The Tumor Microenvironment and the Estrogen Loop in Thyroid Cancer
Abstract
Simple Summary
Abstract
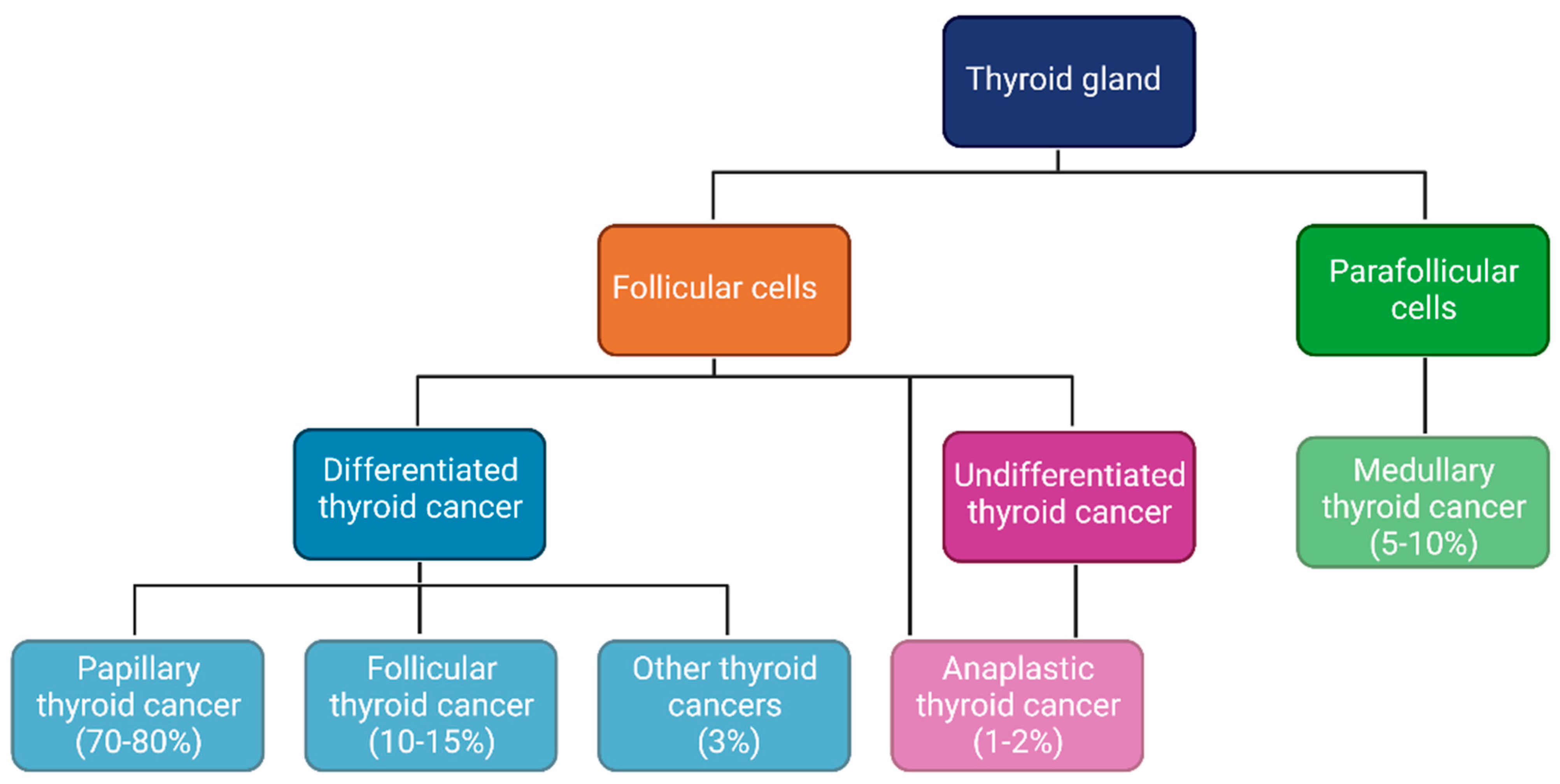
1. Introduction
2. Materials and Methods
3. Results
3.1. TME in TC: Immune Cells, Inflammatory Mediators and Stroma
3.2. TME in TC: The Potential Role of Estrogens
3.3. Estrogens and Thyroid Hormones
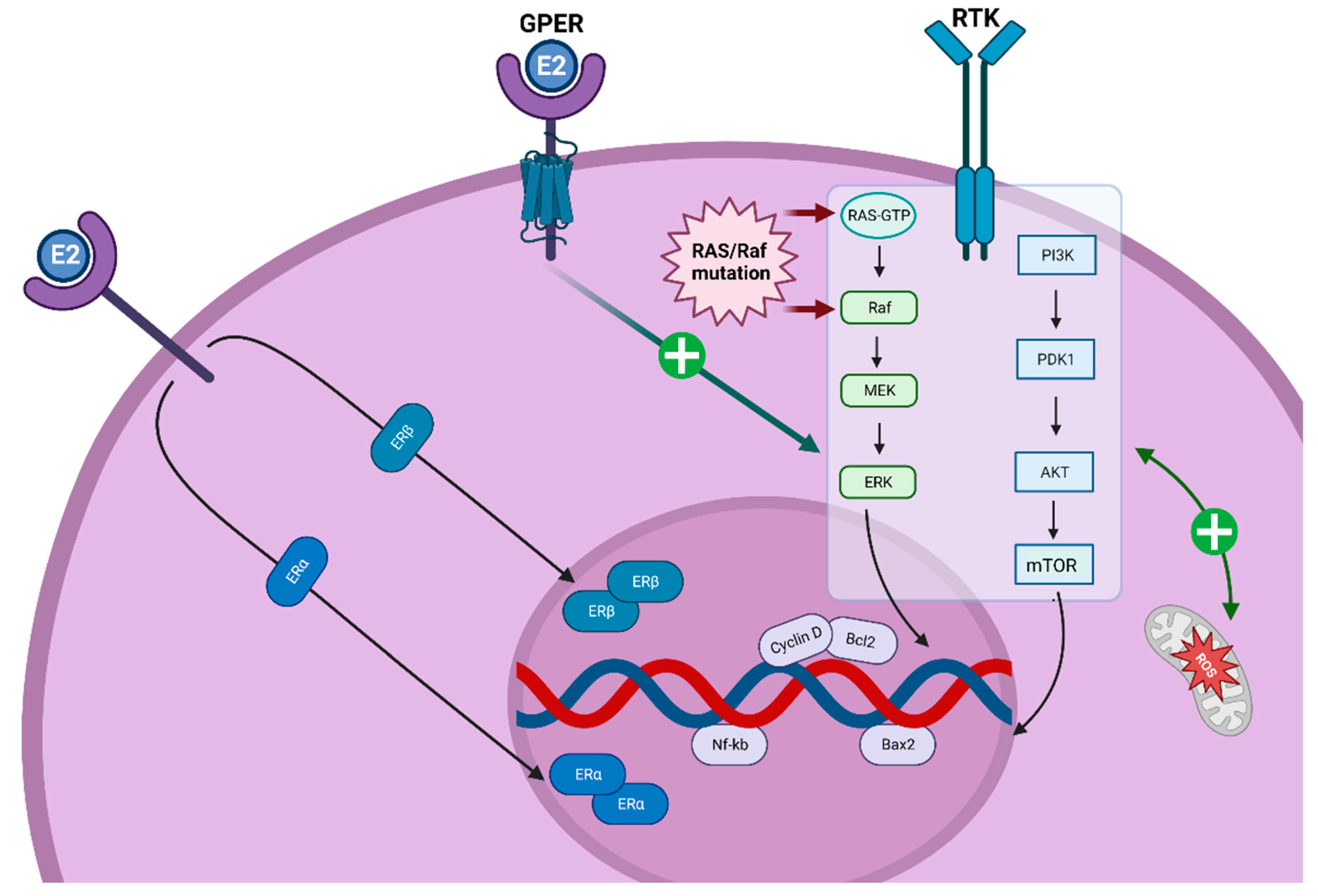
3.4. Estrogen Receptors and TC
3.5. ER-Activated Pathways and TC
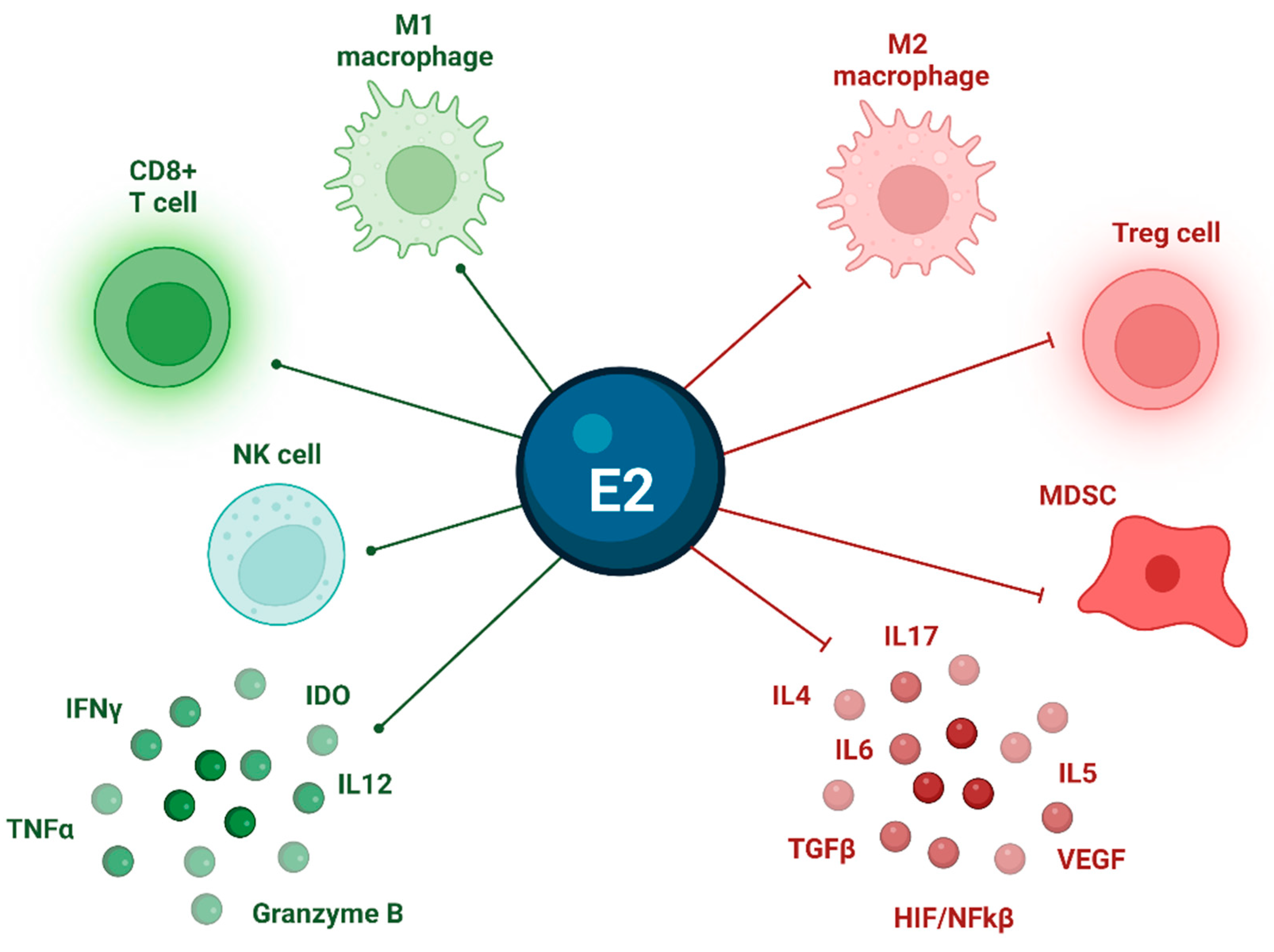
3.6. Estrogens and TME
3.7. TC and Pregnancy
3.8. TC and Metabolic Syndrome
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.; Newbold, K.; Papotti, M.; Berruti, A. ESMO Clinical Practice Guideline update on the use of systemic therapy in advanced thyroid cancer. Ann. Oncol. 2022, 33, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Maso, L.D. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N. Engl. J. Med. 2016, 375, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Koo, J.S. Cell Component and Function of Tumor Microenvironment in Thyroid Cancer. Int. J. Mol. Sci. 2022, 23, 12578. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Shobab, L.; Burman, K.D.; Wartofsky, L. Sex Differences in Differentiated Thyroid Cancer. Thyroid 2022, 32, 224–235. [Google Scholar] [CrossRef]
- Cutolo, M.; Capellino, S.; Sulli, A.; Serioli, B.; Secchi, M.E.; Villaggio, B.; Straub, R.H. Estrogens and Autoimmune Diseases. Ann. N. Y. Acad. Sci. 2006, 1089, 538–547. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Straub, R.H. Estrogen metabolism and autoimmunity. Autoimmun. Rev. 2012, 11, A460–A464. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Lauro, A.; Pironi, D.; Tripodi, D.; Lori, E.; Ferent, I.C.; Amabile, M.I.; Catania, A.; Di Matteo, F.M.; et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers 2021, 13, 5567. [Google Scholar] [CrossRef]
