Impact of BRAFV600E Mutation on Event-Free Survival in Patients with Papillary Thyroid Carcinoma: A Retrospective Study in a Romanian Population
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Pathological Data
2.3. Molecular Analysis
2.4. Follow-Up Data
2.5. Statistical Analysis
3. Results
3.1. Patient’s Characteristics
3.2. Prevalence of BRAFV600E Mutation and Relationship with Demographic, Pathological and Outcomes Characteristics
3.3. Predictive Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Holmes, D. Thyroid Cancer: Incidence Trends in the USA. Nat. Rev. Endocrinol. 2016, 12, 312. [Google Scholar] [CrossRef]
- Mao, Y.; Xing, M. Recent Incidences and Differential Trends of Thyroid Cancer in the USA. Endocr. Relat. Cancer 2016, 23, 313–322. [Google Scholar] [CrossRef]
- Amphlett, B.; Lawson, Z.; Abdulrahman, G.O.; White, C.; Bailey, R.; Premawardhana, L.D.; Okosieme, O.E. Recent Trends in the Incidence, Geographical Distribution, and Survival from Thyroid Cancer in Wales, 1985–2010. Thyroid 2013, 23, 1470–1478. [Google Scholar] [CrossRef]
- Póvoa, A.A.; Teixeira, E.; Bella-Cueto, M.R.; Melo, M.; Oliveira, M.J.; Sobrinho-Simões, M.; Maciel, J.; Soares, P. Clinicopathological Features as Prognostic Predictors of Poor Outcome in Papillary Thyroid Carcinoma. Cancers 2020, 12, 3186. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Lauro, A.; Pironi, D.; Tripodi, D.; Lori, E.; Ferent, I.C.; Amabile, M.I.; Catania, A.; Di Matteo, F.M.; et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers 2021, 13, 5567. [Google Scholar] [CrossRef]
- Bournaud, C.; Descotes, F.; Decaussin-Petrucci, M.; Berthiller, J.; de la Fouchardière, C.; Giraudet, A.L.; Bertholon-Gregoire, M.; Robinson, P.; Lifante, J.C.; Lopez, J.; et al. TERT Promoter Mutations Identify a High-Risk Group in Metastasis-Free Advanced Thyroid Carcinoma. Eur. J. Cancer 2019, 108, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, T.; Liu, Z. Associations between BRAF and Prognostic Factors and Poor Outcomes in Papillary Thyroid Carcinoma: A Meta-Analysis. World J. Surg. Oncol. 2016, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.J.; Marais, R. Guilty as Charged: B-RAF Is a Human Oncogene. Cancer Cell 2004, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Hilger, R.A.; Scheulen, M.E.; Strumberg, D. The Ras-Raf-MEK-ERK Pathway in the Treatment of Cancer. Onkologie 2002, 25, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C.; et al. Association between BRAF V600E Mutation and Recurrence of Papillary Thyroid Cancer. J. Clin. Oncol. 2015, 33, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, K.; Lin, X.; Zhao, L.; An, W.; Wang, C.; Liu, X. The Association between BRAF (V600E) Mutation and Pathological Features in PTC. Eur. Arch. Otorhinolaryngol. 