The Present and Future of Neoadjuvant and Adjuvant Therapy for Locally Advanced Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
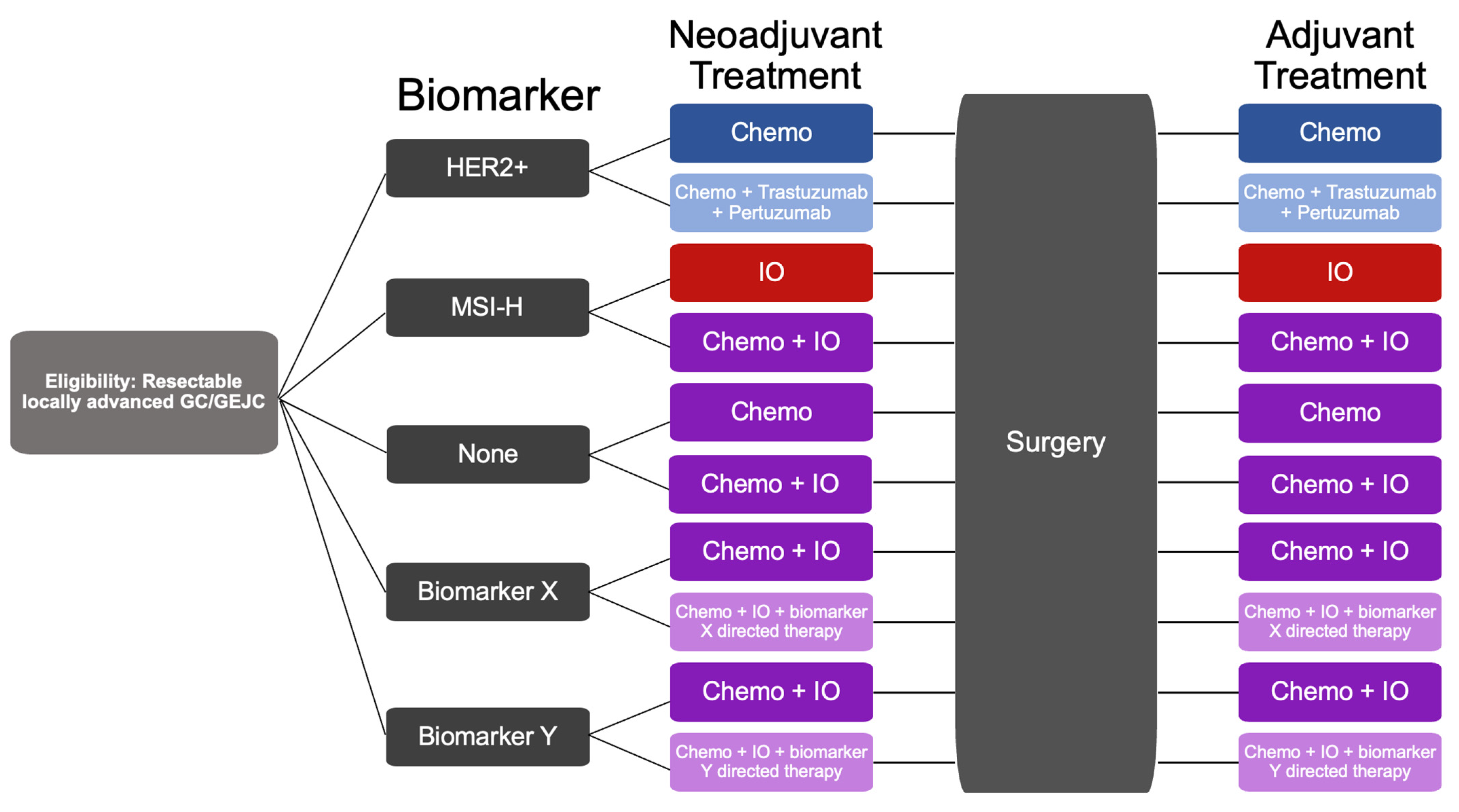
2. Current Perioperative Chemotherapy for LAGC
3. Molecular Subtypes and Biomarkers of GC
4. Immune Checkpoint Blockade for MSI-H LAGC
5. Immune Checkpoint Blockade Plus Chemotherapy for Non-MSI-H LAGC
6. HER2 Blockade for HER2+ LAGC
7. Claudin 18.2: A Newer GC Target
8. FGFR2 Blockade in Advanced GC
9. Future Directions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The Current and Future Incidence and Mortality of Gastric Cancer in 185 Countries, 2020–2040: A Population-Based Modelling Study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; George, R.; Sharma, A.; Graham, D.Y. Changing Trends in Stomach Cancer throughout the World. Curr. Gastroenterol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and PreventionGastric Cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is Gastric Cancer Becoming a Rare Disease? A Global Assessment of Predicted Incidence Trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Przegląd Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Thrift, A.P.; Wenker, T.N.; El-Serag, H.B. Global Burden of Gastric Cancer: Epidemiological Trends, Risk Factors, Screening and Prevention. Nat. Rev. Clin. Oncol. 2023, 20, 338–349. [Google Scholar] [CrossRef]
- Fuccio, L.; Eusebi, L.H.; Bazzoli, F. Gastric Cancer, Helicobacter Pylori Infection and Other Risk Factors. World J. Gastrointest. Oncol. 2010, 2, 342–347. [Google Scholar] [CrossRef]
- Wanebo, H.J.; Kennedy, B.J.; Chmiel, J.; Steele, G., Jr.; Winchester, D.; Osteen, R. Cancer of the Stomach. A Patient Care Study by the American College of Surgeons. Ann. Surg. 1993, 218, 583–592. [Google Scholar] [CrossRef]
- Hundahl, S.A.; Phillips, J.L.; Menck, H.R. The National Cancer Data Base Report on Poor Survival of U.S. Gastric Carcinoma Patients Treated with Gastrectomy: Fifth Edition American Joint Committee on Cancer Staging, Proximal Disease, and the “Different Disease” Hypothesis. Cancer 2000, 88, 921–932. [Google Scholar] [CrossRef]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.-C.; Ho, J.; Unverzagt, S. Chemotherapy for Advanced Gastric Cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef]
- Couto, E.; Marques, A.; Freitas, D.; Nabico, R. Locally Advanced Gastric Cancer: Current and Future Strategies to Improve Outcomes with Multimodality Approach. Surg. Gastroenterol. Oncol. 2020, 25, 17. [Google Scholar] [CrossRef]
- Nashimoto, A.; Akazawa, K.; Isobe, Y.; Miyashiro, I.; Katai, H.; Kodera, Y.; Tsujitani, S.; Seto, Y.; Furukawa, H.; Oda, I.; et al. Gastric Cancer Treated in 2002 in Japan: 2009 Annual Report of the JGCA Nationwide Registry. Gastric Cancer 2013, 16, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Oh, C.-M.; Shin, A.; Lee, J.-S. Survival of Korean Adult Cancer Patients by Stage at Diagnosis, 2006-2010: National Cancer Registry Study. Cancer Res. Treat. 2013, 45, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Wu, C.-W.; Lo, S.-S.; Shen, K.-H.; Hsieh, M.-C.; Chen, J.-H.; Chiang, J.-H.; Lin, H.-J.; Li, A.F.-Y.; Lui, W.-Y. Incidence and Factors Associated with Recurrence Patterns after Intended Curative Surgery for Gastric Cancer. World J. Surg. 2003, 27, 153–158. [Google Scholar] [CrossRef]
- Smalley, S.R.; Benedetti, J.K.; Haller, D.G.; Hundahl, S.A.; Estes, N.C.; Ajani, J.A.; Gunderson, L.L.; Goldman, B.; Martenson, J.A.; Jessup, J.M.; et al. Updated Analysis of SWOG-Directed Intergroup Study 0116: A Phase III Trial of Adjuvant Radiochemotherapy versus Observation after Curative Gastric Cancer Resection. J. Clin. Oncol. 2012, 30, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Elimova, E.; Slack, R.S.; Chen, H.-C.; Planjery, V.; Shiozaki, H.; Shimodaira, Y.; Charalampakis, N.; Lin, Q.; Harada, K.; Wadhwa, R.; et al. Patterns of Relapse in Patients with Localized Gastric Adenocarcinoma Who Had Surgery with or without Adjunctive Therapy: Costs and Effectiveness of Surveillance. Oncotarget 2017, 8, 81430–81440. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, Y.H. Current Approaches to Gastric Cancer in Korea. Gastrointest. Cancer Res. 2008, 2, 137–144. [Google Scholar]
