Clinicopathologic Significance of Heat Shock Protein 60 as a Survival Predictor in Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of Clinicopathologic Factors
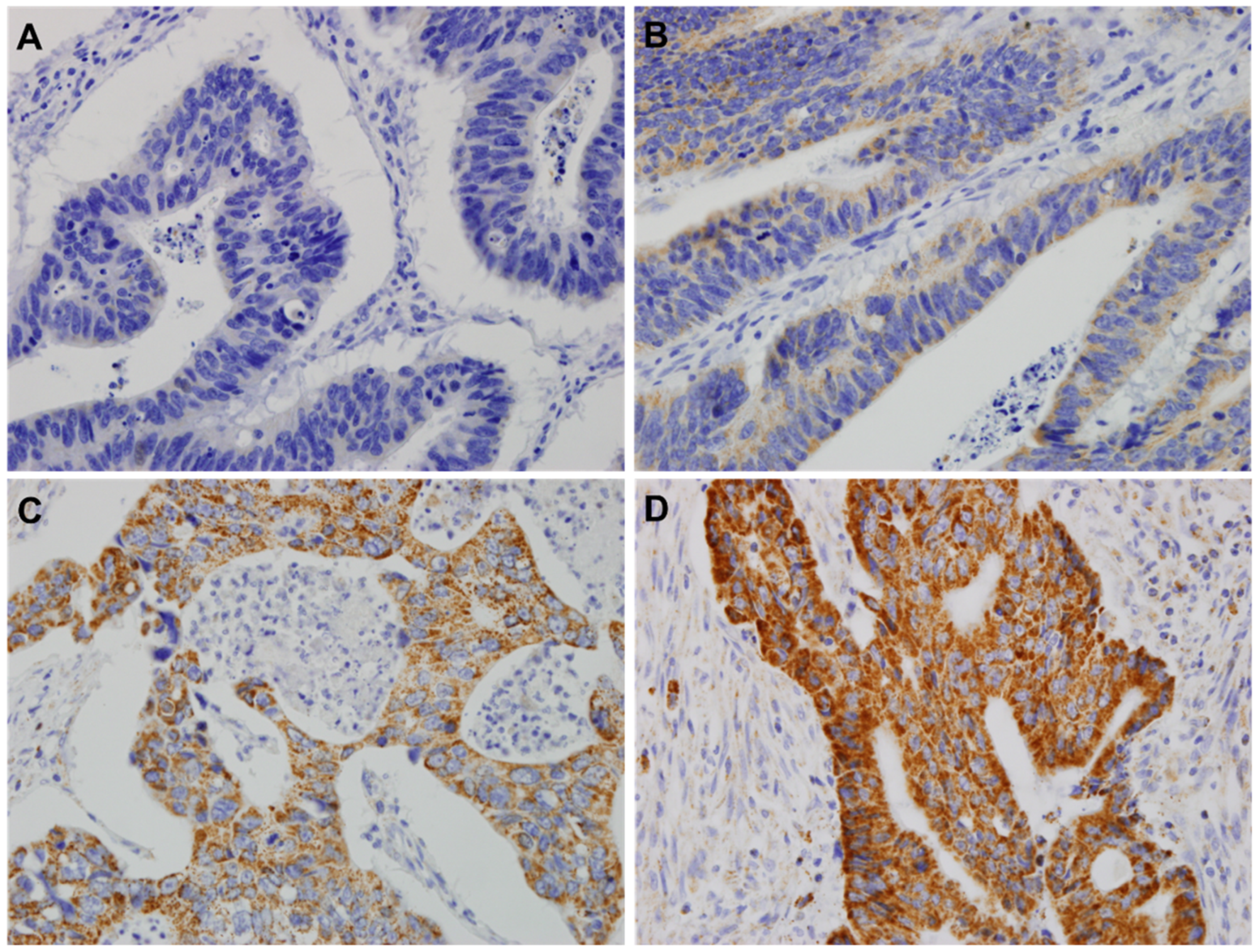
2.2. HSP60 by Tissue Samples
2.2.1. Patient Population and Clinical Specimens
2.2.2. Tissue Microarray (TMA) and IHC
2.2.3. Statistical Analysis
2.3. HSPD1 Statistical Analysis Utilizing the TCGA Data Set
2.4. Mutivariate Analysis
3. Results
3.1. The Association of HSP60 with Clinicopathological Variables
3.2. CRC Prognostication Based on HSP60 Expression
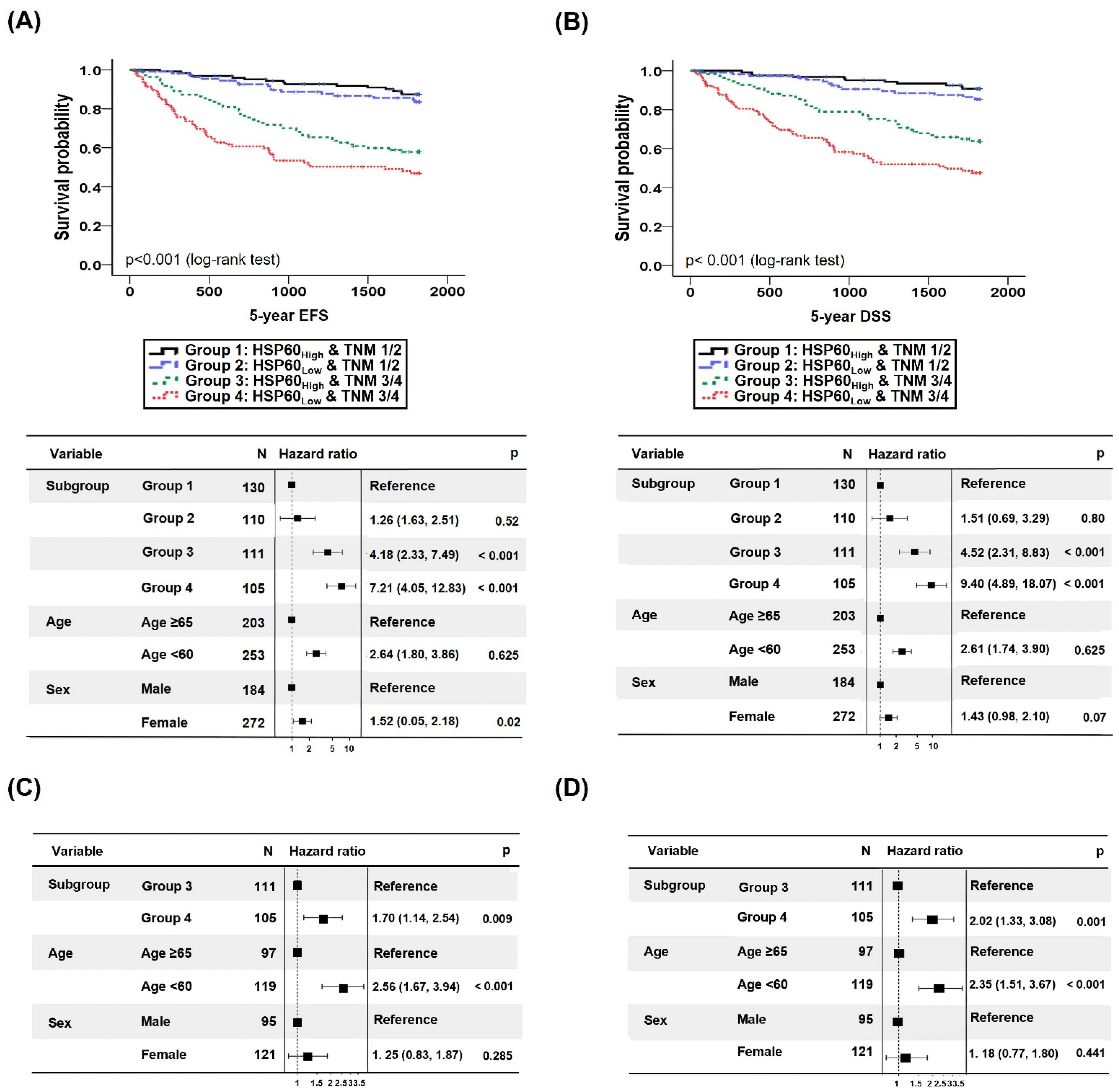
3.2.1. Analysis of Survival Rate according to HSP60 Expression Level
3.2.2. Survival Prediction of CRC Patients Combining TNM Classification and HSP60 Expression Level
3.3. HSPD1 and Colorectal Cancer Patients’ Survival
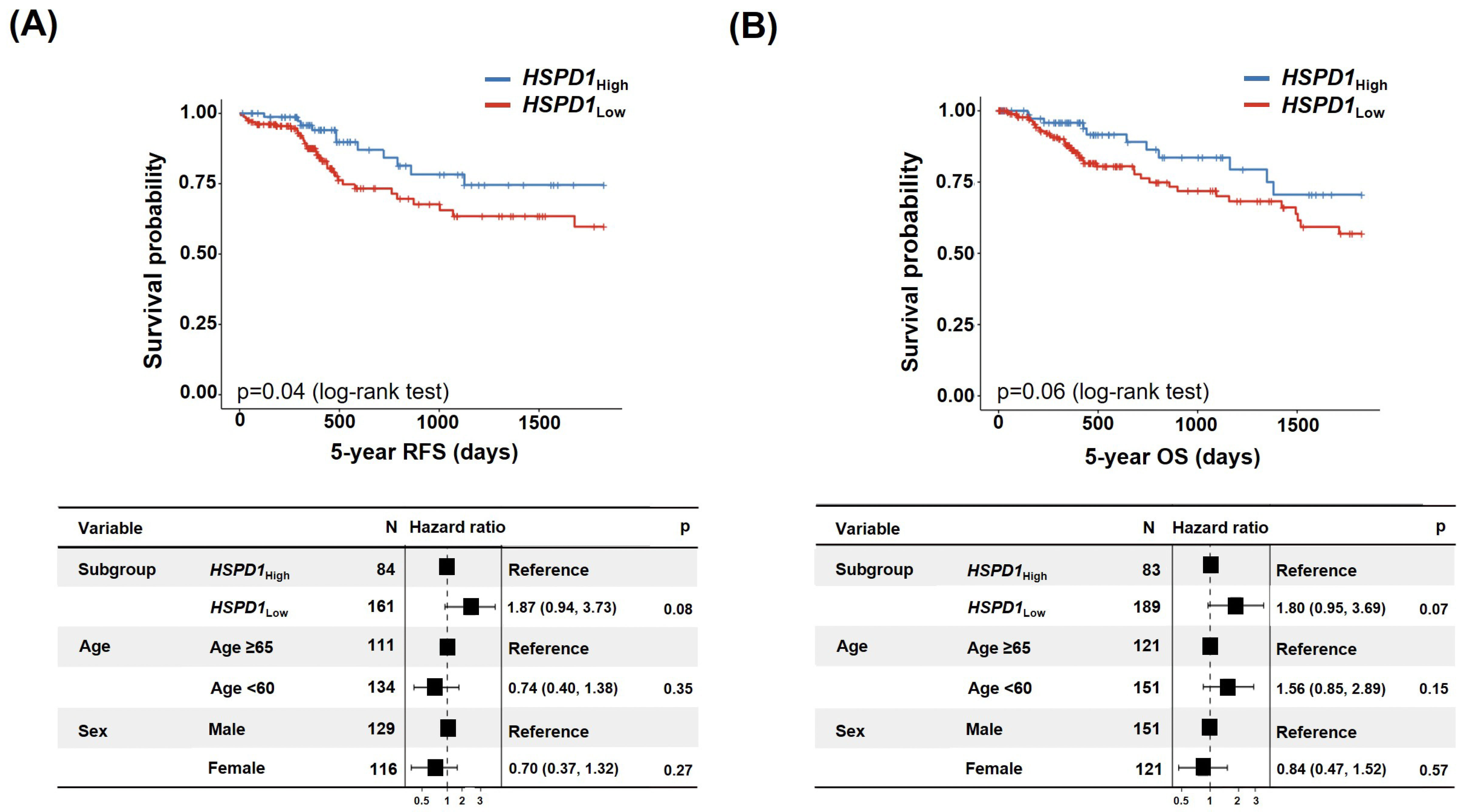
3.3.1. Correlation between HSPD1 Expression Level and Survival Rate
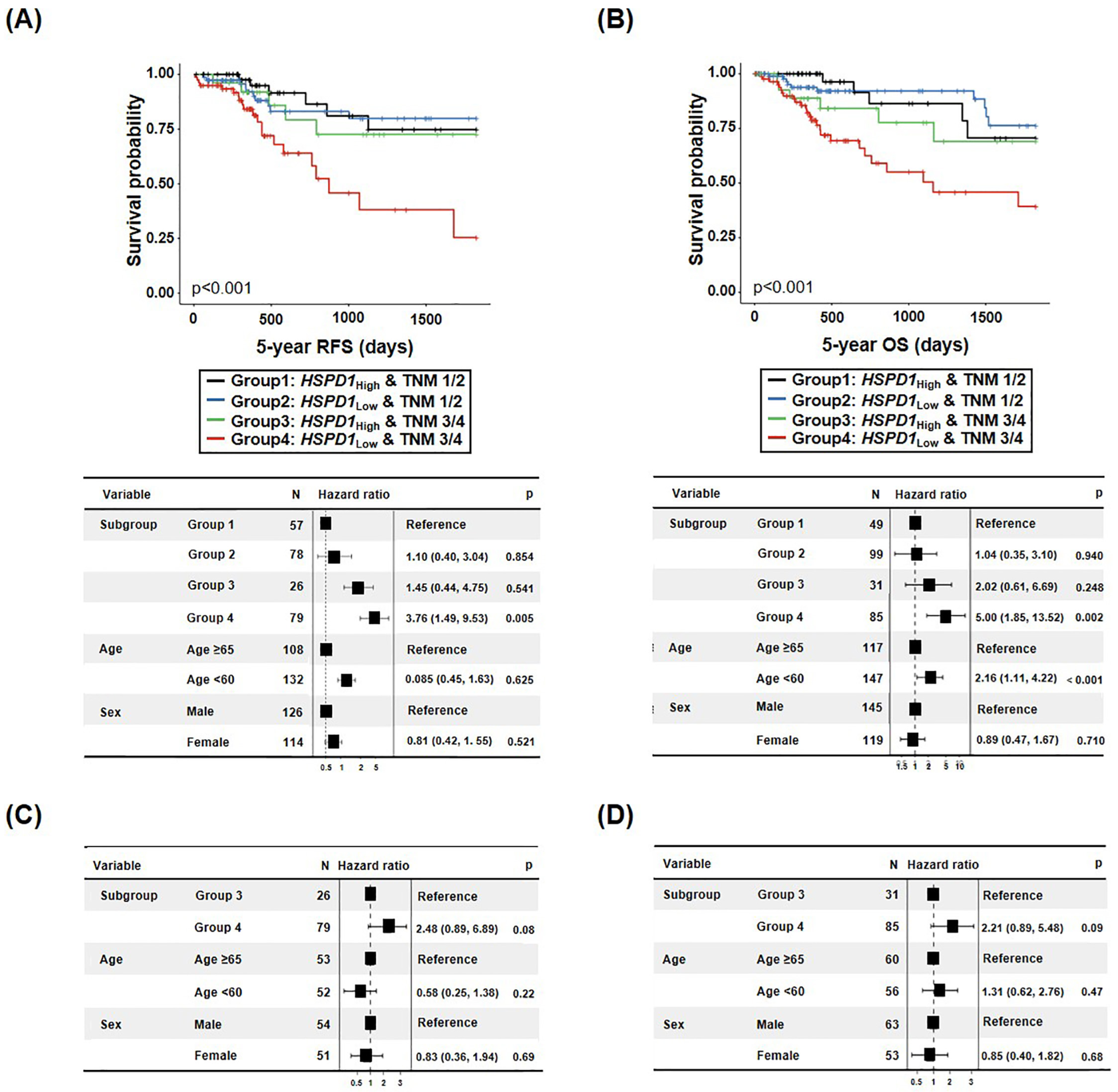
3.3.2. Survival Prediction of CRC Patients Combining TNM Classification and HSPD1 Expression Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar]
- Hoter, A.; Rizk, S.; Naim, H.Y. Heat Shock Protein 60 in Hepatocellular Carcinoma: Insights and Perspectives. Front. Mol. Biosci. 2020, 7, 60. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yeh, C.T. Functional Compartmentalization of HSP60-Survivin Interaction between Mitochondria and Cytosol in Cancer Cells. Cells 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Villanueva, J.F.; Diaz-Molina, R.; Garcia-Gonzalez, V. Protein Folding and Mechanisms of Proteostasis. Int. J. Mol. Sci. 2015, 16, 17193–17230. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Alberti, G.; Vitale, A.M.; Paladino, L.; Campanella, C.; Rappa, F.; Gorska, M.; Conway De Macario, E.; Cappello, F.; Macario, A.J.L.; et al. Hsp60 Post-translational Modifications: Functional and Pathological Consequences. Front. Mol. Biosci. 2020, 7, 95. [Google Scholar]
