1.2) | 2.7 (0.9) | 3.5 (1.2) | |
| Median (IQR) | 3.0 (2.0, 4.0) | 4.0 (3.0, 5.0) | 3.0 (2.0, 4.0) | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.0) | 2.0 (2.0, 3.0) | 3.0 (2.0, 5.0) | |
| Range | 2.0, 5.0 | 2.0, 5.0 | 2.0, 5.0 | 2.0, 5.0 | 2.0, 5.0 | 2.0, 5.0 | 2.0, 5.0 | |
| Sum of Baseline Lesion Diameters (cm) | <0.0001 1 | |||||||
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean (STD) | 11.4 (7.5) | 14.5 (8.4) | 13.3 (8.7) | 14.4 (7.8) | 14.0 (8.1) | 8.6 (5.6) | 13.6 (8.4) | |
| Median (IQR) | 9.5 (5.9, 14.9) | 12.7 (8.2, 19.0) | 11.1 (6.9, 17.7) | 12.7 (8.5, 18.4) | 12.7 (7.9, 18.2) | 7.7 (5.0, 10.2) | 11.7 (7.3, 17.9) | |
| Range | 2.0, 71.5 | 0.8, 72.3 | 1.7, 62.5 | 2.6, 53.2 | 1.8, 58.6 | 2.1, 28.0 | 0.8, 72.3 | |
| Median of Baseline Lesion Diameters (cm) | <0.0001 1 | |||||||
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean (STD) | 3.4 (2.0) | 3.6 (2.0) | 3.9 (2.3) | 3.1 (1.6) | 3.5 (1.9) | 3.2 (1.9) | 3.6 (2.1) | |
| Median (IQR) | 2.9 (2.0, 4.2) | 3.0 (2.1, 4.5) | 3.4 (2.2, 5.0) | 2.8 (2.0, 3.9) | 3.0 (2.2, 4.3) | 2.8 (1.8, 4.1) | 3.0 (2.1, 4.5) | |
| Range | 0.6, 15.3 | 0.4, 18.6 | 0.4, 20.0 | 0.8, 10.8 | 0.9, 12.9 | 1.1, 10.5 | 0.4, 20.0 | |
| Treatment Regimen | LBR12 Response | Median (95% CI) | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Overall | 18.4 (18.1–18.9) | <0.001 1 | ||
| RL | 25.8 (24.1–26.7) | 0.57 (0.53–0.62) | <0.001 2 | |
| RL/SL | 19.9 (19.2–20.5) | 0.81 (0.76–0.85) | <0.001 2 | |
| SL | 16.8 (16.1–17.4) | Reference | ||
| RL/PL | 15.4 (13.2–17.1) | 1.10 (0.96–1.25) | 0.164 2 | |
| SL/PL | 9.0 (8.2–10.1) | 1.98 (1.80–2.18) | <0.001 2 | |
| PL | 8.0 (4.5–10.7) | 2.65 (2.04–3.43) | <0.001 2 | |
| Chemotherapy alone | 16.5 (16.1–17.0) | <0.001 1 | ||
| RL | 22.4 (20.9–24.8) | 0.60 (0.53–0.67) | <0.001 2 | |
| RL/SL | 17.0 (16.3–18.2) | 0.87 (0.79–0.95) | 0.0022 | |
| SL | 15.7 (14.9–16.8) | Reference | ||
| RL/PL | 12.8 (10.3–16.8) | 1.07 (0.88–1.31) | 0.492 2 | |
| SL/PL | 9.3 (8.2–10.4) | 1.80 (1.57–2.05) | <0.001 2 | |
| PL | 8.6 (4.4–14.4) | 2.18 (1.56–3.04) | <0.001 2 | |
| EGFRi | 20.3 (19.4–21.6) | <0.001 1 | ||
| RL | 30.5 (26.7–34.8) | 0.50 (0.42–0.60) | <0.001 2 | |
| RL/SL | 21.1 (19.7–22.9) | 0.76 (0.65–0.87) | <0.001 2 | |
| SL | 17.2 (15.1–18.4) | Reference | ||
| RL/PL | 17.1 (7.6–21.2) | 1.28 (0.92–1.77) | 0.143 2 | |
| SL/PL | 8.3 (5.5–12.1) | 2.18 (1.68–2.82) | <0.001 2 | |
| PL | 8.5 (6.9-NE) | 3.17 (1.48–6.76) | 0.003 2 | |
| VEGFi | 20.2 (19.4–20.9) | <0.001 1 | ||
| RL | 26.3 (23.8–28.6) | 0.57 (0.49–0.66) | <0.001 2 | |
| RL/SL | 21.9 (20.9–22.6) | 0.77 (0.70–0.85) | <0.001 2 | |
| SL | 17.7 (16.5–18.7) | Reference | ||
| RL/PL | 17.0 (14.0–22.8) | 1.09 (0.86–1.37) | 0.466 2 | |
| SL/PL | 8.9 (7.3–11.3) | 2.27 (1.91–2.71) | <0.001 2 | |
| PL | 7.2 (3.0-NE) | 3.73 (2.18–6.37) | <0.001 2 |
| Median (95% CI) 1 | Adjusted Hazard Ratio (95% CI) 2 | Adjusted p-Value | |
