Zoledronic Acid Prevents Bone Resorption Caused by the Combination of Radium-223, Abiraterone Acetate, and Prednisone in an Intratibial Prostate Cancer Mouse Model
Abstract
Simple Summary
Abstract
1. Introduction
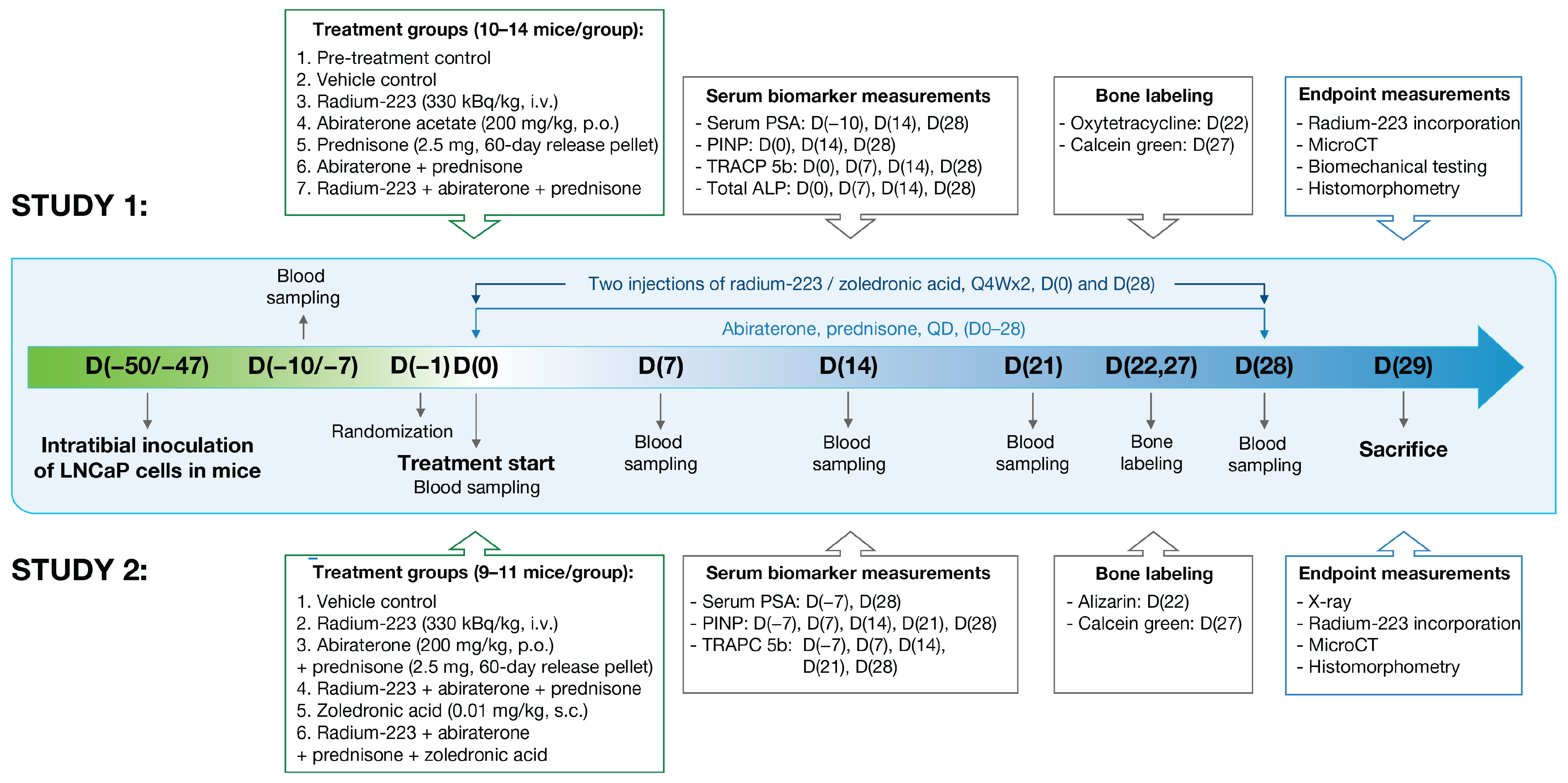
2. Materials and Methods
2.1. Compounds
2.2. Cell Culture
2.3. Animals
2.4. Intratibial LNCaP Model
2.5. Blood Sampling and Biomarkers
2.6. Ex Vivo Radiography
2.7. Radium-223 Incorporation to Bone
2.8. Bone Structure, Quality, and Formation Measurements
2.9. Histology
2.10. Statistical Analyses
3. Results
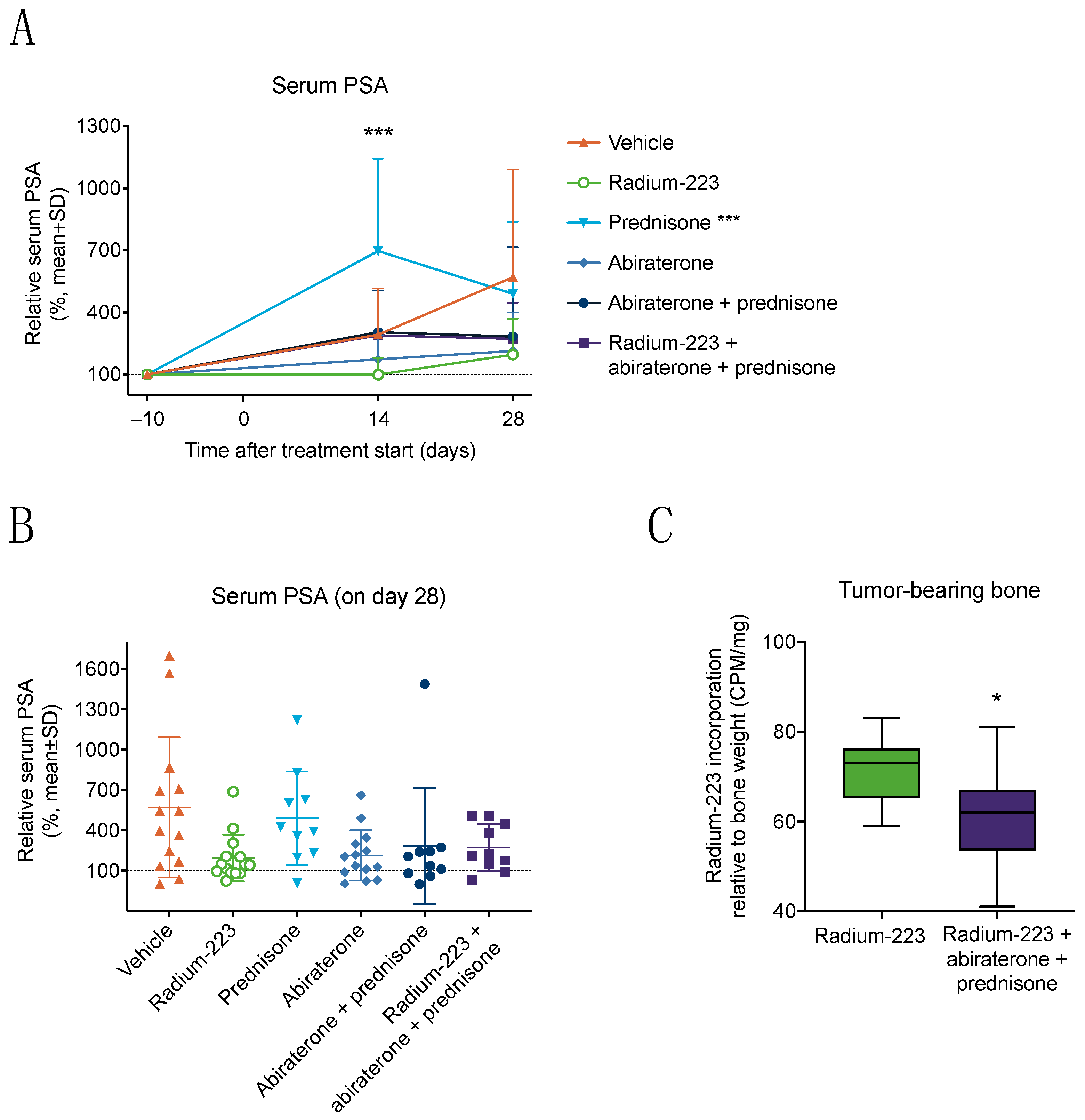
3.1. Combination Treatment with Radium-223, Abiraterone, and Prednisone Has No Additive Effects on Tumor Growth but Reduces Radium-223 Incorporation into Bone
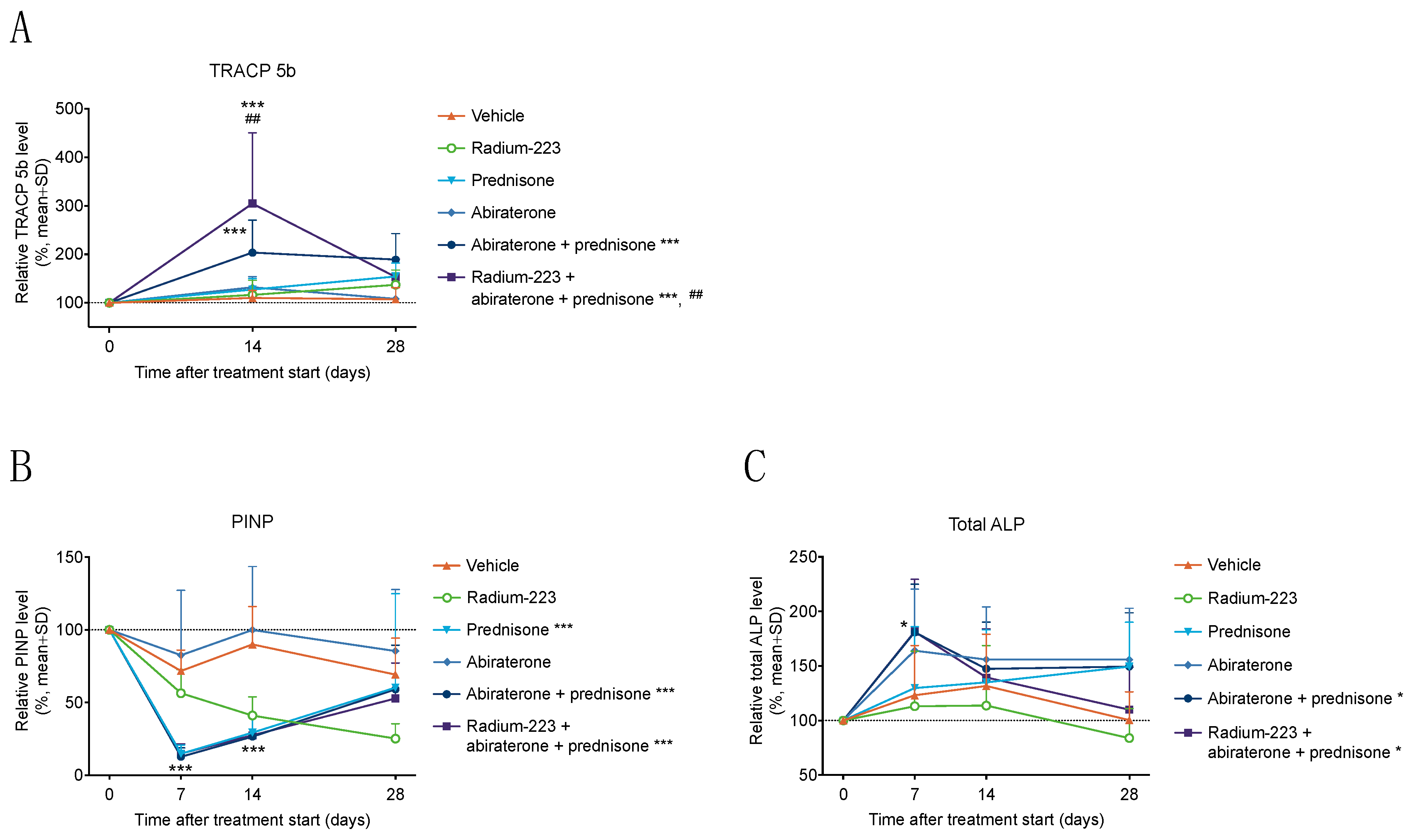
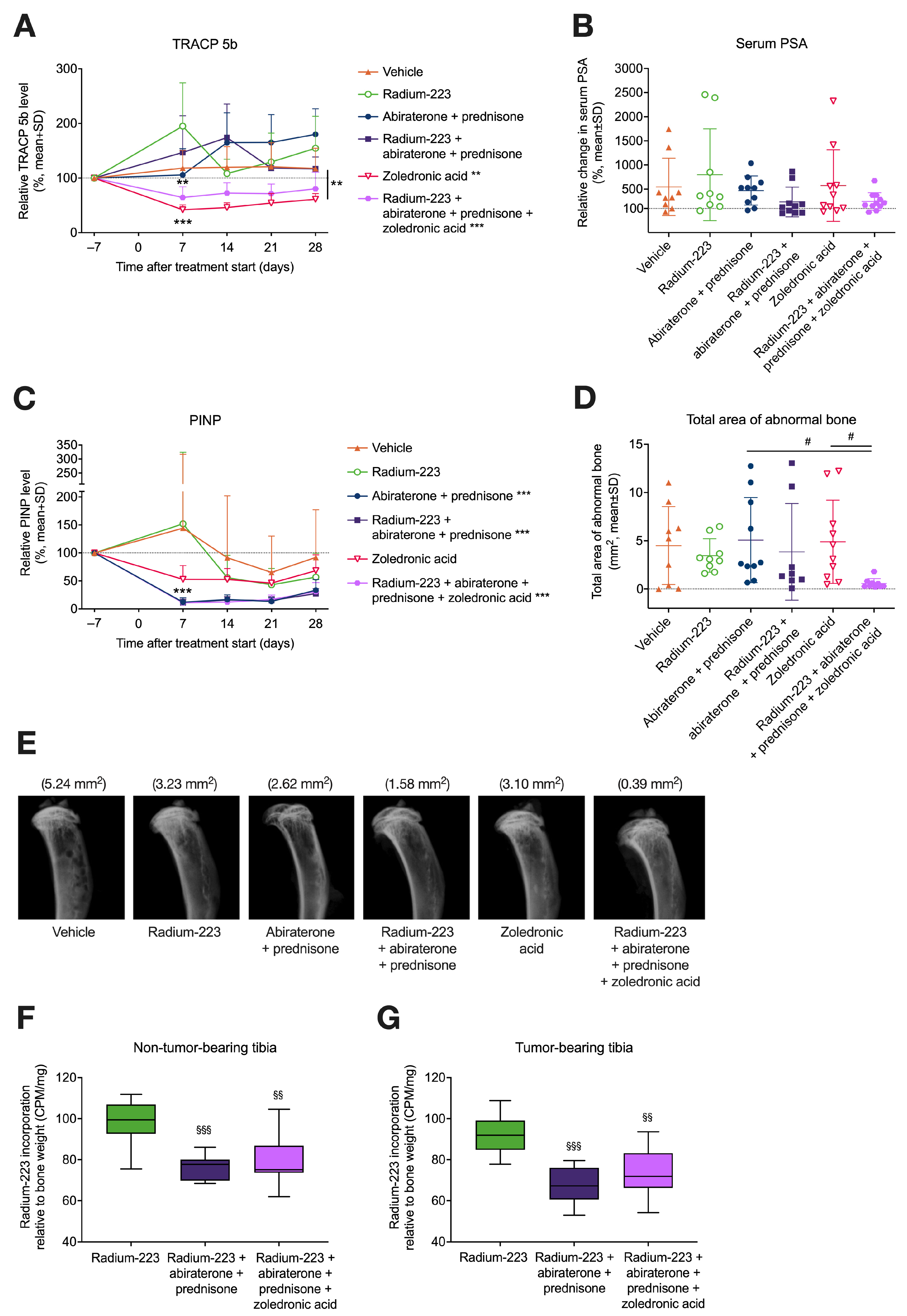
3.2. Radium-223, Abiraterone, and Prednisone Combination Treatment Transiently Increases Bone Resorption
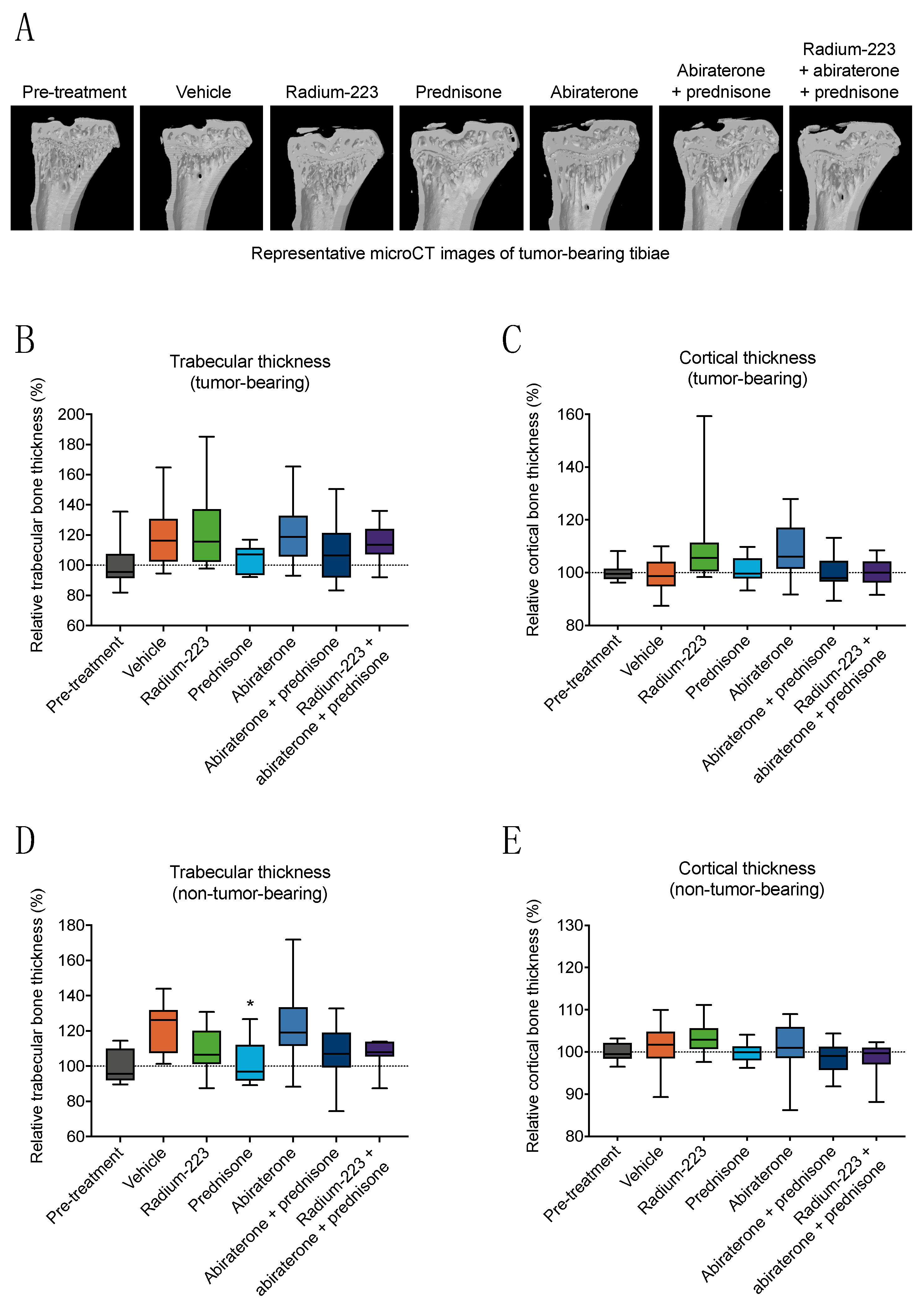
3.3. Radium-223, Abiraterone, and Prednisone Combination Treatment Shows No Treatment-Specific Effects on Bone Structure or Biomechanical Quality
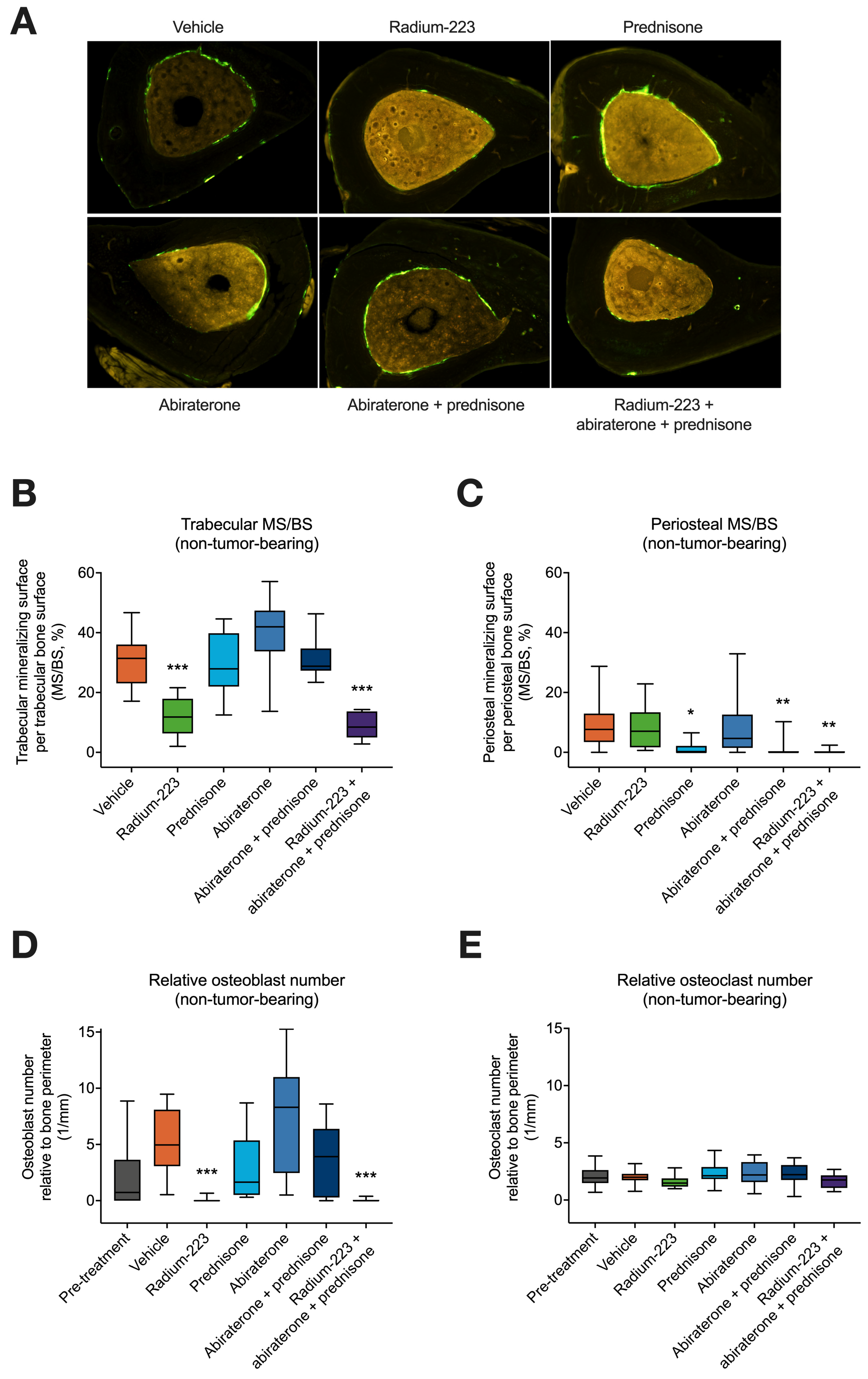
3.4. Radium-223, Abiraterone, and Prednisone Combination Treatment Inhibits Both Trabecular and Cortical Bone Formation in Non-Tumor-Bearing Bone
3.5. Zoledronic Acid Inhibits Increased Bone Resorption Associated with the Combination of Radium-223, Abiraterone Acetate, and Prednisone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suominen, M.I.; Wilson, T.; Kakonen, S.M.; Scholz, A. The Mode-of-Action of Targeted Alpha Therapy Radium-223 as an Enabler for Novel Combinations to Treat Patients with Bone Metastasis. Int. J. Mol. Sci. 2019, 20, 3899. [Google Scholar] [CrossRef] [PubMed]