- Guo, J.; Li, X.; Zhang, W.; Chen, Y.; Zhu, S.; Chen, L.; Xu, R.; Lv, Y.; Wu, D.; Guo, M.; et al. HSP60-regulated Mitochondrial Proteostasis and Protein Translation Promote Tumor Growth of Ovarian Cancer. Sci. Rep. 2019, 9, 12628. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, Q.; Fu, X.Y.; Luo, W.S. Heat shock protein 60 overexpression is associated with the progression and prognosis in gastric cancer. PLoS ONE 2014, 9, e107507. [Google Scholar]
- Huang, Y.H.; Lin, K.H.; Yu, J.S.; Wu, T.J.; Lee, W.C.; Chao, C.C.; Pan, T.L.; Yeh, C.T. Targeting HSP60 by subcutaneous injections of jetPEI/HSP60-shRNA destabilizes cytoplasmic survivin and inhibits hepatocellular carcinoma growth. Mol. Carcinog. 2018, 57, 1087–1101. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, H.; Zheng, C.; Gao, J.; Fu, Q.; Hu, N.; Shao, X.; Zhou, Y.; Xiong, J.; Nie, K.; et al. Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth. Cell Death Dis. 2018, 9, 161. [Google Scholar] [CrossRef]
- Desmetz, C.; Bibeau, F.; Boissiere, F.; Bellet, V.; Rouanet, P.; Maudelonde, T.; Mange, A.; Solassol, J. Proteomics-based identification of HSP60 as a tumor-associated antigen in early stage breast cancer and ductal carcinoma in situ. J. Proteome Res. 2008, 7, 3830–3837. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Cipolla, C.; Graceffa, G.; Barone, R.; Bucchieri, F.; Bulone, D.; Cabibi, D.; Campanella, C.; Marino Gammazza, A.; Pitruzzella, A.; et al. Immunomorphological Pattern of Molecular Chaperones in Normal and Pathological Thyroid Tissues and Circulating Exosomes: Potential Use in Clinics. Int. J. Mol. Sci. 2019, 20, 4496. [Google Scholar]