- Crnčić, T.B.; Tomaš, M.I.; Girotto, N.; Ivanković, S.G. Risk Factors for Thyroid Cancer: What Do We Know So Far? Acta Clin. Croat. 2020, 59, 66–72. [Google Scholar] [CrossRef]
- Pani, F.; Caria, P.; Yasuda, Y.; Makoto, M.; Mariotti, S.; Leenhardt, L.; Roshanmehr, S.; Caturegli, P.; Buffet, C. The Immune Landscape of Papillary Thyroid Cancer in the Context of Autoimmune Thyroiditis. Cancers 2022, 14, 4287. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, T.; Ma, L.; Chang, W. Signal Pathway of Estrogen and Estrogen Receptor in the Development of Thyroid Cancer. Front. Oncol. 2021, 11, 593479. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola, L.; Colombo, C.; Perrino, M.; Muzza, M. Papillary thyroid carcinoma and infammation. Front. Endocrinol. 2011, 2, 88. [Google Scholar] [CrossRef]
- Xi, C.; Zhang, G.-Q.; Sun, Z.-K.; Song, H.-J.; Shen, C.-T.; Chen, X.-Y.; Sun, J.-W.; Qiu, Z.-L.; Luo, Q.-Y. Interleukins in Thyroid Cancer: From Basic Researches to Applications in Clinical Practice. Front. Immunol. 2020, 11, 1124. [Google Scholar] [CrossRef]
- Wen, S.; Qu, N.; Ma, B.; Wang, X.; Luo, Y.; Xu, W.; Jiang, H.; Zhang, Y.; Wang, Y.; Ji, Q. Cancer-Associated Fibroblasts Positively Correlate with Dedifferentiation and Aggressiveness of Thyroid Cancer. OncoTargets Ther. 2021, 14, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, Y.-C.; Ma, B.; Xu, W.-B.; Liao, T.; Wang, Y. Integrated analysis of novel macrophage related signature in anaplastic thyroid cancer. Endocrine 2022, 78, 517–530. [Google Scholar] [CrossRef]
- French, J.D.; Kotnis, G.R.; Said, S.; Raeburn, C.D.; McIntyre, R.C., Jr.; Klopper, J.P.; Haugen, B.R. Programmed Death-1+ T Cells and Regulatory T Cells Are Enriched in Tumor-Involved Lymph Nodes and Associated with Aggressive Features in Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2012, 97, E934–E943. [Google Scholar] [CrossRef]