- Bannik, K.; Madas, B.; Jarzombek, M.; Sutter, A.; Siemeister, G.; Mumberg, D.; Zitzmann-Kolbe, S. Radiobiological effects of the alpha emitter Ra-223 on tumor cells. Sci. Rep. 2019, 9, 18489. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Den, R.B.; George, D.; Pieczonka, C.; McNamara, M. Ra-223 Treatment for Bone Metastases in Castrate-Resistant Prostate Cancer: Practical Management Issues for Patient Selection. Am. J. Clin. Oncol. 2019, 42, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Cursano, M.C.; Iuliani, M.; Casadei, C.; Stellato, M.; Tonini, G.; Paganelli, G.; Santini, D.; De Giorgi, U. Combination radium-223 therapies in patients with bone metastases from castration-resistant prostate cancer: A review. Crit. Rev. Oncol. Hematol. 2020, 146, 102864. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D.; Tutrone, R.F.; Mariados, N.F.; Nordquist, L.T.; Mehlhaff, B.A.; Steere, K.J.; Harrelson, S.S. eRADicAte: A Prospective Evaluation Combining Radium-223 Dichloride and Abiraterone Acetate Plus Prednisone in Patients With Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef]
- Tombal, B.F.; Loriot, Y.; Saad, F.; McDermott, R.S.; Elliott, T.; Rodriguez-Vida, A.; Nole, F.; Fournier, B.; Collette, L.; Gillessen, S. Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: An interim safety analysis. J. Clin. Oncol. 2019, 37, 5007. [Google Scholar] [CrossRef]
