In Patients Treated by Selective Internal Radiotherapy, Cellular In Vitro Immune Function Is Predictive of Survival
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. Lymphocyte Transformation Test
2.3. Statistical Analysis
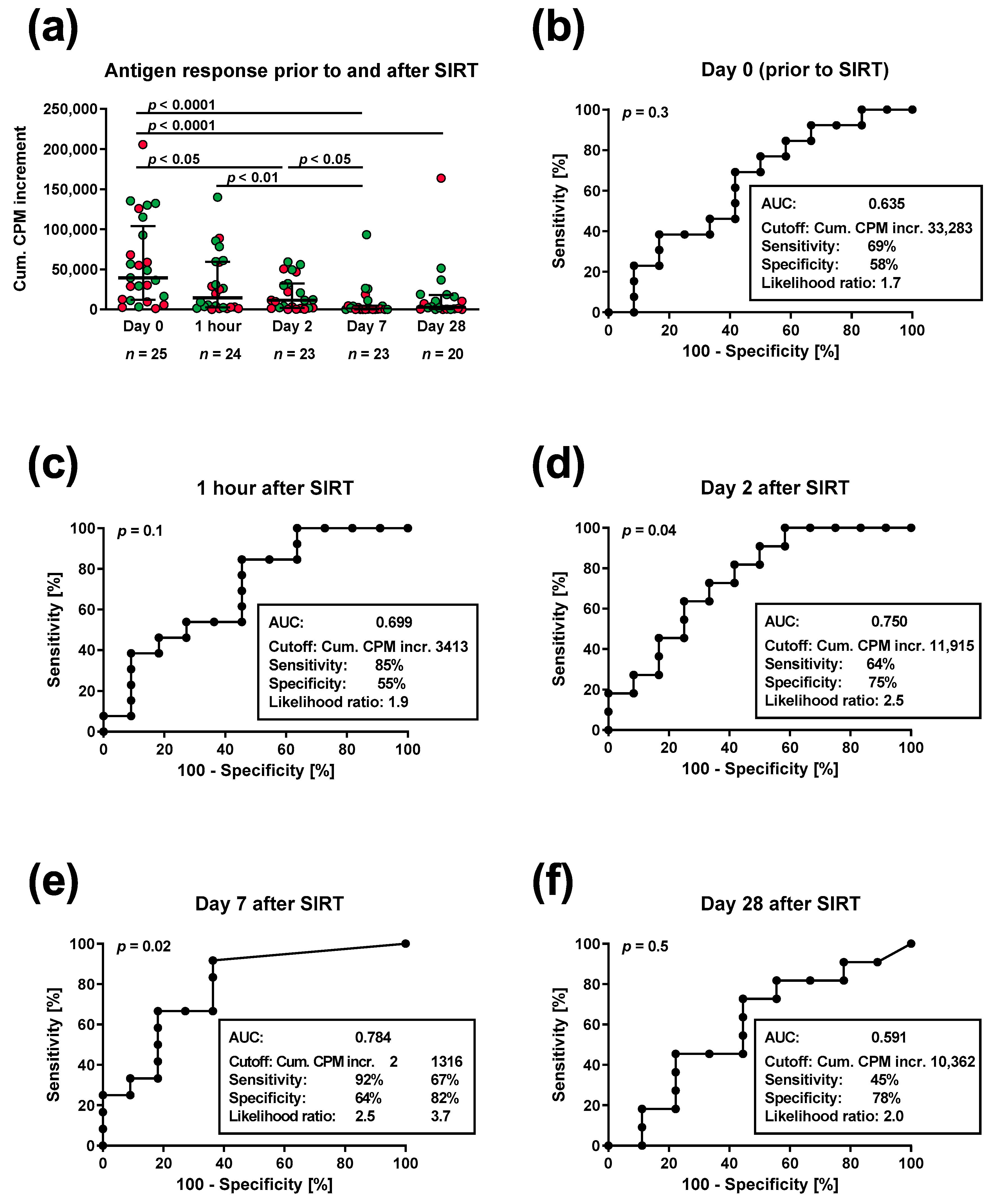
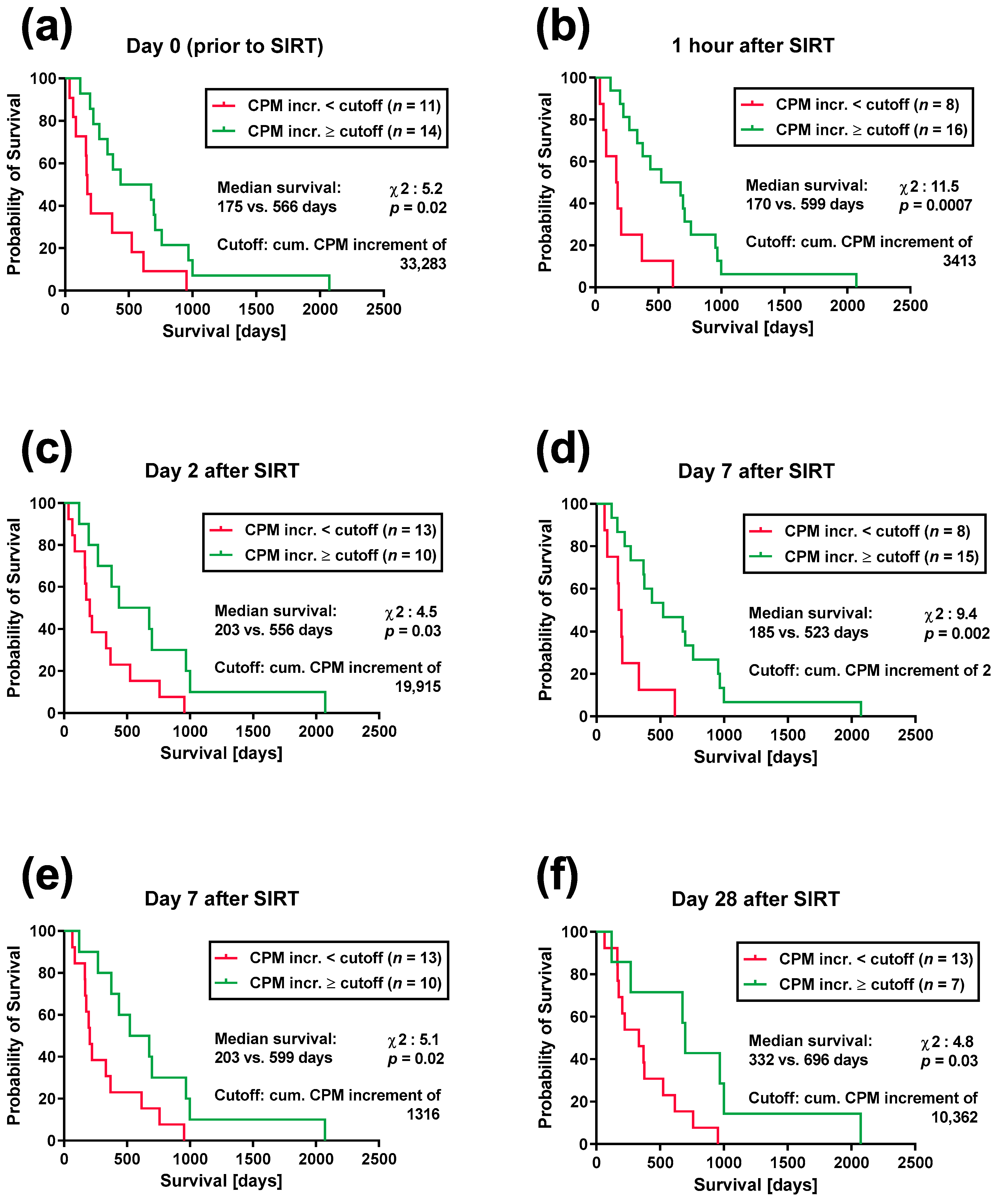
3. Results
3.1. Lymphocyte Proliferation in Patients Prior to and Post SIRT
3.2. Lymphocyte Proliferation and Patient Survival after SIRT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 2993–3036. [Google Scholar] [PubMed]
- Lewandowski, R.J.; Salem, R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin. Interv. Radiol. 2006, 23, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.W.; Alanis, L.; Cho, S.K.; Saab, S. Yttrium-90 Selective Internal Radiation Therapy with Glass Microspheres for Hepatocellular Carcinoma: Current and Updated Literature Review. Korean J. Radiol. 2016, 17, 472–488. [Google Scholar] [CrossRef]
- Theelen, W.; Peulen, H.M.U.; Lalezari, F.; van der Noort, V.; de Vries, J.F.; Aerts, J.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.N.; de Langen, A.J.; et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Barsegian, V.; Hueben, C.; Mueller, S.P.; Poeppel, T.D.; Horn, P.A.; Bockisch, A.; Lindemann, M. Impairment of lymphocyte function following yttrium-90 DOTATOC therapy. Cancer Immunol. Immunother. CII 2015, 64, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Barsegian, V.; Müller, S.P.; Horn, P.A.; Bockisch, A.; Lindemann, M. Lymphocyte function following radioiodine therapy in patients with thyroid carcinoma. Nuklearmedizin-NuclearMedicine 2011, 50, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Barsegian, V.; Müller, S.P.; Möckel, D.; Horn, P.A.; Bockisch, A.; Lindemann, M. Lymphocyte function following radium-223 therapy in patients with metastasized, castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 242–246. [Google Scholar] [CrossRef]