- Sujendran, V.; Wheeler, J.; Baron, R.; Warren, B.F.; Maynard, N. Effect of Neoadjuvant Chemotherapy on Circumferential Margin Positivity and Its Impact on Prognosis in Patients with Resectable Oesophageal Cancer. Br. J. Surg. 2008, 95, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, J.; Doki, Y.; Yasuda, T.; Miyata, H.; Fujiwara, Y.; Takiguchi, S.; Yamasaki, M.; Makari, Y.; Matsuura, N.; Mano, M.; et al. The Effect of Neoadjuvant Chemotherapy on Lymph Node Micrometastases in Squamous Cell Carcinomas of the Thoracic Esophagus. Surgery 2007, 141, 570–580. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative Chemotherapy Compared with Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Chen, J.; Guo, Y.; Fang, M.; Yuan, Y.; Zhu, Y.; Xin, Y.; Zhang, L. Neoadjuvant Chemoradiotherapy for Resectable Gastric Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 927119. [Google Scholar] [CrossRef] [PubMed]
- Trumbull, D.A.; Lemini, R.; Díaz Vico, T.; Jorgensen, M.S.; Attwood, K.; Ji, W.; Brady, M.; Gabriel, E.; Kukar, M. Prognostic Significance of Complete Pathologic Response Obtained with Chemotherapy Versus Chemoradiotherapy in Gastric Cancer. Ann. Surg. Oncol. 2021, 28, 766–773. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Kim, H.-I.; Cheong, J.-H.; Hyung, W.J.; Kim, C.B.; Noh, S.H. Pathologic and Oncologic Outcomes in Locally Advanced Gastric Cancer with Neoadjuvant Chemotherapy or Chemoradiotherapy. Yonsei Med. J. 2013, 54, 888–894. [Google Scholar] [CrossRef]
- Martin-Romano, P.; Sola, J.J.; Diaz-Gonzalez, J.A.; Chopitea, A.; Iragorri, Y.; Martínez-Regueira, F.; Ponz-Sarvise, M.; Arbea, L.; Subtil, J.C.; Cano, D.; et al. Role of Histological Regression Grade after Two Neoadjuvant Approaches with or without Radiotherapy in Locally Advanced Gastric Cancer. Br. J. Cancer 2016, 115, 655–663. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Zhao, L.; Zhou, H.; Wu, C.; Zhang, X.; Zhou, A.; Jin, J.; Zhao, D. The Effect of Neoadjuvant Therapies for Patients with Locally Advanced Gastric Cancer: A Propensity Score Matching Study. J. Cancer 2021, 12, 379–386. [Google Scholar] [CrossRef]
- Park, S.H.; Lim, D.H.; Sohn, T.S.; Lee, J.; Zang, D.Y.; Kim, S.T.; Kang, J.H.; Oh, S.Y.; Hwang, I.G.; Ji, J.H.; et al. A Randomized Phase III Trial Comparing Adjuvant Single-Agent S1, S-1 with Oxaliplatin, and Postoperative Chemoradiation with S-1 and Oxaliplatin in Patients with Node-Positive Gastric Cancer after D2 Resection: The ARTIST 2 Trial☆. Ann. Oncol. 2021, 32, 368–374. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.-M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular Analysis of Gastric Cancer Identifies Subtypes Associated with Distinct Clinical Outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Chen, J.-Y.; Hu, J.; Chen, Q.; Yu, L.-X.; Liu, B.-R.; Qian, X.-P.; Yang, M. Comparison of Microsatellite Status Detection Methods in Colorectal Carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 1431–1438. [Google Scholar]
- Guan, W.-L.; Ma, Y.; Cui, Y.-H.; Liu, T.-S.; Zhang, Y.-Q.; Zhou, Z.-W.; Xu, J.-Y.; Yang, L.-Q.; Li, J.-Y.; Sun, Y.-T.; et al. The Impact of Mismatch Repair Status on Prognosis of Patients With Gastric Cancer: A Multicenter Analysis. Front. Oncol. 2021, 11, 712760. [Google Scholar] [CrossRef] [PubMed]
- Beghelli, S.; de Manzoni, G.; Barbi, S.; Tomezzoli, A.; Roviello, F.; Di Gregorio, C.; Vindigni, C.; Bortesi, L.; Parisi, A.; Saragoni, L.; et al. Microsatellite Instability in Gastric Cancer Is Associated with Better Prognosis in Only Stage II Cancers. Surgery 2006, 139, 347–356. [Google Scholar] [CrossRef]
