A Multidimensional Connectomics- and Radiomics-Based Advanced Machine-Learning Framework to Distinguish Radiation Necrosis from True Progression in Brain Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Inclusion and Clinical Factors
2.2. Imaging Feature Extraction
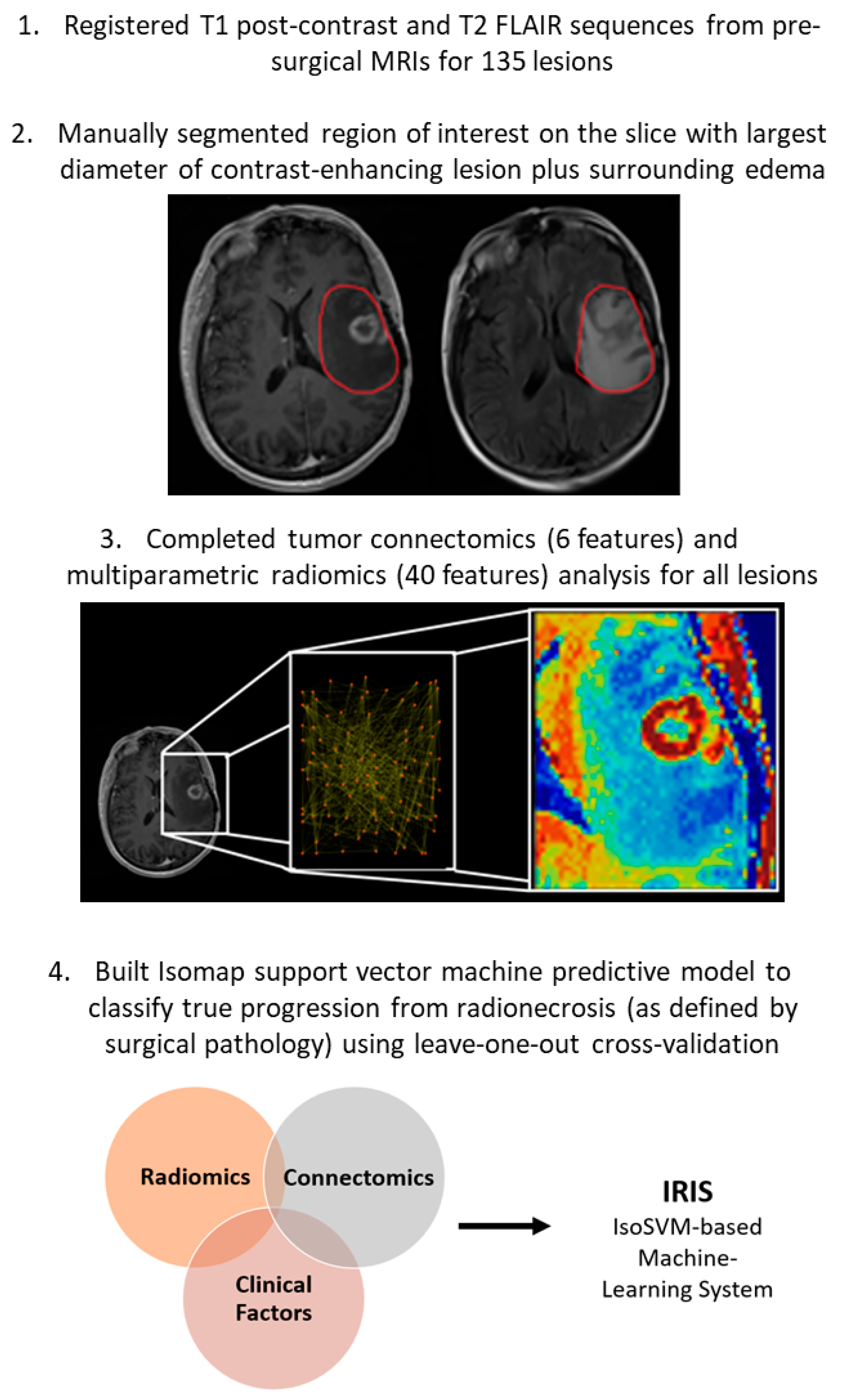
2.2.1. Region of Interest
2.2.2. Tumor Connectomics
- In the first step, the MRI image intensities across both the T1C and corresponding T2 FLAIR images were normalized to values between 0 and 1 (double-precision) to avoid any bias from intensity values.