- Menicali, E.; Guzzetti, M.; Morelli, S.; Moretti, S.; Puxeddu, E. Immune Landscape of Thyroid Cancers: New Insights. Front. Endocrinol. 2021, 11, 637826. [Google Scholar] [CrossRef]
- Song, L.; Zhu, J.; Li, Z.; Wei, T.; Gong, R.; Lei, J. The prognostic value of the lymphocyte-to-monocyte ratio for high-risk papillary thyroid carcinoma. Cancer Manag. Res. 2019, 11, 8451–8462. [Google Scholar] [CrossRef]
- Kim, S.; Cho, S.W.; Min, H.S.; Kim, K.M.; Yeom, G.J.; Kim, E.Y.; Lee, K.E.; Yun, Y.G.; Park, D.J.; Park, Y.J. The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma. Endocrinol. Metab. 2013, 28, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.W.; Gigliotti, B.J.; Pai, S.I.; Parangi, S.; Wachtel, H.; Mino-Kenudson, M.; Gunda, V.; Faquin, W.C. PD-L1 and IDO1 Are Expressed in Poorly Differentiated Thyroid Carcinoma. Endocr. Pathol. 2018, 29, 59–67. [Google Scholar] [CrossRef]
- Brauner, E.; Gunda, V.; Borre, P.V.; Zurakowski, D.; Kim, Y.S.; Dennett, K.V.; Amin, S.; Freeman, G.J.; Parangi, S. Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti tumor immunity in an immunocompetent murine model of anaplastic thyroid cancer. Oncotarget 2016, 7, 17194–17211. [Google Scholar] [CrossRef]
- Angell, T.E.; Lechner, M.G.; Jang, J.K.; Correa, A.J.; LoPresti, J.S.; Epstein, A.L. BRAFV600E in Papillary Thyroid Carcinoma Is Associated with Increased Programmed Death Ligand 1 Expression and Suppressive Immune Cell Infiltration. Thyroid 2014, 24, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Tang, Y.; Guo, Y.; Wen, S. Immune Microenvironment of Thyroid Cancer. J. Cancer 2020, 11, 4884–4896. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Galdiero, M.R.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S.R.; Patrizio, A.; Mazzi, V.; Varricchi, G.; et al. Immune and Inflammatory Cells in Thyroid Cancer Microenvironment. Int. J. Mol. Sci. 2019, 20, 4413. [Google Scholar] [CrossRef] [PubMed]