- Fang, W.-L.; Chang, S.-C.; Lan, Y.-T.; Huang, K.-H.; Chen, J.-H.; Lo, S.-S.; Hsieh, M.-C.; Li, A.F.-Y.; Wu, C.-W.; Chiou, S.-H. Microsatellite Instability Is Associated with a Better Prognosis for Gastric Cancer Patients after Curative Surgery. World J. Surg. 2012, 36, 2131–2138. [Google Scholar] [CrossRef]
- Cortes-Ciriano, I.; Lee, S.; Park, W.-Y.; Kim, T.-M.; Park, P.J. A Molecular Portrait of Microsatellite Instability across Multiple Cancers. Nat. Commun. 2017, 8, 15180. [Google Scholar] [CrossRef]
- Mathiak, M.; Warneke, V.S.; Behrens, H.-M.; Haag, J.; Böger, C.; Krüger, S.; Röcken, C. Clinicopathologic Characteristics of Microsatellite Instable Gastric Carcinomas Revisited: Urgent Need for Standardization. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 12–24. [Google Scholar] [CrossRef]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch Repair Deficiency/Microsatellite Instability-High as a Predictor for Anti-PD-1/PD-L1 Immunotherapy Efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Chen, H.; Castro, F.A.; Hu, J.-K.; Brenner, H. Epstein-Barr Virus Infection and Gastric Cancer: A Systematic Review. Medicine 2015, 94, e792. [Google Scholar] [CrossRef]
- Murphy, G.; Pfeiffer, R.; Camargo, M.C.; Rabkin, C.S. Meta-Analysis Shows that Prevalence of Epstein-Barr Virus-Positive Gastric Cancer Differs Based on Sex and Anatomic Location. Gastroenterology 2009, 137, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Constanza Camargo, M.; Kim, W.-H.; Chiaravalli, A.M.; Kim, K.-M.; Corvalan, A.H.; Matsuo, K.; Yu, J.; Sung, J.J.Y.; Herrera-Goepfert, R.; Meneses-Gonzalez, F.; et al. Improved Survival of Gastric Cancer with Tumour Epstein–Barr Virus Positivity: An International Pooled Analysis. Gut 2014, 63, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xie, T.; Wang, Z.; Tong, S.; Zhao, X.; Zhao, F.; Cai, J.; Wei, X.; Peng, Z.; Shen, L. Efficacy and Predictive Biomarkers of Immunotherapy in Epstein-Barr Virus-Associated Gastric Cancer. J. Immunother. Cancer 2022, 10, e004080. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Jasinski-Bergner, S.; Mandelboim, O.; Wickenhauser, C.; Seliger, B. Epstein–Barr Virus—Associated Malignancies and Immune Escape: The Role of the Tumor Microenvironment and Tumor Cell Evasion Strategies. Cancers 2021, 13, 5189. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Saito, M.; Nakajima, S.; Saito, K.; Nakayama, Y.; Kase, K.; Yamada, L.; Kanke, Y.; Hanayama, H.; Onozawa, H.; et al. PD-L1 Overexpression in EBV-Positive Gastric Cancer is Caused by Unique Genomic or Epigenomic Mechanisms. Sci. Rep. 2021, 11, 1982. [Google Scholar] [CrossRef]
- Hoang, M.P.; Sahin, A.A.; Ordòñez, N.G.; Sneige, N. HER-2/Neu Gene Amplification Compared with HER-2/Neu Protein Overexpression and Interobserver Reproducibility in Invasive Breast Carcinoma. Am. J. Clin. Pathol. 2000, 113, 852–859. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Ghidini, M.; Petrillo, A.; Botticelli, A.; Trapani, D.; Parisi, A.; La Salvia, A.; Sajjadi, E.; Piciotti, R.; Fusco, N.; Khakoo, S. How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches. J. Clin. Med. Res. 2021, 10, 1412. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-Line Nivolumab plus Chemotherapy versus Chemotherapy Alone for Advanced Gastric, Gastro-Oesophageal Junction, and Oesophageal Adenocarcinoma (CheckMate 649): A Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Kim, Y.-W.; Yang, H.-K.; Chung, H.C.; Park, Y.-K.; Lee, K.H.; Lee, K.-W.; Kim, Y.H.; Noh, S.-I.; Cho, J.Y.; et al. Adjuvant Capecitabine and Oxaliplatin for Gastric Cancer after D2 Gastrectomy (CLASSIC): A Phase 3 Open-Label, Randomised Controlled Trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, H.; Shin, S.-J.; Kim, H.Y.; Lee, J.; Yang, H.-K.; Kim, W.H.; Kim, Y.-W.; Kook, M.-C.; Park, Y.K.; et al. Microsatellite Instability and Programmed Cell Death-Ligand 1 Expression in Stage II/III Gastric Cancer: Post Hoc Analysis of the CLASSIC Randomized Controlled Study. Ann. Surg. 2019, 270, 309–316. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.-M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Zhang, S.; Wei, L.; Cheng, H.; Wang, J.; Wang, J. Impacts and Mechanisms of Metabolic Reprogramming of Tumor Microenvironment for Immunotherapy in Gastric Cancer. Cell Death Dis. 2022, 13, 378. [Google Scholar] [CrossRef]