- Hwang, Y.J.; Lee, S.P.; Kim, S.Y.; Choi, Y.H.; Kim, M.J.; Lee, C.H.; Lee, J.Y.; Kim, D.Y. Expression of heat shock protein 60 kDa is upregulated in cervical cancer. Yonsei Med. J. 2009, 50, 399–406. [Google Scholar] [CrossRef]
- Tsai, Y.P.; Yang, M.H.; Huang, C.H.; Chang, S.Y.; Chen, P.M.; Liu, C.J.; Teng, S.C.; Wu, K.J. Interaction between HSP60 and beta-catenin promotes metastasis. Carcinogenesis 2009, 30, 1049–1057. [Google Scholar] [CrossRef]
- Campanella, C.; Rappa, F.; Sciume, C.; Marino Gammazza, A.; Barone, R.; Bucchieri, F.; David, S.; Curcuru, G.; Caruso Bavisotto, C.; Pitruzzella, A.; et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015, 121, 3230–3239. [Google Scholar]
- Rappa, F.; Pitruzzella, A.; Marino Gammazza, A.; Barone, R.; Mocciaro, E.; Tomasello, G.; Carini, F.; Farina, F.; Zummo, G.; Conway De Macario, E.; et al. Quantitative patterns of Hsps in tubular adenoma compared with normal and tumor tissues reveal the value of Hsp10 and Hsp60 in early diagnosis of large bowel cancer. Cell Stress Chaperones 2016, 21, 927–933. [Google Scholar] [PubMed]
- He, Y.; Wu, Y.; Mou, Z.; Li, W.; Zou, L.; Fu, T.; Zhang, A.; Xiang, D.; Xiao, H.; Wang, X. Proteomics-based identification of HSP60 as a tumor-associated antigen in colorectal cancer. Proteom. Clin. Appl. 2007, 1, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Urushibara, M.; Kageyama, Y.; Akashi, T.; Otsuka, Y.; Takizawa, T.; Koike, M.; Kihara, K. HSP60 may predict good pathological response to neoadjuvant chemoradiotherapy in bladder cancer. Jpn. J. Clin. Oncol. 2007, 37, 56–61. [Google Scholar]
- Schneider, J.; Jimenez, E.; Marenbach, K.; Romero, H.; Marx, D.; Meden, H. Immunohistochemical detection of HSP60-expression in human ovarian cancer. Correlation with survival in a series of 247 patients. Anticancer Res. 1999, 19, 2141–2146. [Google Scholar]