- Gillessen, S.; Choudhury, A.; Rodriguez-Vida, A.; Nole, F.; Diaz, E.G.; Roumeguere, T.A.; Daugaard, G.; Loriot, Y.; Saad, F.; McDermott, R.S.; et al. Decreased fracture rate by mandating bone protecting agents in the EORTC 1333/PEACEIII trial combining Ra223 with enzalutamide versus enzalutamide alone: An updated safety analysis. J. Clin. Oncol. 2021, 39, 5002. [Google Scholar] [CrossRef]
- Trieu, J.; Chang, M.; Rojas, V.; Varada, N.; Cao, Y.; Anderson, M.; Vogelzang, N.J. Lower Fracture Rates in Patients Treated with Radium-223, Abiraterone or Enzalutamide, When Given Concurrently with Bone Health Agents: A Real-World Analysis. Clin. Genitourin. Cancer 2022, 20, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.; Higano, C.S.; George, D.J.; Sternberg, C.N.; Saad, F.; Tombal, B.; Miller, K.; Kalinovsky, J.; Jiao, X.; Tangirala, K.; et al. Concurrent or layered treatment with radium-223 and enzalutamide or abiraterone/prednisone: Real-world clinical outcomes in patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Stattin, P.; Westerberg, M.; Lissbrant, I.F.; Eriksson, M.H.; Kjellman, A.; Ullén, A.; Vassilev, Z.; Sandstrom, P.; Weinrib, R.; Martinez, D.; et al. Real World Outcomes in Patients With Metastatic, Castration-Resistant Prostate Cancer Treated With Radium-223 in Routine Clinical Practice in Sweden. Clin. Genitourin. Cancer 2022, 21, 107.e1–107.e9. [Google Scholar] [CrossRef] [PubMed]
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clin. Cancer Res. 2017, 23, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- van’t Hof, R.J. Analysis of bone architecture in rodents using microcomputed tomography. Methods Mol. Biol. 2012, 816, 461–476. [Google Scholar]