- Oya, Y.; Hayakawa, Y.; Koike, K. Tumor Microenvironment in Gastric Cancers. Cancer Sci. 2020, 111, 2696–2707. [Google Scholar] [CrossRef]
- Amedei, A.; Della Bella, C.; Silvestri, E.; Prisco, D.; D’Elios, M.M. T Cells in Gastric Cancer: Friends or Foes. J. Immunol. Res. 2012, 2012, 690571. [Google Scholar] [CrossRef][Green Version]
- Itahashi, K.; Irie, T.; Nishikawa, H. Regulatory T-Cell Development in the Tumor Microenvironment. Eur. J. Immunol. 2022, 52, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, L. Inhibitory B7-Family Molecules in the Tumour Microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Tallón de Lara, P.; Cecconi, V.; Hiltbrunner, S.; Yagita, H.; Friess, M.; Bode, B.; Opitz, I.; Vrugt, B.; Weder, W.; Stolzmann, P.; et al. Gemcitabine Synergizes with Immune Checkpoint Inhibitors and Overcomes Resistance in a Preclinical Model and Mesothelioma PatientsGemcitabine Synergizes with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2018, 24, 6345–6354. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Emens, L.A.; Middleton, G. The Interplay of Immunotherapy and Chemotherapy: Harnessing Potential Synergies. Cancer Immunol. Res 2015, 3, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Huo, G.; Liu, W.; Chen, P. Efficacy of PD-1/PD-L1 Inhibitors in Gastric or Gastro-Oesophageal Junction Cancer Based on Clinical Characteristics: A Meta-Analysis. BMC Cancer 2023, 23, 143. [Google Scholar] [CrossRef]
- Qing, Y.; Li, Q.; Ren, T.; Xia, W.; Peng, Y.; Liu, G.-L.; Luo, H.; Yang, Y.-X.; Dai, X.-Y.; Zhou, S.-F.; et al. Upregulation of PD-L1 and APE1 is Associated with Tumorigenesis and Poor Prognosis of Gastric Cancer. Drug Des. Devel. Ther. 2015, 9, 901–909. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.-J.; Fuchs, C.; Wyrwicz, L.; Lee, K.-W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab plus Chemotherapy vs Chemotherapy Alone for Patients with First-Line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Fuchs, C.S.; Shitara, K.; Tabernero, J.; Muro, K.; Van Cutsem, E.; Bang, Y.-J.; De Vita, F.; Landers, G.; Yen, C.-J.; et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021, 7, 895–902. [Google Scholar] [CrossRef]
- Dubois, M.; Liscia, N.; Brunetti, O.; Ziranu, P.; Lai, E.; Argentiero, A.; Mazza, E.; Cascinu, S.; Silvestris, N.; Casadei-Gardini, A.; et al. The Role of Immune Checkpoint Inhibitors in the Treatment Sequence of Advanced Gastric or Gastro-Esophageal Junction Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Crit. Rev. Oncol. Hematol. 2022, 173, 103674. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Tougeron, D.; Piessen, G.; de la Fouchardière, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; Desrame, J.; et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2023, 41, 255–265. [Google Scholar] [CrossRef]
- Becker, K.; Mueller, J.D.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Böttcher, K.; Siewert, J.R.; Höfler, H. Histomorphology and Grading of Regression in Gastric Carcinoma Treated with Neoadjuvant Chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Raimondi, A.; Lonardi, S.; Murgioni, S.; Cardellino, G.G.; Tamberi, S.; Strippoli, A.; Palermo, F.; Prisciandaro, M.; Randon, G.; et al. INFINITY: A Multicentre, Single-Arm, Multi-Cohort, Phase II Trial of Tremelimumab and Durvalumab as Neoadjuvant Treatment of Patients with Microsatellite Instability-High (MSI) Resectable Gastric or Gastroesophageal Junction Adenocarcinoma (GAC/GEJAC). J. Clin. Orthod. 2023, 41, 358. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, H.; Pan, Y.; Gu, K.; Cang, S.; Han, L.; Shu, Y.; Li, J.; Zhao, J.; Pan, H.; et al. Abstract CT078: First-Line Treatment with Sintilimab (Sin) vs. Placebo in Combination with Chemotherapy (Chemo) in Patients (Pts) with Unresectable Gastric or Gastroesophageal Junction (G/GEJ) Cancer: Final Overall Survival (OS) Results from the Randomized, Phase III ORIENT-16 Trial. Cancer Res. 2023, 83, CT078. [Google Scholar]