2014, 271, 3041–3052. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.S.; Hussein, M.; Issa, P.P.; Elnahla, A.; Farhoud, A.; Magazine, B.M.; Youssef, M.R.; Aboueisha, M.; Shama, M.; Toraih, E.; et al. Association of BRAFV600E Mutation with the Aggressive Behavior of Papillary Thyroid Microcarcinoma: A Meta-Analysis of 33 Studies. Int. J. Mol. Sci. 2022, 23, 15626. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, Y.J.; Lim, J.A.; Ahn, H.Y.; Lee, E.K.; Lee, Y.J.; Kim, K.W.; Hahn, S.K.; Youn, Y.K.; Kim, K.H.; et al. The Association of the BRAF(V600E) Mutation with Prognostic Factors and Poor Clinical Outcome in Papillary Thyroid Cancer: A Meta-Analysis. Cancer 2012, 118, 1764–1773. [Google Scholar] [CrossRef]
- Xing, M. BRAF Mutation in Papillary Thyroid Cancer: Pathogenic Role, Molecular Bases, and Clinical Implications. Endocr. Rev. 2007, 28, 742–762. [Google Scholar] [CrossRef]
- Vuong, H.G.; Duong, U.N.P.; Altibi, A.M.A.; Ngo, H.T.T.; Pham, T.Q.; Tran, H.M.; Gandolfi, G.; Hassell, L. A Meta-Analysis of Prognostic Roles of Molecular Markers in Papillary Thyroid Carcinoma. Endocr. Connect. 2017, 6, R8–R17. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Sorrenti, S.; Barollo, S.; Prinzi, N.; Catania, A.; Nesca, A.; Gnessi, L.; Pelizzo, M.R.; Mian, C.; et al. In Papillary Thyroid Carcinoma BRAFV600E Is Associated with Increased Expression of the Urokinase Plasminogen Activator and Its Cognate Receptor, but Not with Disease-Free Interval. Clin. Endocrinol. 2012, 77, 780–786. [Google Scholar] [CrossRef]
- Gan, X.; Shen, F.; Deng, X.; Feng, J.; Lu, J.; Cai, W.; Peng, L.; Zheng, W.; Wang, W.; Huang, P.; et al. Prognostic Implications of the BRAF-V600E Mutation in Papillary Thyroid Carcinoma Based on a New Cut-off Age Stratification. Oncol. Lett. 2020, 19, 631–640. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. (Eds.) WHO Classification of Tumours of Endocrine Organs; International Agency for Research on Cancer: Lyon, France, 2017; ISBN 978-92-832-4493-6. [Google Scholar]
- American Joint Committee on Cancer; Amin, M.B. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Nechifor-Boila, A.; Loghin, A.; Descotes, F.; Decaussin-Petrucci, M.; Borda, A. Evaluation of a DNA Extraction and Purification Protocol Using Archived Formalin-Fixed Paraffin-Embedded Tissues for BRAF Mutations Analysis in Papillary Thyroid Microcarcinomas. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 70–76. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Costa, V.; Esposito, R.; Pallante, P.; Ciccodicola, A.; Fusco, A. The “next-Generation” Knowledge of Papillary Thyroid Carcinoma. Cell Cycle 2015, 14, 2018–2021. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Kucukodaci, Z.; Akar, E.; Haholu, A.; Baloglu, H. A Valuable Adjunct to FNA Diagnosis of Papillary Thyroid Carcinoma: In-House PCR Assay for BRAF T1799A (V600E). Diagn. Cytopathol. 2011, 39, 424–427. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Xiong, J.; Li, C.; Liao, Y.; Zhu, Y.; Mao, J. Risk and Prognostic Factors for BRAFV600E Mutations in Papillary Thyroid Carcinoma. Biomed. Res. Int. 2022, 2022, 9959649. [Google Scholar] [CrossRef]
- Daliri, M.; Abbaszadegan, M.R.; Mehrabi Bahar, M.; Arabi, A.; Yadollahi, M.; Ghafari, A.; Taghehchian, N.; Zakavi, S.R. The Role of BRAF V600E Mutation as a Potential Marker for Prognostic Stratification of Papillary Thyroid Carcinoma: A Long-Term Follow-up Study. Endocr. Res. 2014, 39, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Al-Masri, M.; Al-Shobaki, T.; Al-Najjar, H.; Iskanderian, R.; Younis, E.; Abdallah, N.; Tbakhi, A.; Haddad, H.; Al-Masri, M.; Obeid, Z.; et al. BRAF V600E Mutation in Papillary Thyroid Carcinoma: It’s Relation to Clinical Features and Oncologic Outcomes in a Single Cancer Centre Experience. Endocr. Connect. 2021, 10, 1531–1537. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.Z.; Guan, Y.X.; Chen, Q.J.; Zhu, Q.Y. Meta-Analyses of Association between BRAF(V600E) Mutation and Clinicopathological Features of Papillary Thyroid Carcinoma. Cell. Physiol. Biochem. 2016, 38, 763–776. [Google Scholar] [CrossRef]
- Xing, M.; Westra, W.H.; Tufano, R.P.; Cohen, Y.; Rosenbaum, E.; Rhoden, K.J.; Carson, K.A.; Vasko, V.; Larin, A.; Tallini, G.; et al. BRAF Mutation Predicts a Poorer Clinical Prognosis for Papillary Thyroid Cancer. J Clin. Endocrinol. Metab. 2005, 90, 6373–6379. [Google Scholar] [CrossRef]
- Damiani, L.; Lupo, S.; Rossi, R.; Bruni, S.; Bartolomei, M.; Panareo, S.; Franceschetti, P.; Carcoforo, P.; Lanza, G.; Pelucchi, S.; et al. Evaluation of the Role of BRAF V600E Somatic Mutation on Papillary Thyroid Cancer Disease Persistence: A Prospective Study. Eur. Thyroid J. 2018, 7, 251–257. [Google Scholar] [CrossRef]
- Li, X.; Kwon, H. The Impact of BRAF Mutation on the Recurrence of Papillary Thyroid Carcinoma: A Meta-Analysis. Cancers 2020, 12, 2056. [Google Scholar] [CrossRef] [PubMed]
- Scheffel, R.S.; Dora, J.M.; Maia, A.L. BRAF Mutations in Thyroid Cancer. Curr. Opin. Oncol. 2022, 34, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Viola, D.; Torregrossa, L.; Giannini, R.; Romei, C.; Ugolini, C.; Molinaro, E.; Agate, L.; Biagini, A.; Lupi, C.; et al. The BRAF(V600E) Mutation Is an Independent, Poor Prognostic Factor for the Outcome of Patients with Low-Risk Intrathyroid Papillary Thyroid Carcinoma: Single-Institution Results from a Large Cohort Study. J. Clin. Endocrinol. Metab. 