- The second step involved computing an inter-voxel pair-wise Euclidean distance matrix. A threshold of 0.1 was empirically selected to transform the Euclidean distance into geodesic distance matrix using Dijkstra’s algorithm [14].
- The geodesic distance matrix was then evaluated to extract six different quantitative graph metrics of degree centrality, betweenness centrality, eigenvector centrality, node strength, average path length, and clustering coefficient.
2.2.3. Multiparametric Radiomics
- In the first step, the MRI image intensities across both sequence images were normalized to values between 0 and 1 (double-precision) to avoid any bias from the intensity values.
- The second step involved the extraction of tissue signature first-order statistics (TSFOS), tissue signature probability matrix (TSPM), and tissue signature co-occurrence matrix (TSCM) features, resulting in a total of 40 unique mpRad features. These features consisted of 2 TSPM, 15 TSFOS, and 23 TSCM features, as previously described [15].
2.3. IsoSVM Classification
- We used a combination of the AUC-ROC and MCC as our optimization metric instead of just the conventionally used AUC-ROC. This is because the MCC metric is a balanced statistical metric that produces a good score only when the prediction accuracy across all classes is high, ensuring that the classifier does not overfit towards the over-represented class [13,19].
2.4. Statistical Analysis
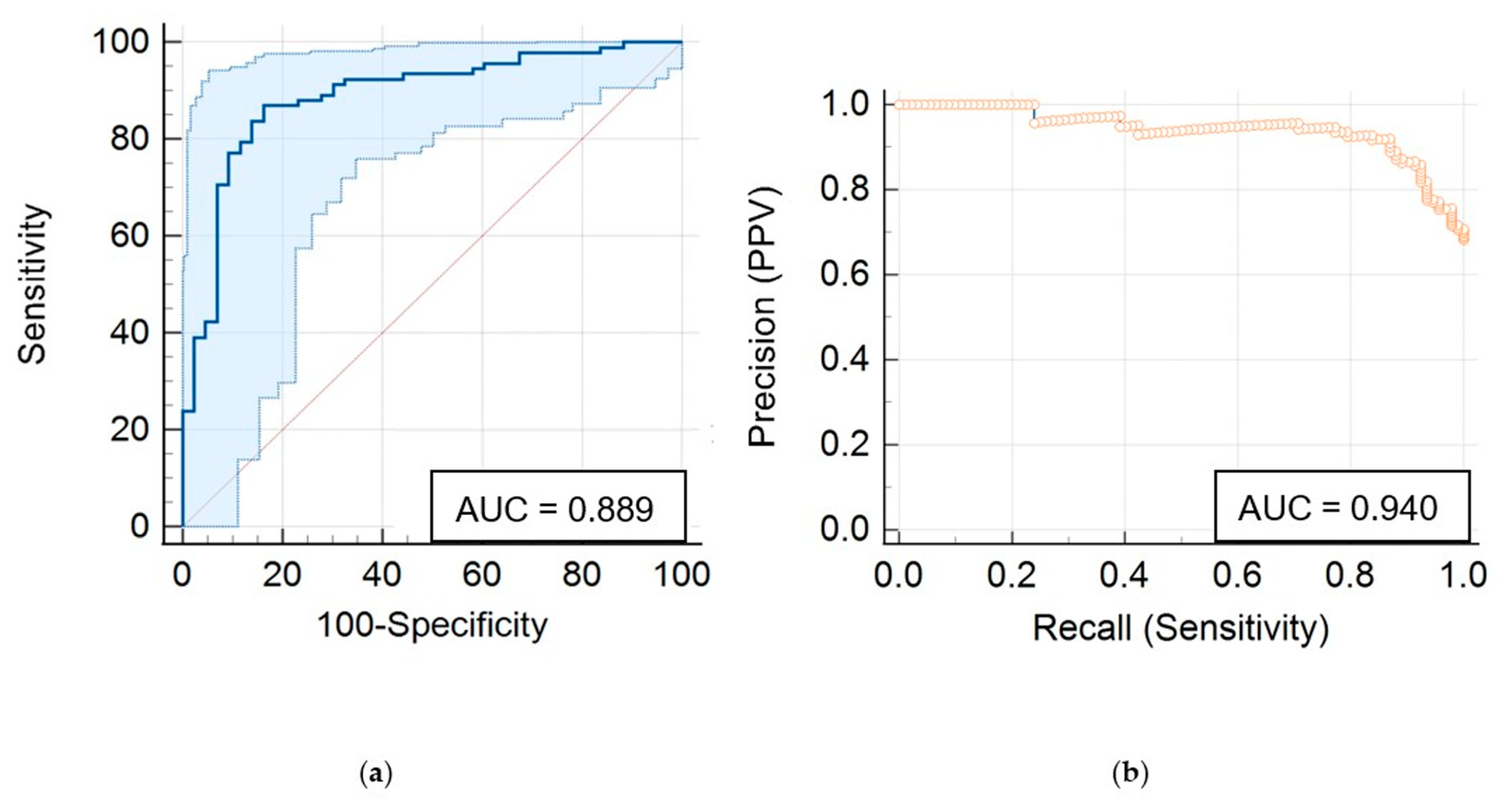
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, J.J.-W.; Hird, A.; Kirou-Mauro, A.; Napolskikh, J.; Chow, E. Quality of life in brain metastases radiation trials: A literature review. Curr. Oncol. 2008, 15, 25–45. [Google Scholar] [CrossRef]
- Redmond, K.J.; Gui, C.; Benedict, S.; Milano, M.T.; Grimm, J.; Vargo, J.A.; Soltys, S.G.; Yorke, E.; Jackson, A.; El Naqa, I.; et al. Tumor Control Probability of Radiosurgery and Fractionated Stereotactic Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. 2021, 110, 53–67. [Google Scholar] [CrossRef]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Ahluwalia, M.S.; Gurewitz, J.; Bernstein, K.; Kondziolka, D.; Niranjan, A.; Wei, Z.; Lunsford, L.D.; Fakhoury, K.R.; Rusthoven, C.G.; et al. Imaging-defined necrosis after treatment with single-fraction stereotactic radiosurgery and immune checkpoint inhibitors and its potential association with improved outcomes in patients with brain metastases: An international multicenter study of 697 patients. J. Neurosurg. 2023, 138, 1178–1187. [Google Scholar] [CrossRef]