- Domouchtsidou, A.; Barsegian, V.; Mueller, S.P.; Best, J.; Ertle, J.; Bedreli, S.; Horn, P.A.; Bockisch, A.; Lindemann, M. Impaired lymphocyte function in patients with hepatic malignancies after selective internal radiotherapy. Cancer Immunol. Immunother. CII 2018, 67, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Matula, Z.; Gonczi, M.; Beko, G.; Kadar, B.; Ajzner, E.; Uher, F.; Valyi-Nagy, I. Antibody and T Cell Responses against SARS-CoV-2 Elicited by the Third Dose of BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) Vaccines Using a Homologous or Heterologous Booster Vaccination Strategy. Vaccines 2022, 10, 539. [Google Scholar] [CrossRef]
- Lindemann, M.; Witzke, O.; Winterhagen, T.; Ross, B.; Kreuzfelder, E.; Reinhardt, W.; Roggendorf, M.; Mann, K.; Philipp, T.; Grosse-Wilde, H. T-cell function after interleukin-2 therapy in HIV-infected patients is correlated with serum cortisol concentrations. Aids 2004, 18, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Domouchtsidou, A.; Barsegian, V.; Mueller, S.P.; Lobachevsky, P.; Best, J.; Horn, P.A.; Bockisch, A.; Lindemann, M. DNA lesions correlate with lymphocyte function after selective internal radiotherapy. Cancer Immunol. Immunother. CII 2019, 68, 907–915. [Google Scholar] [CrossRef]
- Kousholt, A.N.; Menzel, T.; Sorensen, C.S. Pathways for genome integrity in G2 phase of the cell cycle. Biomolecules 2012, 2, 579–607. [Google Scholar] [CrossRef]
- Lin, S.Z.; Chen, K.J.; Xu, Z.Y.; Chen, H.; Zhou, L.; Xie, H.Y.; Zheng, S.S. Prediction of recurrence and survival in hepatocellular carcinoma based on two Cox models mainly determined by FoxP3+ regulatory T cells. Cancer Prev. Res. 2013, 6, 594–602. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, F.M.; Wang, T.; Wang, Y.J.; Zhu, Z.Y.; Gao, Y.T.; Du, Z. Tumor-infiltrating FoxP3+ Tregs and CD8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion 2012, 86, 329–337. [Google Scholar] [CrossRef]
- Jochems, C.; Schlom, J. Tumor-infiltrating immune cells and prognosis: The potential link between conventional cancer therapy and immunity. Exp. Biol. Med. 2011, 236, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.F.; Ding, Y.H.; Ying, X.H.; Wu, F.Z.; Zhou, X.M.; Zhang, D.K.; Zou, H.; Ji, J.S. Regulatory T cells, especially ICOS(+) FOXP3(+) regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci. Rep. 2016, 6, 35056. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Cucchetti, A.; La Barba, G.; Ravaioli, M.; Del Gaudio, M.; Lauro, A.; Grazi, G.L.; Pinna, A.D. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: Reassessment of risk factors for tumor recurrence. Ann. Surg. 2008, 248, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Decaens, T.; Roudot-Thoraval, F.; Bresson-Hadni, S.; Meyer, C.; Gugenheim, J.; Durand, F.; Bernard, P.H.; Boillot, O.; Compagnon, P.; Calmus, Y.; et al. Role of immunosuppression and tumor differentiation in predicting recurrence after liver transplantation for hepatocellular carcinoma: A multicenter study of 412 patients. World J. Gastroenterol. 2006, 12, 7319–7325. [Google Scholar] [CrossRef]
- Vivarelli, M.; Bellusci, R.; Cucchetti, A.; Cavrini, G.; De Ruvo, N.; Aden, A.A.; La Barba, G.; Brillanti, S.; Cavallari, A. Low recurrence rate of hepatocellular carcinoma after liver transplantation: Better patient selection or lower immunosuppression? Transplantation 2002, 74, 1746–1751. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; García-Caparrós, C.; O’Beirne, J.; Poyato-González, A.; Ferrín-Sánchez, G.; Montero-Álvarez, J.L.; Patch, D.; et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1193–1199. [Google Scholar] [CrossRef]
- Ossami Saidy, R.R.; Postel, M.P.; Pflüger, M.J.; Schoening, W.; Öllinger, R.; Gül-Klein, S.; Schmelzle, M.; Tacke, F.; Pratschke, J.; Eurich, D. Minimization of Immunosuppressive Therapy Is Associated with Improved Survival of Liver Transplant Patients with Recurrent Hepatocellular Carcinoma. Cancers 2021, 13, 1617. [Google Scholar] [CrossRef]
- Pesthy, S.; Wegener, E.; Ossami Saidy, R.R.; Timmermann, L.; Uluk, D.; Aydin, M.; Dziodzio, T.; Schoening, W.; Lurje, G.; Öllinger, R.; et al. Reducing Immunosuppression in Patients with De Novo Lung Carcinoma after Liver Transplantation Could Significantly Prolong Survival. Cancers 2022, 14, 2748. [Google Scholar] [CrossRef]