2012, 97, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Altibi, A.M.A.; Duong, U.N.P.; Hassell, L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin. Endocrinol. 2017, 87, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, M.; Fiorentino, V.; Martini, M.; Capodimonti, S.; Cenci, T.; Lombardi, C.P.; Raffaelli, M.; Pontecorvi, A.; Fadda, G.; Pantanowitz, L.; et al. How limited molecular testing can also offer diagnostic and prognostic evaluation of thyroid nodules processed with liquid-based cytology: Role of TERT promoter and BRAF V600E mutation analysis. Cancer Cytopathol. 2021, 129, 819–829. [Google Scholar] [CrossRef]
- Monti, E.; Gay, S.; Dono, M.; Giusti, M.; Pigozzi, S.; De Luca, G.; Anselmi, G.; Mora, M.; Spina, B.; Minuto, M.N.; et al. PD-L1 expression, BRAF and TERT mutation in a cohort of aggressive thyroid cancers: Case series from a single-centre experience. J. Endocrinol. Investig. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Pizzimenti, C.; Fiorentino, V.; Ieni, A.; Rossi, E.D.; Germanà, E.; Giovanella, L.; Lentini, M.; Alessi, Y.; Tuccari, G.; Campennì, A.; et al. BRAF-AXL-PD-L1 Signaling Axis as a Possible Biological Marker for RAI Treatment in the Thyroid Cancer ATA Intermediate Risk Category. Int. J. Mol. Sci. 2023, 24, 10024. [Google Scholar] [CrossRef]
- Romei, C.; Elisei, R. A Narrative Review of Genetic Alterations in Primary Thyroid Epithelial Cancer. Int. J. Mol. Sci. 2021, 22, 1726. [Google Scholar] [CrossRef]
- Guenter, R.; Patel, Z.; Chen, H. Notch Signaling in Thyroid Cancer. Adv. Exp. Med. Biol. 2021, 1287, 155–168. [Google Scholar] [CrossRef]
- Boufraqech, M.; Patel, D.; Nilubol, N.; Powers, A.; King, T.; Shell, J.; Lack, J.; Zhang, L.; Gara, S.K.; Gunda, V.; et al. Lysyl Oxidase Is a Key Player in BRAF/MAPK Pathway-Driven Thyroid Cancer Aggressiveness. Thyroid 2019, 29, 79–92. [Google Scholar] [CrossRef]
- Saqcena, M.; Leandro-Garcia, L.J.; Maag, J.L.V.; Tchekmedyian, V.; Krishnamoorthy, G.P.; Tamarapu, P.P.; Tiedje, V.; Reuter, V.; Knauf, J.A.; de Stanchina, E.; et al. SWI/SNF Complex Mutations Promote Thyroid Tumor Progression and Insensitivity to Redifferentiation Therapies. Cancer Discov. 2021, 11, 1158–1175. [Google Scholar] [CrossRef] [PubMed]

| Factors | Total n = 127 | BRAFV600E Wild-Type n = 60 | BRAFV600E Mutated n = 67 | p a |
|---|---|---|---|---|
| Age at surgery (mean ± SD, years) | 48.6 ± 1.28 | 46.18 ± 1.17 | 50.76 ± 1.72 | 0.075 * |
| Age (n, %) | 0.037 | |||
| <55 years | 79 (62.2) | 43 (71.7) | 36 (53.7) | |
| ≥55 years | 48 (37.8) | 17 (28.3) | 31 (46.3) | |
| Gender, female (n, %) | 110 (86.6) | 56 (93.3) | 54 (80.6) | 0.035 |
| Tumor size (mean ± SD, mm) | 22.88 ± 1.5 | 23.35 ± 1.42 | 21.90 ± 1.33 | 0.458 * |
| Tumor size (n, %) | ||||