- Moehler, M.H.; Kato, K.; Arkenau, H.-T.; Oh, D.-Y.; Tabernero, J.; Cruz-Correa, M.; Wang, H.; Xu, H.; Li, J.; Yang, S.; et al. Rationale 305: Phase 3 Study of Tislelizumab plus Chemotherapy vs Placebo plus Chemotherapy as First-Line Treatment (1L) of Advanced Gastric or Gastroesophageal Junction Adenocarcinoma (GC/GEJC). J. Clin. Orthod. 2023, 41, 286. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Chen, L.-T.; Ryu, M.-H.; Oh, D.-Y.; Oh, S.C.; Chung, H.C.; Lee, K.-W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus Chemotherapy versus Placebo plus Chemotherapy in Patients with HER2-Negative, Untreated, Unresectable Advanced or Recurrent Gastric or Gastro-Oesophageal Junction Cancer (ATTRACTION-4): A Randomised, Multicentre, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef]
- Raufi, A.G.; Lee, S.; May, M.; Portillo, A.D.; Sender, N.; Ana, S.S.; Gautier, K.; Alouani, E.; Park, H.; Oberstein, P.; et al. Abstract CT009: Phase II Trial of Perioperative Pembrolizumab plus Capecitabine and Oxaliplatin Followed by Adjuvant Pembrolizumab for Resectable Gastric and Gastroesophageal Junction (GC/GEJ) Adenocarcinoma. Cancer Res. 2022, 82, CT009. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Lorenzen, S.; Thuss-Patience, P.C.; Homann, N.; Schenk, M.; Lindig, U.; Heuer, V.; Kretzschmar, A.; Goekkurt, E.; Haag, G.M.; et al. Surgical and Pathological Outcome, and Pathological Regression, in Patients Receiving Perioperative Atezolizumab in Combination with FLOT Chemotherapy versus FLOT Alone for Resectable Esophagogastric Adenocarcinoma: Interim Results from DANTE, a Randomized, Multicenter, Phase IIb Trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J. Clin. Orthod. 2022, 40, 4003. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Fuchs, C.S.; Ohtsu, A.; Tabernero, J.; Ilson, D.H.; Hyung, W.J.; Strong, V.E.; Goetze, T.O.; Yoshikawa, T.; et al. KEYNOTE-585: Phase III Study of Perioperative Chemotherapy with or without Pembrolizumab for Gastric Cancer. Future Oncol. 2019, 15, 943–952. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Van Cutsem, E.; Muro, K.; Wainberg, Z.; Al-Batran, S.-E.; Hyung, W.J.; Molena, D.; Marcovitz, M.; Ruscica, D.; Robbins, S.H.; et al. MATTERHORN: Phase III Study of Durvalumab plus FLOT Chemotherapy in Resectable Gastric/Gastroesophageal Junction Cancer. Future Oncol. 2022, 18, 2465–2473. [Google Scholar] [CrossRef]
- Hollebecque, A.; Wainberg, Z.A.; Ajani, J.A.; Marshall, J.; Cunningham, D.; Ou, S.-H.I.; Lutzky, J.; Jamal, R.; Curigliano, G.; Gutierrez, M.; et al. Safety and Clinical Activity of Durvalumab Monotherapy in Patients with Gastroesophageal Cancers. J. Clin. Orthod. 2018, 36, 4032. [Google Scholar] [CrossRef]
- Kelly, R.J.; Lee, J.; Bang, Y.-J.; Almhanna, K.; Blum-Murphy, M.; Catenacci, D.V.T.; Chung, H.C.; Wainberg, Z.A.; Gibson, M.K.; Lee, K.-W.; et al. Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients with Advanced Gastric and Gastroesophageal Junction Adenocarcinoma. Clin. Cancer Res. 2020, 26, 846–854. [Google Scholar] [CrossRef]
- Terashima, M.; Kang, Y.-K.; Kim, Y.-W.; Boku, N.; Chung, H.C.C.; Chen, J.-S.; Ji, J.; Yeh, T.-S.; Chen, L.-T.; Ryu, M.-H.; et al. ATTRACTION-5: A Phase 3 Study of Nivolumab plus Chemotherapy as Postoperative Adjuvant Treatment for Pathological Stage III (PStage III) Gastric or Gastroesophageal Junction (G/GEJ) Cancer. J. Clin. Orthod. 2023, 41, 4000. [Google Scholar] [CrossRef]