- Salajegheh, A.; Dolan-Evans, E.; Sullivan, E.; Irani, S.; Rahman, A.; Vosgha, H.; Gopalan, V.; Smith, R.A.; Lam, A.K.-Y. The expression profiles of the galectin gene family in primary and metastatic papillary thyroid carcinoma with particular emphasis on galectin-1 and galectin-3 expression. Exp. Mol. Pathol. 2014, 96, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Arcolia, V.; Journe, F.; Wattier, A.; Leteurtre, E.; Renaud, F.; Gabius, H.-J.; Remmelink, M.; Decaestecker, C.; Rodriguez, A.; Boutry, S.; et al. Galectin-1 is a diagnostic marker involved in thyroid cancer progression. Int. J. Oncol. 2017, 51, 760–770. [Google Scholar] [CrossRef]
- Giuliani, C.; Verrocchio, S.; Verginelli, F.; Bucci, I.; Grassadonia, A.; Napolitano, G. Hormonal Regulation of the MHC Class I Gene in Thyroid Cells: Role of the Promoter “Tissue-Specific” Region. Front. Endocrinol. 2021, 12, 749609. [Google Scholar] [CrossRef]
- Scheuba, C.; Kaserer, K.; Kaczirek, K.; Asari, R.; Niederle, B. Desmoplastic Stromal Reaction in Medullary Thyroid Cancer—An Intraoperative “Marker” for Lymph Node Metastases. World J. Surg. 2006, 30, 853–859. [Google Scholar] [CrossRef]
- Koperek, O.; Asari, R.; Niederle, B.; Kaserer, K. Desmoplastic stromal reaction in papillary thyroid microcarcinoma. Histopathology 2011, 58, 919–924. [Google Scholar] [CrossRef]
- Jolly, L.A.; Novitskiy, S.; Owens, P.; Massoll, N.; Cheng, N.; Fang, W.; Moses, H.L.; Franco, A.T. Fibroblast-Mediated Collagen Remodeling Within the Tumor Microenvironment Facilitates Progression of Thyroid Cancers Driven by BrafV600E and Pten Loss. Cancer Res. 2016, 76, 1804–1813. [Google Scholar] [CrossRef]