- Li, D.Q.; Wang, L.; Fei, F.; Hou, Y.F.; Luo, J.M.; Wei, C.; Zeng, R.; Wu, J.; Lu, J.S.; Di, G.H.; et al. Identification of breast cancer metastasis-associated proteins in an isogenic tumor metastasis model using two-dimensional gel electrophoresis and liquid chromatography-ion trap-mass spectrometry. Proteomics 2006, 6, 3352–3368. [Google Scholar] [PubMed]
- Slotta-Huspenina, J.; Wolff, C.; Drecoll, E.; Feith, M.; Bettstetter, M.; Malinowsky, K.; Bauer, L.; Becker, K.; Ott, K.; Hofler, H.; et al. A specific expression profile of heat-shock proteins and glucose-regulated proteins is associated with response to neoadjuvant chemotherapy in oesophageal adenocarcinomas. Br. J. Cancer 2013, 109, 370–378. [Google Scholar]
- Guo, J.; Zhu, S.; Deng, H.; Xu, R. HSP60-knockdown suppresses proliferation in colorectal cancer cells via activating the adenine/AMPK/mTOR signaling pathway. Oncol. Lett. 2021, 22, 630. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Shao, S. The Clinical Value of HSP60 in Digestive System Cancers: A Systematic Review and Meta-Analysis. Clin. Lab. 2019, 65, 523. [Google Scholar] [CrossRef]
- Cappello, F.; David, S.; Rappa, F.; Bucchieri, F.; Marasa, L.; Bartolotta, T.E.; Farina, F.; Zummo, G. The expression of HSP60 and HSP10 in large bowel carcinomas with lymph node metastase. BMC Cancer 2005, 5, 139. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Lausen, B. On the exact distribution of maximally selected rank statistics. Comput. Stat. Data Anal. 2003, 43, 121–137. [Google Scholar]
- Li, J.; Guo, B.C.; Sun, L.R.; Wang, J.W.; Fu, X.H.; Zhang, S.Z.; Poston, G.; Ding, K.F. TNM staging of colorectal cancer should be reconsidered by T stage weighting. World J. Gastroenterol. 2014, 20, 5104–5112. [Google Scholar]
- Hong, Y.; Kim, J.; Choi, Y.J.; Kang, J.G. Clinical study of colorectal cancer operation: Survival analysis. Korean J. Clin. Oncol. 2020, 16, 3–8. [Google Scholar] [CrossRef]