- Vellayappan, B.; Tan, C.L.; Yong, C.; Khor, L.K.; Koh, W.Y.; Yeo, T.T.; Detsky, J.; Lo, S.; Sahgal, A. Diagnosis and Management of Radiation Necrosis in Patients With Brain Metastases. Front. Oncol. 2018, 8, 395. [Google Scholar] [CrossRef]
- Hettal, L.; Stefani, A.; Salleron, J.; Courrech, F.; Behm-Ansmant, I.; Constans, J.M.; Gauchotte, G.; Vogin, G. Radiomics Method for the Differential Diagnosis of Radionecrosis Versus Progression after Fractionated Stereotactic Body Radiotherapy for Brain Oligometastasis. Radiat. Res. 2020, 193, 471–480. [Google Scholar] [CrossRef]
- Karami, E.; Ruschin, M.; Soliman, H.; Sahgal, A.; Stanisz, G.J.; Sadeghi-Naini, A. An MR Radiomics Framework for Predicting the Outcome of Stereotactic Radiation Therapy in Brain Metastasis. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 1022–1025. [Google Scholar] [CrossRef]
- Larroza, A.; Moratal, D.; Paredes-Sánchez, A.; Soria-Olivas, E.; Chust, M.L.; Arribas, L.A.; Arana, E. Support vector machine classification of brain metastasis and radiation necrosis based on texture analysis in MRI. J. Magn. Reson. Imaging 2015, 42, 1362–1368. [Google Scholar] [CrossRef]
- Lohmann, P.; Kocher, M.; Ceccon, G.; Bauer, E.K.; Stoffels, G.; Viswanathan, S.; Ruge, M.I.; Neumaier, B.; Shah, N.J.; Fink, G.R.; et al. Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. NeuroImage Clin. 2018, 20, 537–542. [Google Scholar] [CrossRef]
- Mouraviev, A.; Detsky, J.; Sahgal, A.; Ruschin, M.; Lee, Y.K.; Karam, I.; Heyn, C.; Stanisz, G.J.; Martel, A.L. Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neuro-Oncology 2020, 22, 797–805. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Ho, A.; Jiang, W.; Logan, J.; Wang, X.; Brown, P.D.; McGovern, S.L.; Guha-Thakurta, N.; Ferguson, S.D.; et al. A predictive model for distinguishing radiation necrosis from tumour progression after gamma knife radiosurgery based on radiomic features from MR images. Eur. Radiol. 2018, 28, 2255–2263. [Google Scholar] [CrossRef]
- Parekh, V.; Jacobs, M.A. Radiomics: A new application from established techniques. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 207–226. [Google Scholar] [CrossRef]
- Parekh, V.S.; Pillai, J.J.; Macura, K.J.; LaViolette, P.S.; Jacobs, M.A. Tumor Connectomics: Mapping the Intra-Tumoral Complex Interaction Network Using Machine Learning. Cancers 2022, 14, 1481. [Google Scholar] [CrossRef]
- Dijkstra, E.W. A Note on Two Problems in Connexion with Graphs. Numer. Mat. 1959, 1, 269–271. [Google Scholar] [CrossRef]