| 11–20 mm | 67 (52.8) | 29 (48.3) | 38 (56.7) | 0.442 |
| 21–40 mm | 51 (40.2) | 27 (45.0) | 24 (35.8) | 0.225 |
| >40 mm | 9 (7.1) | 4 (6.7) | 5 (7.5) | 0.864 |
| Multifocality (n, %) | 51 (40.2) | 20 (33.3) | 31 (46.3) | 0.138 |
| Histological variant (n, %) | ||||
| Conventional | 88 (69.3) | 32 (53.3) | 56 (83.6) | 0.0005 |
| Tall cell variant | 9 (7.1) | 3 (5) | 6 (9) | 0.596 |
| Warthin-like | 7 (5.5) | 3 (5) | 4 (6) | 0.886 |
| Oncocytic | 3 (2.4) | 3 (5) | 0 | 0.205 |
| Solid | 2 (1.6) | 2 (3.3) | 0 | 0.434 |
| Follicular variant, infiltrative | 13 (10.2) | 12 (20) | 1 (1.5) | 0.001 |
| Follicular variant, encapsulated, invasive | 5 (3.9) | 5 (8.3) | 0 | 0.051 |
| Extrathyroidal extension (n, %) | 24 (18.9) | 5 (8.3) | 19 (28.4) | 0.004 |
| Primary tumor, pT (n, %) | ||||
| 1b | 51 (40.2) | 25 (41.7) | 26 (38.8) | 0.879 |
| 2 | 44 (34.6) | 25 (41.7) | 19 (28.4) | 0.165 |
| 3a | 8 (6.3) | 5 (8.3) | 3 (4.5) | 0.607 |
| 3b | 24 (18.9) | 5 (8.3) | 19 (28.4) | 0.007 |
| Lymph node involvement (n, %) | 26/39 | 2/8 (25) | 24/31 (77.4) | 0.001 |
| Vascular invasion (n, %) | 4 (3.3) | 2 (3.1) | 2 (3) | 0.911 |
| Positive surgical resection margin (n, %) | 18 (14.2) | 4 (6.7) | 14 (20.9) | 0.022 |
| Stage grouping | ||||
| I | 101 (79.5) | 54 (90) | 47 (70.1) | 0.010 |
| II | 22 (17.3) | 6 (10) | 16 (23.9) | 0.067 |
| III | 4 (3.1) | 0 | 4 (6) | 0.155 |
| Type of surgery (n, %) | ||||
| Lobectomy | 0 | 0 | 0 | - |
| Total thyroidectomy | 88 (69.3) | 52 (86.7) | 36 (53.7) | 0.0001 |
| Total thyroidectomy with lymph node dissection | 39 (30.7) | 8 (13.3) | 31 (46.2) | 0.0001 |
| Follow-up data (n, median, months) | 57 (CI: 9–130) | 58 (CI: 17–114) | 57 (CI: 9–130) | - |
| I131 therapy (n, %) | 133 (100) | 66 (100) | 67 (100) | - |
| Disease free (n, %) | 107 (84.3) | 56 (93.4) | 51 (76.1) | 0.008 |
| Persistent disease (n, %) | 14 (11) | 2 (3.3) | 12 (17.9) | 0.009 |
| Recurrence (n, %) | 6 (4.7) | 2 (3.3) | 4 (6) | 0.484 |
| Distant metastasis (n, %) | 5 (3.9) | 0 | 5 (7.5) | 0.031 |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Factors | BRAFV600E Positive (67)/N | OR | [95%CI] | p | OR | [95%CI] | p |
| Age ≥ 55 years | 31/48 | 2.18 | [1.04–4.56] | 0.039 | 2.20 | [0.90–5.37] | 0.081 |
| Sex, male | 13/17 | 3.37 | [1.03–10.98] | 0.043 | 2.81 | [0.67–11.72] | 0.155 |
| PTC, conventional | 56/88 | 4.44 | [1.95–10.13] | 0.008 | 6.33 | [2.18–18.40] | <0.001 |
| PTC, “tall cell” | 4/9 | 1.88 | [0.44–7.82] | 0.392 | |||
| Tumor size > 40 mm | 5/9 | 1.13 | [0.28–4.41] | 0.861 | |||
| Extrathyroidal extension | 19/24 | 4.35 | [1.51–12.54] | 0.006 | 5.83 | [1.60–21.27] | 0.007 |
| Multifocality | 31/51 | 1.72 | [0.83–3.53] | 0.139 | |||
| Lymph node metastasis | 24/26 | 7.81 | [2.52–24.20] | <0.001 | 4.77 | [1.44–15.79] | 0.010 |