- Berenguer, M.; Burra, P.; Ghobrial, M.; Hibi, T.; Metselaar, H.; Sapisochin, G.; Bhoori, S.; Kwan Man, N.; Mas, V.; Ohira, M.; et al. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1143–1149. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Ling, Y.; Wang, H.Y. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. Precis. Oncol. 2018, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; Messa, C.; Guerra, V.; Carr, B.I.; D’Alessandro, R. Inflammatory Mechanisms of HCC Development. Cancers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Ailia, M.J.; Heo, J.; Yoo, S.Y. Navigating through the PD-1/PDL-1 Landscape: A Systematic Review and Meta-Analysis of Clinical Outcomes in Hepatocellular Carcinoma and Their Influence on Immunotherapy and Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 6495. [Google Scholar] [CrossRef]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-thermal ablations—Boosting the anticancer immune response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef]
- Donisi, C.; Puzzoni, M.; Ziranu, P.; Lai, E.; Mariani, S.; Saba, G.; Impera, V.; Dubois, M.; Persano, M.; Migliari, M.; et al. Immune Checkpoint Inhibitors in the Treatment of HCC. Front. Oncol. 2020, 10, 601240. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Kovács, S.A.; Fekete, J.T.; Győrffy, B. Predictive biomarkers of immunotherapy response with pharmacological applications in solid tumors. Acta Pharmacol. Sin. 2023, 1–11. [Google Scholar] [CrossRef]
- Marei, H.E.; Hasan, A.; Pozzoli, G.; Cenciarelli, C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): Potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 2023, 23, 64. [Google Scholar] [CrossRef]
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef]
| Parameter | Absolute Number or Median (Range) | |
|---|---|---|
| Age (years) | 73 (33–84) | |
| Sex | Female | 5 |
| Male | 20 | |
| Body mass index (kg/m2) | 26.6 (20.1–38.3) | |
| Comorbidities | Kidney insufficiency | 5 |
| Diabetes mellitus | 13 | |
| Hypertension | 22 | |
| Coronary heart disease | 11 | |
| ECOG performance status | 0 | 14 |
| 1 | 9 | |
| 2 | 2 | |
| Kidney function | Serum creatinine (mg/dL) b | 1.0 (0.8–2.2) |