- Yuan, S.; Nie, R.-C.; Jin, Y.; Liang, C.-C.; Jian, R.; Li, Y.-F.; Qiu, H.; Wang, W.; Chen, S.; Zhang, D.-S.; et al. Perioperative PD-1 Antibody Toripalimab plus SOX or XELOX Chemotherapy versus SOX or XELOX Alone for Locally Advanced Gastric or Gastro-Oesophageal Junction Cancer: Results from a Prospective, Randomized, Open-Label, Phase II Trial. J. Clin. Orthod. 2023, 41, 4001. [Google Scholar] [CrossRef]
- Moasser, M.M. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric CancerAdvances in HER2-Targeted Therapy. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Matsuura, N.; Kimura, Y.; Adachi, S.; Fujita, J.; Imamura, H.; Kobayashi, K.; Yokoyama, Y.; Shaker, M.N.; Takiguchi, S.; et al. Multicenter Large-Scale Study of Prognostic Impact of HER2 Expression in Patients with Resectable Gastric Cancer. Gastric Cancer 2015, 18, 691–697. [Google Scholar] [CrossRef]
- Jørgensen, J.T.; Hersom, M. HER2 as a Prognostic Marker in Gastric Cancer—A Systematic Analysis of Data from the Literature. J. Cancer 2012, 3, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Unni, N.; Peng, Y. The Changing Paradigm for the Treatment of HER2-Positive Breast Cancer. Cancers 2020, 12, 2081. [Google Scholar] [CrossRef]
- Junttila, T.T.; Akita, R.W.; Parsons, K.; Fields, C.; Lewis Phillips, G.D.; Friedman, L.S.; Sampath, D.; Sliwkowski, M.X. Ligand-Independent HER2/HER3/PI3K Complex Is Disrupted by Trastuzumab and is Effectively Inhibited by the PI3K Inhibitor GDC-0941. Cancer Cell 2009, 15, 429–440. [Google Scholar] [CrossRef]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; de Vos, A.M.; Sliwkowski, M.X. Insights into ErbB Signaling from the Structure of the ErbB2-Pertuzumab Complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef]
- Yamashita-Kashima, Y.; Iijima, S.; Yorozu, K.; Furugaki, K.; Kurasawa, M.; Ohta, M.; Fujimoto-Ouchi, K. Pertuzumab in Combination with Trastuzumab Shows Significantly Enhanced Antitumor Activity in HER2-Positive Human Gastric Cancer Xenograft Models. Clin. Cancer Res. 2011, 17, 5060–5070. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.-J.; Feng-Yi, F.; Xu, J.M.; Lee, K.-W.; Jiao, S.-C.; Chong, J.L.; López-Sanchez, R.I.; Price, T.; Gladkov, O.; et al. HER2 Screening Data from ToGA: Targeting HER2 in Gastric and Gastroesophageal Junction Cancer. Gastric Cancer 2015, 18, 476–484. [Google Scholar] [CrossRef]
- Gall, V.A.; Philips, A.V.; Qiao, N.A.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic CellsTrastuzumab Increases HER2 Cross-Presentation. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef] [PubMed]
- zum Büschenfelde, C.M.; Hermann, C.; Schmidt, B.; Peschel, C.; Bernhard, H. Antihuman Epidermal Growth Factor Receptor 2 (HER2) Monoclonal Antibody Trastuzumab Enhances Cytolytic Activity of Class I-Restricted HER2-Specific T Lymphocytes against HER2-Overexpressing Tumor Cells. Cancer Res. 2002, 62, 2244–2247. [Google Scholar]
- Varadan, V.; Gilmore, H.; Miskimen, K.L.S.; Tuck, D.; Parsai, S.; Awadallah, A.; Krop, I.E.; Winer, E.P.; Bossuyt, V.; Somlo, G.; et al. Immune Signatures Following Single Dose Trastuzumab Predict Pathologic Response to PreoperativeTrastuzumab and Chemotherapy in HER2-Positive Early Breast CancerChange in Immune Signature Predicts Response to Trastuzumab. Clin. Cancer Res. 2016, 22, 3249–3259. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 Trial of Dual PD-1 and HER2 Blockade in HER2-Positive Gastric Cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Koganemaru, S.; Shitara, K. Antibody–Drug Conjugates to Treat Gastric Cancer. Expert Opin. Biol. Ther. 2021, 21, 923–930. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- FDA. FDA Approves Fam-Trastuzumab Deruxtecan-Nxki for HER2-Positive Gastric Adenocarcinomas. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-positive-gastric-adenocarcinomas (accessed on 15 January 2021).