|---|---|---|---|
| Most prevalent tumor response | 0.0148 3 | ||
| More RL | 17.2 (14.6–21.6) | 0.93 (0.67–1.28) | |
| Equal RL/PL | 15.4 (12.0–18.6) | Reference | |
| More PL | 8.6 (5.6–15.0) | 2.04 (1.25–3.33) |
| Median (95% CI) 1 | Adjusted Hazard Ratio (95% CI) 2 | Adjusted p-Value | |
|---|---|---|---|
| Patients with partial response by RECIST 1.1 | <0.0001 3 | ||
| RL | 25.7 (24.1–26.7) | Reference | |
| RL/SL | 21.9 (20.6–22.9) | 1.26 (1.16–1.38) | |
| RL/PL | 16.9 (12.5–42.0) | 1.47 (1.04–2.08) | |
| Patients with stable disease by RECIST 1.1 | <0.0001 3 | ||
| RL/SL | 18.2 (17.5–19.1) | Reference | |
| SL | 16.7 (16.1–17.4) | 1.09 (1.02–1.17) | |
| RL/PL | 14.7 (12.8–17.2) | 1.26 (1.08–1.45) | |
| SL/PL | 10.2 (9.2–11.3) | 1.93 (1.73–2.16) |
| Concordance (95% CI) | ||
|---|---|---|
| LBR12 $ | RECIST 1.1 $ | |
| Overall | 0.626 (0.583–0.669) | 0.617 (0.574–0.660) |
| Chemotherapy alone | 0.618 (0.573–0.664) | 0.603 (0.557–0.648) |
| EGFRi | 0.645 (0.611–0.678) | 0.641 (0.608–0.674) |
| VEGFi | 0.622 (0.576–0.667) | 0.612 (0.567–0.658) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, F.-S.; Ahn, D.H.; Dixon, J.G.; Grothey, A.; Lou, Y.; Kasi, P.M.; Hubbard, J.M.; Van Cutsem, E.; Saltz, L.B.; Schmoll, H.-J.; et al. Evaluation of Intratumoral Response Heterogeneity in Metastatic Colorectal Cancer and Its Impact on Patient Overall Survival: Findings from 10,551 Patients in the ARCAD Database. Cancers 2023, 15, 4117. https://doi.org/10.3390/cancers15164117
Ou F-S, Ahn DH, Dixon JG, Grothey A, Lou Y, Kasi PM, Hubbard JM, Van Cutsem E, Saltz LB, Schmoll H-J, et al. Evaluation of Intratumoral Response Heterogeneity in Metastatic Colorectal Cancer and Its Impact on Patient Overall Survival: Findings from 10,551 Patients in the ARCAD Database. Cancers. 2023; 15(16):4117. https://doi.org/10.3390/cancers15164117
Chicago/Turabian StyleOu, Fang-Shu, Daniel H. Ahn, Jesse G. Dixon, Axel Grothey, Yiyue Lou, Pashtoon M. Kasi, Joleen M. Hubbard, Eric Van Cutsem, Leonard B. Saltz, Hans-Joachim Schmoll, and et al. 2023. "Evaluation of Intratumoral Response Heterogeneity in Metastatic Colorectal Cancer and Its Impact on Patient Overall Survival: Findings from 10,551 Patients in the ARCAD Database" Cancers 15, no. 16: 4117. https://doi.org/10.3390/cancers15164117
APA StyleOu, F.-S., Ahn, D. H., Dixon, J. G., Grothey, A., Lou, Y., Kasi, P. M., Hubbard, J. M., Van Cutsem, E., Saltz, L. B., Schmoll, H.-J., Goldberg, R. M., Venook, A. P., Hoff, P., Douillard, J.-Y., Hecht, J. R., Hurwitz, H., Punt, C. J. A., Koopman, M., Bokemeyer, C., ... Shi, Q. (2023). Evaluation of Intratumoral Response Heterogeneity in Metastatic Colorectal Cancer and Its Impact on Patient Overall Survival: Findings from 10,551 Patients in the ARCAD Database. Cancers, 15(16), 4117. https://doi.org/10.3390/cancers15164117