- Engelke, K.; Prevrhal, S.; Genant, H.K. Macro- and microimaging of bone architecture. In Principles of Bone Biology; John, P., Bilezikian, L.G.R., John Martin, T., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1905–1942. [Google Scholar]
- Goodyear, S.R.; Aspden, R.M. Mechanical properties of bone ex vivo. Methods Mol. Biol. 2012, 816, 555–571. [Google Scholar]
- Jepsen, K.J.; Silva, M.J.; Vashishth, D.; Guo, X.E.; van der Meulen, M.C. Establishing biomechanical mechanisms in mouse models: Practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J. Bone Miner. Res. 2015, 30, 951–966. [Google Scholar] [CrossRef]
- Morgan, E.F.; Bouxsein, M.L. Biomechanics of Bone and Age-Related Fractures. In Principles of Bone Biology, 3rd ed.; John, P., Bilezikian, L.G.R., John Martin, T., Eds.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Peng, Z.; Tuukkanen, J.; Zhang, H.; Jamsa, T.; Vaananen, H.K. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone 1994, 15, 523–532. [Google Scholar] [CrossRef]
- Turner, C.H.; Burr, D.B. Basic biomechanical measurements of bone: A tutorial. Bone 1993, 14, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W. Histomorphometric analysis of bone remodeling. In Principles of Bone Biology; John, P., Bilezikian, L.G.R., John Martin, T., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 447–463. [Google Scholar]