- Farhan, Y.a.A.; Arabdin, M.; Khan, A. The Role of Heat Shock Proteins in Cellular Homeostasis and Cell Survival. Cureus 2021, 13, e18316. [Google Scholar]
- Ray, A.M.; Salim, N.; Stevens, M.; Chitre, S.; Abdeen, S.; Washburn, A.; Sivinski, J.; O’hagan, H.M.; Chapman, E.; Johnson, S.M. Exploiting the HSP60/10 chaperonin system as a chemotherapeutic target for colorectal cancer. Bioorg. Med. Chem. 2021, 40, 116129. [Google Scholar] [CrossRef]
- Druck, T.; Cheung, D.G.; Park, D.; Trapasso, F.; Pichiorri, F.; Gaspari, M.; Palumbo, T.; Aqeilan, R.I.; Gaudio, E.; Okumura, H.; et al. Fhit-Fdxr interaction in the mitochondria: Modulation of reactive oxygen species generation and apoptosis in cancer cells. Cell Death Dis. 2019, 10, 147. [Google Scholar] [CrossRef]
- Sane, S.; Hafner, A.; Srinivasan, R.; Masood, D.; Slunecka, J.L.; Noldner, C.J.; Hanson, A.D.; Kruisselbrink, T.; Wang, X.; Wang, Y.; et al. UBXN2A enhances CHIP-mediated proteasomal degradation of oncoprotein mortalin-2 in cancer cells. Mol. Oncol. 2018, 12, 1753–1777. [Google Scholar] [CrossRef]
- Jalili, A.; Makowski, M.; Switaj, T.; Nowis, D.; Wilczynski, G.M.; Wilczek, E.; Chorazy-Massalska, M.; Radzikowska, A.; Maslinski, W.; Bialy, L.; et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin. Cancer Res. 2004, 10, 4498–4508. [Google Scholar] [PubMed]
- Teng, R.; Liu, Z.; Tang, H.; Zhang, W.; Chen, Y.; Xu, R.; Chen, L.; Song, J.; Liu, X.; Deng, H. HSP60 silencing promotes Warburg-like phenotypes and switches the mitochondrial function from ATP production to biosynthesis in ccRCC cells. Redox Biol. 2019, 24, 101218. [Google Scholar] [PubMed]
- Kang, B.H.; Shu, C.W.; Chao, J.K.; Lee, C.H.; Fu, T.Y.; Liou, H.H.; Ger, L.P.; Liu, P.F. HSPD1 repressed E-cadherin expression to promote cell invasion and migration for poor prognosis in oral squamous cell carcinoma. Sci. Rep. 2019, 9, 8932. [Google Scholar]