| Positive resection margin | 14/18 | 3.69 | [1.14–11.95] | 0.028 | 2.25 | [0.56–9.00] | 0.249 |
| BRAFV600E Wild Type | BRAFV600E Mutated | Log Rank | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | * 12 Months (%) (95%CI) | * 24 Months (%) (95%CI) | * 48 Months (%) (95%CI) | * 60 Months (%) (95%CI) | * 12 Months (%) (95%CI) | * 24 Months (%) (95%CI) | * 48 Months (%) (95%CI) | * 60 Months (%) (95%CI) | ||
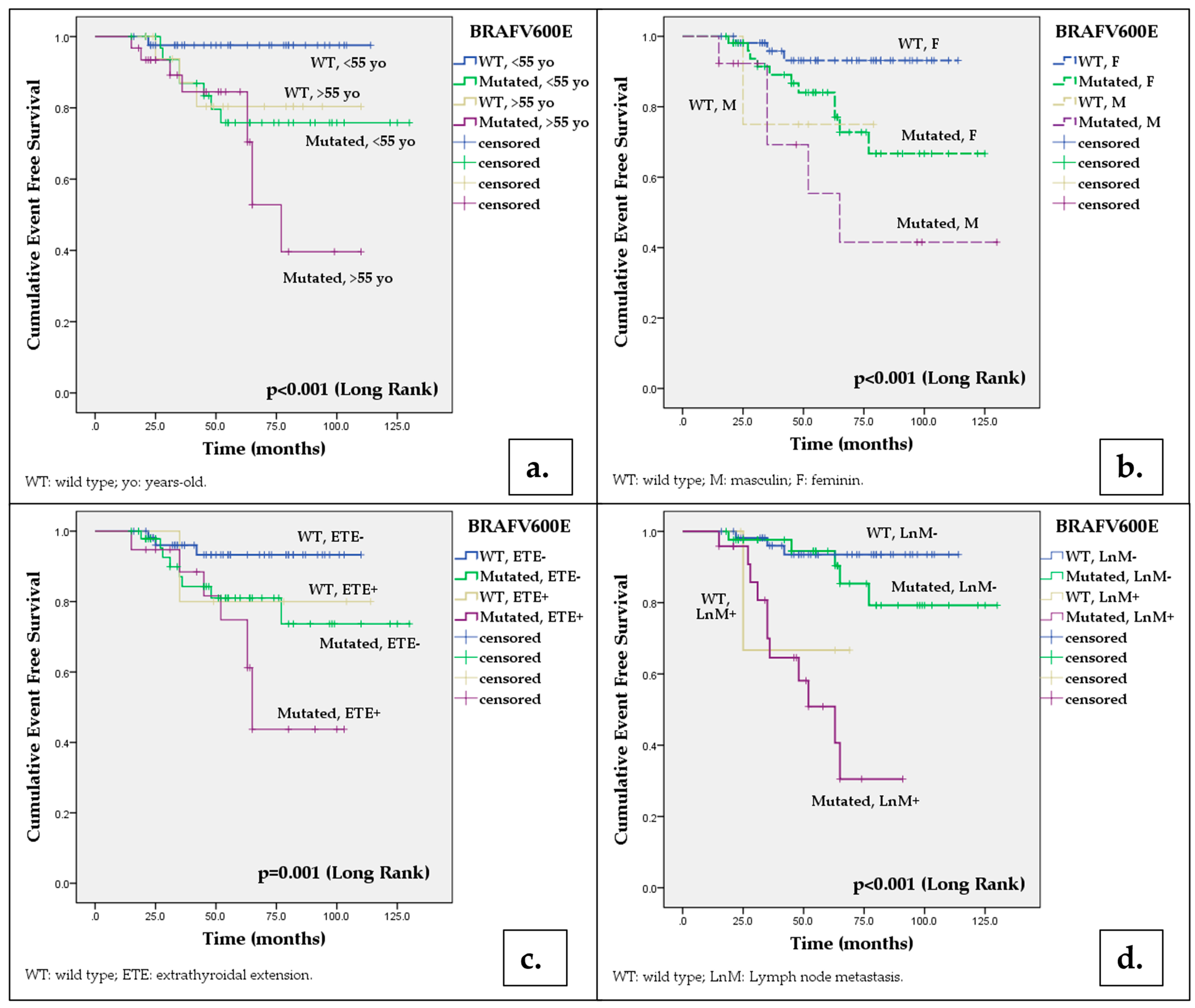
| Total | 98.3 (96.6–100) | 96.4 (93.9–98.4) | 94.2 (90.9–97.5) | 91.7 (87.7–95.7) | 98.5 (97–100) | 95.1 (92.3–97.9) | 81.4 (76–86.8) | 62.4 (54.2–70.6) | 6.581 | 0.010 |
| Age (n,%) | ||||||||||
| <55 years | 97.6 (95.2–100) | 97.6 (95.2–100) | 97.6 (95.2–100) | 97.6 (95.2–100) | 96.8 (96.4–100) | 93.5 (89.1–97.8) | 79.6 (72.1–87.1) | 75.8 (67.8–83.8) | 5.314 a | 0.021 |
| ≥55 years | 93.8 (93.1–100) | 93.8 (93.1–100) | 80.4 (70.2–90.6) | 80.4 (70.2–90.6) | 96.8 (93.6–100) | 93.4 (88.9–97.9) | 84.5 (77.2–91.8) | 52.8 (39.3–66.3) | 12.641 a | <0.001 |
| Gender (n, %) | ||||||||||
| Female | 98.1 (96.3–100) | 98.1 (96.3–100) | 93.1 (89.2–97) | 93.1 (89.2–97) | 98.1 (96.2–100) | 95.9 (93–98.8) | 84 (78.4–89.6) | 72.8 (65–80.6) | 5.274 b | 0.022 |
| Male | 75 (53.3–96.7) | 75 (53.3–96.7) | 75 (53.3–96.7) | 75 (53.3–96.7) | 92.3 (84.9–100) | 92.3 (84.9–100) | 69.2 (47.7–78.1) | 55.4 (38.1–72.7) | 13.427 b | <0.001 |
| Histology | ||||||||||
| PTC conventional | 96.7 (93.4–100) | 94.3 (90–98.6) | 92.1 (87.2–97) | 89.3 (83.7–94.9) | 98.1 (96.2–100) | 93.6 (90–97.2) | 78.6 (72.2–85) | 68.5 (59.8–77.2) | 7.973 | 0.005 |
| PTC tall cell variant | 98.4 (96.8–100) | 85.4 (81.5–89.3) | 66.7 (39.5–93.9) | 66.7 (39.5–93.9) | 83.3 (68.1–98.5) | 83.3 (68.1–96.5) | 62.5 (41.2–83.8) | 41.7 (19.5–63.9) | 5.997 | 0.014 |
| Tumor size | ||||||||||
| ≤40 mm | 98.2(96.4–100) | 96.2 (93.5–98.9) | 91.2 (86.9–95.5) | 91.2(86.9–95.5) | 98.4(96.8–100) | 96.7 (94.4–99) | 81.7 (76.1–87.3) | 68.3 (60.6–76) | 4.505 c | 0.034 |
| >40 mm | - | - | - | - | - | - | 80 (62.1–97.9) | 26.7 (4.1–49.3) | 8.221 c | 0.004 |
| Extrathyroidal extension | ||||||||||
| Absent | 98.1 (96.3–100) | 96 (93.2–98.8) | 93.3 (89.5–97.1) | 93.3 (89.5–97.1) | 97.8 (95.6–100) | 95.2 (91.9–98.5) | 81 (74.5–87.5) | 73.7 (64.5–82.9) | 3.608 d | 0.057 |
| Present | 99 (98–100) | 80 (62.1–97.9) | 80 (62.1–97.9) | 80 (62.1–97.9) | 94.7 (89.6–100) | 88.4 (80.6–96.2) | 74.8 (63.8–85.8) | 43.7 (29.9–57.5) | 11.243 d | 0.001 |
| Multifocality | ||||||||||
| Absent | 97.2 (94.5–100) | 97.2 (94.5–100) | 97.2 (94.5–100) | 97.2 (94.5–100) | 97.2 (94.5–100) | 97.2 (94.5–100) | 85.8 (79.1–92.5) | 74 (64.3–83.7) | 5.193 e | 0.023 |