- Erben, R.G.; Glosmann, M. Histomorphometry in rodents. Methods Mol. Biol. 2012, 816, 279–303. [Google Scholar]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Spratt, D.E. Combination therapies in prostate cancer: Proceed with caution. Lancet Oncol. 2019, 20, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.J.; Ghasem-Zadeh, A.; Gianatti, E.; Lim-Joon, D.; Bolton, D.; Zebaze, R.; Seeman, E.; Zajac, J.D.; Grossmann, M. Structural decay of bone microarchitecture in men with prostate cancer treated with androgen deprivation therapy. J. Clin. Endocrinol. Metab. 2010, 95, E456–E463. [Google Scholar] [CrossRef]
- Alibhai, S.M.; Mohamedali, H.Z.; Gulamhusein, H.; Panju, A.H.; Breunis, H.; Timilshina, N.; Fleshner, N.; Krahn, M.D.; Naglie, G.; Tannock, I.F.; et al. Changes in bone mineral density in men starting androgen deprivation therapy and the protective role of vitamin D. Osteoporos. Int. 2013, 24, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- TOMBAL, B.F.; Armstrong, A.J.; Barrus, J.K.; Beer, T.M.; Chowdhury, S.; Evans, C.P.; Heidenreich, A.; Higano, C.S.; Kamba, T.; Lin, P.; et al. Adverse events of special interest assessed by review of safety data in enzalutamide castration-resistant prostate cancer (CRPC) trials. J. Clin. Oncol. 2019, 37, 237. [Google Scholar] [CrossRef]
- Suominen, M.I.; Knuuttila, M.; Schatz, C.A.; Schlicker, A.; Vääräniemi, J.; Sjöholm, B.; Alhoniemi, E.; Haendler, B.; Mumberg, D.; Käkönen, S.M.; et al. Enhanced Antitumor Efficacy of Radium-223 and Enzalutamide in the Intratibial LNCaP Prostate Cancer Model. Int. J. Mol. Sci. 2023, 24, 2189. [Google Scholar] [CrossRef]