| GFR (mL/min/1.73 m2) b | 69 (31–113) | |
| Albumin (g/dL) b | 4.1 (3.4–4.9) | |
| Liver function | Aspartate aminotransferase (U/L) b | 52 (28–227) |
| Alanine aminotransferase (U/L) b | 36 (17–208) | |
| Alkaline phosphatase (U/L) b | 148 (64–775) | |
| Bilirubin, total (mg/dL) a | 0.8 (0.4–1.8) | |
| Gamma-glutamyl transferase (U/L) b | 184 (20–1467) | |
| Quick (%) | 97 (42–120) | |
| Median administered activity (GBq) | 2.9 (1.0–6.4) | |
| Therapy volume (Gy) | 119 (110–282) | |
| Dose to the lungs (Gy) a | 6.5 (1.0–21.4) | |
| Location of primary tumor | Liver | 24 |
| CUP | 1 | |
| Metastases | Without | 10 |
| Lungs | 4 | |
| Adrenal gland | 2 | |
| Bones | 2 | |
| Pancreas | 1 | |
| Stomach | 1 | |
| Other | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domouchtsidou, A.; Beckmann, F.; Marenbach, B.; Mueller, S.P.; Best, J.; Herrmann, K.; Horn, P.A.; Barsegian, V.; Lindemann, M. In Patients Treated by Selective Internal Radiotherapy, Cellular In Vitro Immune Function Is Predictive of Survival. Cancers 2023, 15, 4055. https://doi.org/10.3390/cancers15164055
Domouchtsidou A, Beckmann F, Marenbach B, Mueller SP, Best J, Herrmann K, Horn PA, Barsegian V, Lindemann M. In Patients Treated by Selective Internal Radiotherapy, Cellular In Vitro Immune Function Is Predictive of Survival. Cancers. 2023; 15(16):4055. https://doi.org/10.3390/cancers15164055
Chicago/Turabian StyleDomouchtsidou, Aglaia, Ferdinand Beckmann, Beate Marenbach, Stefan P. Mueller, Jan Best, Ken Herrmann, Peter A. Horn, Vahé Barsegian, and Monika Lindemann. 2023. "In Patients Treated by Selective Internal Radiotherapy, Cellular In Vitro Immune Function Is Predictive of Survival" Cancers 15, no. 16: 4055. https://doi.org/10.3390/cancers15164055
APA StyleDomouchtsidou, A., Beckmann, F., Marenbach, B., Mueller, S. P., Best, J., Herrmann, K., Horn, P. A., Barsegian, V., & Lindemann, M. (2023). In Patients Treated by Selective Internal Radiotherapy, Cellular In Vitro Immune Function Is Predictive of Survival. Cancers, 15(16), 4055. https://doi.org/10.3390/cancers15164055