- Cho, J.-G.; Byeon, H.K.; Oh, K.H.; Baek, S.-K.; Kwon, S.-Y.; Jung, K.-Y.; Woo, J.-S. Clinicopathological significance of cancer-associated fibroblasts in papillary thyroid carcinoma: A predictive marker of cervical lymph node metastasis. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer—Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bai, Q.; Lu, Y.; Lu, Y.; Zhu, L.; Zhou, X.; Wu, L. Expression and function of cxcl12/cxcr4/cxcr7 in thyroid cancer. Int. J. Oncol. 2016, 48, 2321–2329. [Google Scholar] [CrossRef]
- Sun, W.-Y.; Jung, W.-H.; Koo, J.S. Expression of cancer-associated fibroblast-related proteins in thyroid papillary carcinoma. Tumor Biol. 2016, 37, 8197–8207. [Google Scholar] [CrossRef]
- Avagliano, A.; Fiume, G.; Bellevicine, C.; Troncone, G.; Venuta, A.; Acampora, V.; De Lella, S.; Ruocco, M.R.; Masone, S.; Velotti, N.; et al. Thyroid Cancer and Fibroblasts. Cancers 2022, 14, 4172. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, X.; Pan, Y.; Xu, J.; Si, Y.; Min, Z.; Yu, B. A new risk factor indicator for papillary thyroid cancer based on immune infiltration. Cell Death Dis. 2021, 12, 51. [Google Scholar] [CrossRef]
- Mannathazhathu, A.S.; George, P.S.; Sudhakaran, S.; Vasudevan, D.; Km, J.K.; Booth, C.; Mathew, A. Reproductive factors and thyroid cancer risk: Meta-analysis. Head Neck 2019, 41, 4199–4208. [Google Scholar] [CrossRef]
- Tsatsakis, A.M. Toxicological Risk Assessment and Multi-System Health Impacts from Exposure; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- McInerney, E.M.; Tsai, M.J.; O’Malley, B.W.; Katzenellenbogen, B.S. Analysis of estrogen receptor transcriptional enhancement by a nuclear hormone receptor coactivator. Proc. Natl. Acad. Sci. USA 1996, 93, 10069–10073. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Spencer, T.; Oñate, S.A.; Jenster, G.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog. Horm. Res. 1997, 52, 141164. [Google Scholar]