- Veldscholte, J.; Ris-Stalpers, C.; Kuiper, G.G.; Jenster, G.; Berrevoets, C.; Claassen, E.; van Rooij, H.C.; Trapman, J.; Brinkmann, A.O.; Mulder, E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem. Biophys. Res. Commun. 1990, 173, 534–540. [Google Scholar] [CrossRef]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [PubMed]
- Halleen, J.M.; Tiitinen, S.L.; Ylipahkala, H.; Fagerlund, K.M.; Vaananen, H.K. Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin. Lab. 2006, 52, 499–509. [Google Scholar] [PubMed]
- Mikah, P.; Krabbe, L.M.; Eminaga, O.; Herrmann, E.; Papavassilis, P.; Hinkelammert, R.; Semjonow, A.; Schrader, A.J.; Boegemann, M. Dynamic changes of alkaline phosphatase are strongly associated with PSA-decline and predict best clinical benefit earlier than PSA-changes under therapy with abiraterone acetate in bone metastatic castration resistant prostate cancer. BMC Cancer 2016, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Cursano, M.C.; Santini, D.; Iuliani, M.; Paganelli, G.; De Giorgi, U. Early use of abiraterone and radium-223 in metastatic prostate cancer. Lancet Oncol. 2019, 20, e228. [Google Scholar] [CrossRef] [PubMed]
- Dalla Volta, A.; Formenti, A.M.; Berruti, A. Higher Risk of Fragility Fractures in Prostate Cancer Patients Treated with Combined Radium-223 and Abiraterone: Prednisone May Be the Culprit. Eur. Urol. 2019, 75, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Brabnikova Maresova, K.; Pavelka, K.; Stepan, J.J. Acute effects of glucocorticoids on serum markers of osteoclasts, osteoblasts, and osteocytes. Calcif. Tissue Int. 2013, 92, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ton, F.N.; Gunawardene, S.C.; Lee, H.; Neer, R.M. Effects of low-dose prednisone on bone metabolism. J. Bone Miner. Res. 2005, 20, 464–470. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Jia, D.; Plotkin, L.I.; Bellido, T.; Powers, C.C.; Stewart, S.A.; Manolagas, S.C.; Weinstein, R.S. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 2004, 145, 1835–1841. [Google Scholar] [CrossRef]
- Rissanen, J.P.; Suominen, M.I.; Peng, Z.; Morko, J.; Rasi, S.; Risteli, J.; Halleen, J.M. Short-term changes in serum PINP predict long-term changes in trabecular bone in the rat ovariectomy model. Calcif. Tissue Int. 2008, 82, 155–161. [Google Scholar] [CrossRef]
- Suominen, M.I.; Rissanen, J.P.; Kakonen, R.; Fagerlund, K.M.; Alhoniemi, E.; Mumberg, D.; Ziegelbauer, K.; Halleen, J.M.; Kakonen, S.M.; Scholz, A. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J. Natl. Cancer Inst. 2013, 105, 908–916. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Monari, F.; Mascia, M.; Costa, R.; Rubini, G.; Spanu, A.; Farcomeni, A.; Lodi Rizzini, E.; Cindolo, L.; Licari, M.; et al. Overall survival in mCPRC patients treated with Radium-223 in association with bone health agents: A national multicenter study. Int. J. Radiat. Biol. 2020, 96, 1608–1613. [Google Scholar] [CrossRef]
- Caffo, O.; Frantellizzi, V.; Tucci, M.; Galli, L.; Monari, F.; Baldari, S.; Masini, C.; Bortolus, R.; Facchini, G.; Alongi, P.; et al. Fracture risk and survival outcomes in metastatic castration-resistant prostate cancer patients sequentially treated with abiraterone acetate and RADIUM-223. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2633–2638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suominen, M.I.; Knuuttila, M.; Sjöholm, B.; Wilson, T.; Alhoniemi, E.; Mumberg, D.; Käkönen, S.-M.; Scholz, A. Zoledronic Acid Prevents Bone Resorption Caused by the Combination of Radium-223, Abiraterone Acetate, and Prednisone in an Intratibial Prostate Cancer Mouse Model. Cancers 2023, 15, 4115. https://doi.org/10.3390/cancers15164115
Suominen MI, Knuuttila M, Sjöholm B, Wilson T, Alhoniemi E, Mumberg D, Käkönen S-M, Scholz A. Zoledronic Acid Prevents Bone Resorption Caused by the Combination of Radium-223, Abiraterone Acetate, and Prednisone in an Intratibial Prostate Cancer Mouse Model. Cancers. 2023; 15(16):4115. https://doi.org/10.3390/cancers15164115
Chicago/Turabian StyleSuominen, Mari I., Matias Knuuttila, Birgitta Sjöholm, Timothy Wilson, Esa Alhoniemi, Dominik Mumberg, Sanna-Maria Käkönen, and Arne Scholz. 2023. "Zoledronic Acid Prevents Bone Resorption Caused by the Combination of Radium-223, Abiraterone Acetate, and Prednisone in an Intratibial Prostate Cancer Mouse Model" Cancers 15, no. 16: 4115. https://doi.org/10.3390/cancers15164115
APA StyleSuominen, M. I., Knuuttila, M., Sjöholm, B., Wilson, T., Alhoniemi, E., Mumberg, D., Käkönen, S.-M., & Scholz, A. (2023). Zoledronic Acid Prevents Bone Resorption Caused by the Combination of Radium-223, Abiraterone Acetate, and Prednisone in an Intratibial Prostate Cancer Mouse Model. Cancers, 15(16), 4115. https://doi.org/10.3390/cancers15164115