- Peng, L.; Parekh, V.; Huang, P.; Lin, D.D.; Sheikh, K.; Baker, B.; Kirschbaum, T.; Silvestri, F.; Son, J.; Robinson, A.; et al. Distinguishing True Progression From Radionecrosis After Stereotactic Radiation Therapy for Brain Metastases With Machine Learning and Radiomics. Int. J. Radiat. Oncol. 2018, 102, 1236–1243. [Google Scholar] [CrossRef]
- Parekh, V.S.; Jacobs, M.A. Integrated radiomic framework for breast cancer and tumor biology using advanced machine learning and multiparametric MRI. NPJ Breast Cancer 2017, 3, 43. [Google Scholar] [CrossRef]
- Jacobs, M.A.; Umbricht, C.B.; Parekh, V.S.; El Khouli, R.H.; Cope, L.; Macura, K.J.; Harvey, S.; Wolff, A.C. Integrated Multiparametric Radiomics and Informatics System for Characterizing Breast Tumor Characteristics with the OncotypeDX Gene Assay. Cancers 2020, 12, 2772. [Google Scholar] [CrossRef]
- Jacobs, M.A.; Parekh, V.S. Informatics Radiomics Integration System (IRIS): A Novel Combined Informatics and Radiomics Method for Integration of Many Types of Data for Classification into Different Groups for Improved Visualization. U.S. Patent 11,324,469, 10 May 2022. [Google Scholar]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef]
- Chen, X.; Parekh, V.S.; Peng, L.; Chan, M.D.; Redmond, K.J.; Soike, M.; McTyre, E.; Lin, D.; Jacobs, M.A.; Kleinberg, L.R. Multiparametric radiomic tissue signature and machine learning for distinguishing radiation necrosis from tumor progression after stereotactic radiosurgery. Neuro-Oncol. Adv. 2021, 3, vdab150. [Google Scholar] [CrossRef]
- Hotta, M.; Minamimoto, R.; Miwa, K. 11C-methionine-PET for differentiating recurrent brain tumor from radiation necrosis: Radiomics approach with random forest classifier. Sci. Rep. 2019, 9, 15666. [Google Scholar] [CrossRef]
- Lohmann, P.; Stoffels, G.; Ceccon, G.; Rapp, M.; Sabel, M.; Filss, C.P.; Kamp, M.A.; Stegmayr, C.; Neumaier, B.; Shah, N.J.; et al. Radiation injury vs. recurrent brain metastasis: Combining textural feature radiomics analysis and standard parameters may increase 18F-FET PET accuracy without dynamic scans. Eur. Radiol. 2017, 27, 2916–2927. [Google Scholar] [CrossRef]
- Serizawa, T.; Saeki, N.; Higuchi, Y.; Ono, J.; Matsuda, S.; Sato, M.; Yanagisawa, M.; Iuchi, T.; Nagano, O.; Yamaura, A.; et al. Diagnostic value of thallium-201 chloride single-photon emission computerized tomography in differentiating tumor recurrence from radiation injury after gamma knife surgery for metastatic brain tumors. J. Neurosurg. 2005, 102, 266–271. [Google Scholar] [CrossRef]
- Lai, G.; Mahadevan, A.; Hackney, D.; Warnke, P.; Nigim, F.; Kasper, E.; Wong, E.; Carter, B.; Chen, C. Diagnostic Accuracy of PET, SPECT, and Arterial Spin-Labeling in Differentiating Tumor Recurrence from Necrosis in Cerebral Metastasis after Stereotactic Radiosurgery. Am. J. Neuroradiol. 2015, 36, 2250–2255. [Google Scholar] [CrossRef]