- Faried, A.; Sohda, M.; Nakajima, M.; Miyazaki, T.; Kato, H.; Kuwano, H. Expression of heat-shock protein Hsp60 correlated with the apoptotic index and patient prognosis in human oesophageal squamous cell carcinoma. Eur. J. Cancer 2004, 40, 2804–2811. [Google Scholar] [CrossRef]
- Mori, D.; Nakafusa, Y.; Miyazaki, K.; Tokunaga, O. Differential expression of Janus kinase 3 (JAK3), matrix metalloproteinase 13 (MMP13), heat shock protein 60 (HSP60), and mouse double minute 2 (MDM2) in human colorectal cancer progression using human cancer cDNA microarrays. Pathol. Res. Pract. 2005, 201, 777–789. [Google Scholar] [CrossRef]
- Tang, Y.; Whow, Y.; Fan, S.; Wen, Q. The multiple roles and therapeutic potential of HSP60 in cancer. Biochem Parmacol. 2022, 201, 115096. [Google Scholar] [CrossRef]
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A review of the Role of Carcinoembryonic Actigen in Clinical Practice. Ann Coloprotol. 2019, 35, 294–305. [Google Scholar]
- Ruan, W.; Wang, Y.; Ma, Y.; Xing, X.; Lin, J.; Cui, J.; Lai, M. HSP60, a protein downregulated by IGFBP7 in colorectal carcinoma. J. Exp. Clin. Cancer Res. 2010, 29, 41. [Google Scholar] [CrossRef]


| Total (n = 456) | |
|---|---|
| Age | 65.0 ± 11.4 |
| Sex | |
| Male | 272 (59.6%) |
| Female | 184 (40.4%) |
| Laboratory findings | |
| Hemoglobin (g/dL) | 12.3 ± 2.4 |
| WBC (103/μL) | 11,747.9 ± 4031.8 |
| CEA (ng/mL) | 80.7 ± 405.2 |
| Histology | |
| Size (mm) | 51.8 ± 21.6 |
| Location | |
| Cecum | 9 (2.0%) |
| Ascending colon | 79 (17.3%) |
| Hepatic flexure | 8 (1.8%) |
| Transverse colon | 28 (6.1%) |
| Splenic flexure | 4 (0.9%) |
| Descending colon | 12 (2.6%) |
| Sigmoid-descending | 5 (1.1%) |
| Sigmoid colon | 110 (24.1%) |