- Hofheinz, R.-D.; Merx, K.; Haag, G.M.; Springfeld, C.; Ettrich, T.; Borchert, K.; Kretzschmar, A.; Teschendorf, C.; Siegler, G.; Ebert, M.P.; et al. FLOT Versus FLOT/Trastuzumab/Pertuzumab Perioperative Therapy of Human Epidermal Growth Factor Receptor 2-Positive Resectable Esophagogastric Adenocarcinoma: A Randomized Phase II Trial of the AIO EGA Study Group. J. Clin. Oncol. 2022, 40, 3750–3761. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus Trastuzumab and Chemotherapy for HER2-Positive Metastatic Gastric or Gastro-Oesophageal Junction Cancer (JACOB): Final Analysis of a Double-Blind, Randomised, Placebo-Controlled Phase 3 Study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef]
- Wagner, A.D.; Grabsch, H.I.; Mauer, M.; Marreaud, S.; Caballero, C.; Thuss-Patience, P.; Mueller, L.; Elme, A.; Moehler, M.H.; Martens, U.; et al. EORTC-1203-GITCG–the “INNOVATION”-Trial: Effect of Chemotherapy Alone versus Chemotherapy plus Trastuzumab, versus Chemotherapy plus Trastuzumab plus Pertuzumab, in the Perioperative Treatment of HER2 Positive, Gastric and Gastroesophageal Junction Adenocarcinoma on Pathologic Response Rate: A Randomized Phase II-Intergroup Trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer Group. BMC Cancer 2019, 19, 494. [Google Scholar] [CrossRef]
- Coati, I.; Lotz, G.; Fanelli, G.N.; Brignola, S.; Lanza, C.; Cappellesso, R.; Pellino, A.; Pucciarelli, S.; Spolverato, G.; Guzzardo, V.; et al. Claudin-18 Expression in Oesophagogastric Adenocarcinomas: A Tissue Microarray Study of 523 Molecularly Profiled Cases. Br. J. Cancer 2019, 121, 257–263. [Google Scholar] [CrossRef]
- Sahin, U.; Koslowski, M.; Dhaene, K.; Usener, D.; Brandenburg, G.; Seitz, G.; Huber, C.; Türeci, O. Claudin-18 Splice Variant 2 is a Pan-Cancer Target Suitable for Therapeutic Antibody Development. Clin. Cancer Res. 2008, 14, 7624–7634. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-T.; Jeng, Y.-M.; Yang, C.-Y. Claudin-18 as a Marker for Identifying the Stomach and Pancreatobiliary Tract as the Primary Sites of Metastatic Adenocarcinoma. Am. J. Surg. Pathol. 2020, 44, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Kyuno, D.; Takasawa, A.; Takasawa, K.; Ono, Y.; Aoyama, T.; Magara, K.; Nakamori, Y.; Takemasa, I.; Osanai, M. Claudin-18.2 as a Therapeutic Target in Cancers: Cumulative Findings from Basic Research and Clinical Trials. Tissue Barriers 2021, 10, 1967080. [Google Scholar] [CrossRef] [PubMed]
- Pellino, A.; Brignola, S.; Riello, E.; Niero, M.; Murgioni, S.; Guido, M.; Nappo, F.; Businello, G.; Sbaraglia, M.; Bergamo, F.; et al. Association of CLDN18 Protein Expression with Clinicopathological Features and Prognosis in Advanced Gastric and Gastroesophageal Junction Adenocarcinomas. J. Pers. Med. 2021, 11, 1095. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A Randomised Phase II Study of Zolbetuximab (IMAB362) plus EOX versus EOX Alone for First-Line Treatment of Advanced CLDN18.2-Positive Gastric and Gastro-Oesophageal Adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.C.; Ilson, D.H.; Shah, M.A.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J.; et al. Zolbetuximab + MFOLFOX6 as First-Line (1L) Treatment for Patients (Pts) Withclaudin-18.2+ (CLDN18.2+)/HER2− Locally Advanced (LA) Unresectable or Metastatic Gastric or Gastroesophageal Junction (MG/GEJ) Adenocarcinoma: Primary Results from Phase 3 SPOTLIGHT Study. J. Clin. Orthod. 2023, 41, LBA292. [Google Scholar] [CrossRef]
- Xu, R.-H.; Shitara, K.; Ajani, J.A.; Bang, Y.-J.; Enzinger, P.C.; Ilson, D.H.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab + CAPOX in 1L Claudin-18.2+ (CLDN18.2+)/HER2− Locally Advanced (LA) or Metastatic Gastric or Gastroesophageal Junction (MG/GEJ) Adenocarcinoma: Primary Phase 3 Results from GLOW. J. Clin. Orthod. 2023, 41, 405736. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. “Off-the-Shelf” Allogeneic CAR T Cells: Development and Challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell 2020, 38, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-Specific CAR T Cells in Gastrointestinal Cancers: Phase 1 Trial Interim Results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xing, H.; Li, Y.; Tian, W.; Song, Y.; Jiang, Z.; Yu, J. Claudin18.2 is a Novel Molecular Biomarker for Tumor-Targeted Immunotherapy. Biomark. Res. 2022, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Li, N.-G.; Zhang, Y.-M.; Xie, W.-C.; Yang, S.-P.; Lu, T.; Shi, Z.-H. Recent Advance in the Development of Novel, Selective and Potent FGFR Inhibitors. Eur. J. Med. Chem. 2020, 186, 111884. [Google Scholar] [CrossRef] [PubMed]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.-S.; Li, X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Helsten, T.; Schwaederle, M.; Kurzrock, R. Fibroblast Growth Factor Receptor Signaling in Hereditary and Neoplastic Disease: Biologic and Clinical Implications. Cancer Metastasis Rev. 2015, 34, 479–496. [Google Scholar] [CrossRef]