- Hatzoglou, V.; Yang, T.J.; Omuro, A.; Gavrilovic, I.; Ulaner, G.; Rubel, J.; Schneider, T.; Woo, K.M.; Zhang, Z.; Peck, K.K.; et al. A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro-Oncology 2016, 18, 873–880. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cho, S.J.; Sunwoo, L.; Baik, S.H.; Bae, Y.J.; Choi, B.S.; Jung, C.; Kim, J.H. Classification of true progression after radiotherapy of brain metastasis on MRI using artificial intelligence: A systematic review and meta-analysis. Neuro-Oncol. Adv. 2021, 3, vdab080. [Google Scholar] [CrossRef]
- Leeman, J.E.; Clump, D.A.; Flickinger, J.C.; Mintz, A.H.; Burton, S.A.; Heron, D.E. Extent of perilesional edema differentiates radionecrosis from tumor recurrence following stereotactic radiosurgery for brain metastases. Neuro-Oncology 2013, 15, 1732–1738. [Google Scholar] [CrossRef]
- Narloch, J.L.; Farber, S.H.; Sammons, S.; McSherry, F.; Herndon, J.E.; Hoang, J.K.; Yin, F.-F.; Sampson, J.H.; Fecci, P.E.; Blackwell, K.L.; et al. Biopsy of enlarging lesions after stereotactic radiosurgery for brain metastases frequently reveals radiation necrosis. Neuro-Oncology 2017, 19, 1391–1397. [Google Scholar] [CrossRef]
- Sneed, P.K.; Mendez, J.; Hoek, J.G.M.V.-V.D.; Seymour, Z.A.; Ma, L.; Molinaro, A.M.; Fogh, S.E.; Nakamura, J.L.; McDermott, M.W.; Ii, B.F.; et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: Incidence, time course, and risk factors. J. Neurosurg. 2015, 123, 373–386. [Google Scholar] [CrossRef]
- Miller, J.A.; Bennett, E.E.; Xiao, R.; Kotecha, R.; Chao, S.T.; Vogelbaum, M.A.; Barnett, G.H.; Angelov, L.; Murphy, E.S.; Yu, J.S.; et al. Association between Radiation Necrosis and Tumor Biology after Stereotactic Radiosurgery for Brain Metastasis. Int. J. Radiat. Oncol. 2016, 96, 1060–1069. [Google Scholar] [CrossRef]
- Kirkpatrick, J.P.; Wang, Z.; Sampson, J.H.; McSherry, F.; Herndon, J.E.; Allen, K.J.; Duffy, E.; Hoang, J.K.; Chang, Z.; Yoo, D.S.; et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. Int. J. Radiat. Oncol. 2015, 91, 100–108. [Google Scholar] [CrossRef]
- Dequesada, I.M.; Quisling, R.G.; Yachnis, A.; Friedman, W.A. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 2008, 63, 898–903; discussion 904. [Google Scholar] [CrossRef]
- Stockham, A.L.; Tievsky, A.L.; Koyfman, S.A.; Reddy, C.A.; Suh, J.H.; Vogelbaum, M.A.; Barnett, G.H.; Chao, S.T. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J. Neuro-Oncol. 2012, 109, 149–158. [Google Scholar] [CrossRef]