| Rectosigmoid colon | 81 (17.8%) |
| Rectum | 120 (26.3%) |
| Pathology | |
| WD | 29 (6.4%) |
| MD | 387 (84.9%) |
| PD | 20 (4.4%) |
| Mucinous | 18 (3.9%) |
| SRC | 2 (0.4%) |
| TNM stage | |
| Ⅰ | 77 (16.9%) |
| Ⅱ | 163 (35.7%) |
| Ⅲ | 160 (35.1%) |
| Ⅳ | 56 (12.3%) |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Total (n = 456) | HSP60 (n = 215), Low | HSP60 (n = 241), High | p-Value | OR (CI) | p-Value | |
| Age (yrs) | 0.609 | |||||
| <65 | 203 (44.5%) | 93 (43.3%) | 110 (45.6%) | |||
| ≥65 | 253 (55.5%) | 122 (56.7%) | 131 (54.4%) | |||
| Sex | 0.473 | |||||
| Male | 272 (59.6%) | 132 (61.4%) | 140 (58.1%) | |||
| Female | 184 (40.4%) | 83 (38.6%) | 101 (41.9%) | |||
| Diabetes mellitus | 0.735 | |||||
| No | 366 (80.3%) | 174 (80.9%) | 192 (79.7%) | |||
| Yes | 90 (19.7%) | 41 (19.1%) | 49 (20.3%) | |||
| Smoking | 0.983 | |||||
| No | 354 (77.6%) | 167 (77.7%) | 187 (77.6%) | |||
| Yes | 102 (22.4%) | 48 (22.3%) | 54 (22.4%) | |||
| Family history | 0.625 | |||||
| No | 437 (95.8%) | 205 (95.3%) | 231 (96.3%) | |||
| Yes | 19 (4.2%) | 10 (4.7%) | 9 (3.7%) | |||
| Anemia (Hemoglobin, g/dL) | 0.249 | |||||
| No | 233 (51.1%) | 116 (54.0%) | 117 (48.5%) | |||
| Yes | 223 (48.9%) | 99 (46.0%) | 124 (51.5%) | |||
| WBC counts (103/μL) | 0.693 | |||||
| Normal | 385 (84.4%) | 180 (83.7%) | 205 (85.1%) | |||
| Abnormal | 71 (15.6%) | 35 (16.3%) | 36 (14.9%) | |||
| Serum CEA (ng/mL) | 0.653 | |||||
| Normal | 337 (73.9%) | 161 (74.9%) | 176 (73.0%) | |||
| Abnormal | 119 (26.1%) | 54 (25.1%) | 65 (27.0%) | |||
| Tumor size (mm) | 0.539 | |||||
| <50 | 220 (48.2%) | 107 (49.8%) | 113 (46.9%) | |||
| ≥50 | 236 (51.8%) | 108 (50.2%) | 128 (53.1%) | |||
| Tumor location | 0.044 | 0.164 | ||||
| LCC | 332 (72.8%) | 147 (68.4%) | 185 (76.8%) | 1 | ||
| RCC | 124 (23.2%) | 68 (31.6%) | 56 (23.2%) | 0.732 (0.477–1.124) | ||