- Hosoda, K.; Yamashita, K.; Ushiku, H.; Ema, A.; Moriya, H.; Mieno, H.; Washio, M.; Watanabe, M. Prognostic Relevance of FGFR2 Expression in Stage II/III Gastric Cancer with Curative Resection and S-1 Chemotherapy. Oncol. Lett. 2017, 15, 1853–1860. [Google Scholar] [CrossRef]
- Seo, S.; Park, S.J.; Ryu, M.-H.; Park, S.R.; Ryoo, B.-Y.; Park, Y.S.; Na, Y.-S.; Lee, C.-W.; Lee, J.-K.; Kang, Y.-K. Prognostic Impact of Fibroblast Growth Factor Receptor 2 Gene Amplification in Patients Receiving Fluoropyrimidine and Platinum Chemotherapy for Metastatic and Locally Advanced Unresectable Gastric Cancers. Oncotarget 2017, 8, 33844–33854. [Google Scholar] [CrossRef]
- Powers, J.; Palencia, S.; Foy, S.; Sennino, B.; Hidalgo, T.R.; Gemo, A.; Brennan, T.; Pierce, K. Abstract 1407: FPA144, a Therapeutic Monoclonal Antibody Targeting the FGFR2b Receptor, Promotes Antibody Dependent Cell-Mediated Cytotoxicity and Stimulates Sensitivity to PD-1 in the 4T1 Syngeneic Tumor Model. Cancer Res. 2016, 76, 1407. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.-K.; Qin, S.; Yamaguchi, K.; Kim, I.-H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab in Patients with FGFR2b-Selected Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FIGHT): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Study. Lancet Oncol. 2022, 23, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Chao, J.; Muro, K.; Yen, P.; Yanes, R.E.; Zahlten-Kumeli, A.; Rha, S.Y. Trial in Progress: Phase 3 Study of Bemarituzumab + MFOLFOX6 versus Placebo + MFOLFOX6 in Previously Untreated Advanced Gastric or Gastroesophageal Junction (GEJ) Cancer with FGFR2b Overexpression (FORTITUDE-101). J. Clin. Orthod. 2022, 40, TPS4164. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Van Cutsem, E.; Moehler, M.H.; Kang, Y.-K.; Yen, P.; Finger, E.; Keegan, A.; Shitara, K. Trial in Progress: Phase 1b/3 Study of Bemarituzumab + MFOLFOX6 + Nivolumab versus MFOLFOX6 + Nivolumab in Previously Untreated Advanced Gastric and Gastroesophageal Junction (GEJ) Cancer with FGFR2b Overexpression (FORTITUDE-102). J. Clin. Orthod. 2022, 40, TPS4165. [Google Scholar] [CrossRef]
| Clinical Trial Information | Arms | Patient Population | Status | Primary Outcomes |
|---|---|---|---|---|
| NCT04006262 GERCOR NEONIPIGA Phase II | Arm 1: Perioperative nivolumab plus neoadjuvant ipilimumab | cT2-T4/NX/M0 resectable GC/GEJC, dMMR/MSI-H+ | Recruiting | pCR rate 58.6% |
| NCT04817826 INFINITY Phase II | Arm 1: Neoadjuvant tremelimumab and durvalumab | MSI-H+, c ≥ T2, any N, any M, GC/GEJC | Recruiting | Overall pCR rate 60%, T2-3 tumor pCR rate 89% |
| NCT03421288 DANTE Phase II | Arm 1: Perioperative atezolizumab and FLOT 1 Arm 2: Perioperative FLOT | c ≥ T2 or N+ GC/GEJC | Active, not recruiting | PFS—pending data |
| NCT03221426 KEYNOTE-585 Phase III | Arm 1: Perioperative pembrolizumab and XP 2/FP 3/FLOT Arm 2: Perioperative XP/FP/FLOT | Stage II-IVa GC/GEJC | Active, not recruiting | Event-free survival, pCR, OS, rate of adverse events—pending data |
| NCT04592913 MATTERHORN Phase III | Arm 1: Perioperative durvalumab plus FLOT Arm 2: Perioperative FLOT | ≥Stage II GC/GEJC | Active, not recruiting | Event-free survival—pending data |
| NCT04250948 Phase II | Arm 1: Perioperative toripalimab and SOX/XELOX 4 Arm 2: Perioperative SOX/XELOX | Resectable cT3-4a/N+/M0 GC/GEJC | Recruiting | Pathological complete regression/moderate regression rate (TRG 0–1): Arm 1 44.4% vs. Arm 2 20.4%, p = 0.009 |
| NCT02581462 PETRARCA Phase II | Arm 1: Perioperative trastuzumab and pertuzumab and FLOT Arm 2: Perioperative FLOT | cT2-T4 and/or N+ GC/GEJC with HER2 overexpression | Completed | pCR: Arm 1 35% vs. Arm 2 12%, p = 0.02 |
| NCT02205047 INNOVATION Phase II | Arm 1: Perioperative trastuzumab and FLOT Arm 2: Perioperative trastuzumab, pertuzumab, and FLOT Arm 3: Perioperative FLOT | Resectable GC with HER2 overexpression | Active, not recruiting | Major pathological response rate (<10% vital residual tumor cells)—pending data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koerner, A.S.; Moy, R.H.; Ryeom, S.W.; Yoon, S.S. The Present and Future of Neoadjuvant and Adjuvant Therapy for Locally Advanced Gastric Cancer. Cancers 2023, 15, 4114. https://doi.org/10.3390/cancers15164114
Koerner AS, Moy RH, Ryeom SW, Yoon SS. The Present and Future of Neoadjuvant and Adjuvant Therapy for Locally Advanced Gastric Cancer. Cancers. 2023; 15(16):4114. https://doi.org/10.3390/cancers15164114
Chicago/Turabian StyleKoerner, Anna S., Ryan H. Moy, Sandra W. Ryeom, and Sam S. Yoon. 2023. "The Present and Future of Neoadjuvant and Adjuvant Therapy for Locally Advanced Gastric Cancer" Cancers 15, no. 16: 4114. https://doi.org/10.3390/cancers15164114
APA StyleKoerner, A. S., Moy, R. H., Ryeom, S. W., & Yoon, S. S. (2023). The Present and Future of Neoadjuvant and Adjuvant Therapy for Locally Advanced Gastric Cancer. Cancers, 15(16), 4114. https://doi.org/10.3390/cancers15164114