- Kano, H.; Kondziolka, D.; Lobato-Polo, J.; Zorro, O.; Flickinger, J.C.; Lunsford, L.D. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 2010, 66, 486–491; discussion 491–492. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef]
| Entire Cohort (n = 135) | Radiation Necrosis Cohort (n = 43) | True Progression Cohort (n = 92) | p-Value | |
|---|---|---|---|---|
| Primary histology | 0.08 | |||
| NSCLC | 48 (35.6%) | 10 (23.3%) | 38 (41.3%) | |
| Breast | 28 (20.7%) | 10 (23.3%) | 18 (19.6%) | |
| Melanoma | 27 (20.0%) | 14 (32.6%) | 13 (14.1%) | |
| SCLC | 9 (6.7%) | 2 (4.7%) | 7 (7.6%) | |
| Other | 23 (17.0%) | 7 (16.3%) | 16 (17.4%) | |
| RT in addition to SRS to the same area | 29 (21.5%) | 11 (25.6%) | 18 (19.6%) | 0.43 |
| Mean SRS isodose line, % (SD) | 68.3 (8.8) | 67.3 (9.7) | 68.8 (8.4) | 0.37 |
| Mean SRS BED10, Gy (SD) | 45.9 (8.2) | 48.3 (8.9) | 44.8 (7.7) | 0.03 |
| Mean SRS PTV volume, cc (SD) | 11.0 (13.2) | 9.8 (13.0) | 11.5 (13.3) | 0.50 |
| Mean time from SRS to surgery, months (SD) | 12.2 (10.0) | 15.2 (10.2) | 10.8 (9.7) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Parekh, V.S.; Lee, E.; Chen, X.; Redmond, K.J.; Pillai, J.J.; Peng, L.; Jacobs, M.A.; Kleinberg, L.R. A Multidimensional Connectomics- and Radiomics-Based Advanced Machine-Learning Framework to Distinguish Radiation Necrosis from True Progression in Brain Metastases. Cancers 2023, 15, 4113. https://doi.org/10.3390/cancers15164113
Cao Y, Parekh VS, Lee E, Chen X, Redmond KJ, Pillai JJ, Peng L, Jacobs MA, Kleinberg LR. A Multidimensional Connectomics- and Radiomics-Based Advanced Machine-Learning Framework to Distinguish Radiation Necrosis from True Progression in Brain Metastases. Cancers. 2023; 15(16):4113. https://doi.org/10.3390/cancers15164113
Chicago/Turabian StyleCao, Yilin, Vishwa S. Parekh, Emerson Lee, Xuguang Chen, Kristin J. Redmond, Jay J. Pillai, Luke Peng, Michael A. Jacobs, and Lawrence R. Kleinberg. 2023. "A Multidimensional Connectomics- and Radiomics-Based Advanced Machine-Learning Framework to Distinguish Radiation Necrosis from True Progression in Brain Metastases" Cancers 15, no. 16: 4113. https://doi.org/10.3390/cancers15164113
APA StyleCao, Y., Parekh, V. S., Lee, E., Chen, X., Redmond, K. J., Pillai, J. J., Peng, L., Jacobs, M. A., & Kleinberg, L. R. (2023). A Multidimensional Connectomics- and Radiomics-Based Advanced Machine-Learning Framework to Distinguish Radiation Necrosis from True Progression in Brain Metastases. Cancers, 15(16), 4113. https://doi.org/10.3390/cancers15164113