| Prezent | 95 (90–100) | 95 (90–100) | 88.7 (81.1–96.3) | 81.8 (72.2–91.4) | 96.7 (93.4–100) | 92.9 (88.1–97.7) | 77.4 (69.2–85.6) | 59.7 (48.7–70.7) | 11.639 e | 0.001 |
| Lymph node metastases | ||||||||||
| Absent | 98.1 (96.3–100) | 98.1 (96.3–100) | 93.5 (89.8–97.2) | 93.5 (89.8–97.2) | 97.6 (95.2–100) | 97.5 (95.2–100) | 94.5 (90.7–98.3) | 85.3 (92.4–78.2) | 1.155 f | 0.282 |
| Present | - | 66.7(39.5–93.9) | 66.7(39.5–93.9) | - | 95.8 (91.7–100) | 90.8 (84.6–97) | 58.1 (46.6–69.6) | 30.5 (17.2–43.8) | 23.84 f | <0.001 |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Factors | Event/N (Total = 127) | HR | [95%CI] | p | HR | [95%CI] | p |
| Age ≥ 55 years | 12/48 | 2.74 | [1.12–6.73] | 0.027 | 2.39 | [0.82–6.96] | 0.109 |
| Sex, male | 6/17 | 3.91 | [1.4–10.21] | 0.005 | 3.80 | [1.30–11.06] | 0.014 |
| PTC, conventional | 12/88 | 0.82 | [0.33–2.01] | 0.620 | |||
| PTC, “tall cell” variant | 4/9 | 2.71 | [0.91–8.12] | 0.075 | |||
| Tumor size > 40 mm | 3/9 | 2.40 | [0.70–8.23] | 0.163 | |||
| Extrathyroidal extension | 9/24 | 2.96 | [1.22–7.15] | 0.016 | 1.02 | [0.31–3.47] | 0.905 |
| Multifocality | 13/51 | 2.81 | [1.12–7.05] | 0.027 | 3.11 | [0.97–9.95] | 0.055 |
| Lymph node metastasis | 12/26 | 9.14 | [3.63–22.97] | <0.001 | 6.71 | [2.29–19.69] | <0.001 |
| Positive resection margin | 5/18 | 2.79 | [0.99–7.89] | 0.05 | |||
| Positive BRAFV600E mutation | 16/67 | 3.74 | [1.25–11.21] | 0.018 | 1.02 | [0.27–3.61] | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechifor-Boilă, A.; Zahan, A.; Bănescu, C.; Moldovan, V.; Piciu, D.; Voidăzan, S.; Borda, A. Impact of BRAFV600E Mutation on Event-Free Survival in Patients with Papillary Thyroid Carcinoma: A Retrospective Study in a Romanian Population. Cancers 2023, 15, 4053. https://doi.org/10.3390/cancers15164053
Nechifor-Boilă A, Zahan A, Bănescu C, Moldovan V, Piciu D, Voidăzan S, Borda A. Impact of BRAFV600E Mutation on Event-Free Survival in Patients with Papillary Thyroid Carcinoma: A Retrospective Study in a Romanian Population. Cancers. 2023; 15(16):4053. https://doi.org/10.3390/cancers15164053
Chicago/Turabian StyleNechifor-Boilă, Adela, Ancuţa Zahan, Claudia Bănescu, Valeriu Moldovan, Doina Piciu, Septimiu Voidăzan, and Angela Borda. 2023. "Impact of BRAFV600E Mutation on Event-Free Survival in Patients with Papillary Thyroid Carcinoma: A Retrospective Study in a Romanian Population" Cancers 15, no. 16: 4053. https://doi.org/10.3390/cancers15164053
APA StyleNechifor-Boilă, A., Zahan, A., Bănescu, C., Moldovan, V., Piciu, D., Voidăzan, S., & Borda, A. (2023). Impact of BRAFV600E Mutation on Event-Free Survival in Patients with Papillary Thyroid Carcinoma: A Retrospective Study in a Romanian Population. Cancers, 15(16), 4053. https://doi.org/10.3390/cancers15164053