| Tumor differentiation | <0.001 | 0.001 | ||||
| Differentiation | 416 (91.2%) | 185 (86.0%) | 231 (95.9%) | 1 | ||
| Undifferentiation | 40 (8.8%) | 30 (14.0%) | 10 (4.1%) | 0.269 (0.127–0.571) | ||
| p53 expression a | 0.045 | 0.033 | ||||
| Negative | 93 (20.7%) | 52 (24.8%) | 41 (17.1%) | 1 | ||
| Positive | 357 (79.3%) | 158 (75.2%) | 199 (82.9%) | 1.662 (1.042–2.651) | ||
| EGFR mutation b | 0.074 | |||||
| Negative | 306 (64.2%) | 152 (72.0%) | 154 (64.2%) | |||
| Positive | 145 (32.2%) | 59 (28.0%) | 86 (35.8%) | |||
| TNM stage | 0.553 | |||||
| Ⅰ/Ⅱ | 240 (52.6%) | 110 (51.2%) | 130 (53.9%) | |||
| Ⅲ/Ⅳ | 216 (47.4%) | 105 (48.8%) | 111 (46.1%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.; Jeong, S.; An, J.; Park, S.; Nam, S.; Kwon, K.A.; Sahoo, D.; Ghosh, P.; Kim, J.H. Clinicopathologic Significance of Heat Shock Protein 60 as a Survival Predictor in Colorectal Cancer. Cancers 2023, 15, 4052. https://doi.org/10.3390/cancers15164052
Kang M, Jeong S, An J, Park S, Nam S, Kwon KA, Sahoo D, Ghosh P, Kim JH. Clinicopathologic Significance of Heat Shock Protein 60 as a Survival Predictor in Colorectal Cancer. Cancers. 2023; 15(16):4052. https://doi.org/10.3390/cancers15164052
Chicago/Turabian StyleKang, Myunghee, Soyeon Jeong, Jungsuk An, Sungjin Park, Seungyoon Nam, Kwang An Kwon, Debashis Sahoo, Pradipta Ghosh, and Jung Ho Kim. 2023. "Clinicopathologic Significance of Heat Shock Protein 60 as a Survival Predictor in Colorectal Cancer" Cancers 15, no. 16: 4052. https://doi.org/10.3390/cancers15164052
APA StyleKang, M., Jeong, S., An, J., Park, S., Nam, S., Kwon, K. A., Sahoo, D., Ghosh, P., & Kim, J. H. (2023). Clinicopathologic Significance of Heat Shock Protein 60 as a Survival Predictor in Colorectal Cancer. Cancers, 15(16), 4052. https://doi.org/10.3390/cancers15164052