- Northrop, J.P.; Nguyen, D.; Piplani, S.; Olivan, S.E.; Kwan, S.T.-S.; Go, N.F.; Hart, C.P.; Schatz, P.J. Selection of estrogen receptor beta- and thyroid hormone receptor beta-specific coactivator-mimetic peptides using recombinant peptide libraries. Mol. Endocrinol. 2000, 14, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Zhu, Y. A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. Int. J. Mol. Sci. 2022, 23, 2708. [Google Scholar] [CrossRef]
- Figueiredo, N.B.; Cestari, S.H.; Conde, S.J.; Luvizotto, R.A.M.; De Sibio, M.T.; Perone, D.; Katayama, M.L.H.; Carraro, D.M.; Brentani, H.P.; Brentani, M.M.; et al. Estrogen-Responsive Genes Overlap with Triiodothyronine-Responsive Genes in a Breast Carcinoma Cell Line. Sci. World J. 2014, 2014, 969404. [Google Scholar] [CrossRef] [PubMed]
- Desmawati, D.; Sulastri, D. A Phytoestrogens and Their Health Effect. Open Access Maced. J. Med. Sci. 2019, 7, 495–499. [Google Scholar] [CrossRef]
- Bulotta, S.; Capriglione, F.; Celano, M.; Pecce, V.; Russo, D.; Maggisano, V. Phytochemicals in thyroid cancer: Analysis of the preclinical studies. Endocrine 2021, 73, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Granata, R.; Locati, L.D.; Licitra, L. Fosbretabulin for the treatment of anaplastic thyroid cancer. Futur. Oncol. 2014, 10, 2015–2021. [Google Scholar] [CrossRef]
- Sosa, J.A.; Elisei, R.; Jarząb, B.; Balkissoon, J.; Lu, S.-P.; Bal, C.; Marur, S.; Gramza, A.; Ben Yosef, R.; Gitlitz, B.; et al. Randomized Safety and Efficacy Study of Fosbretabulin with Paclitaxel/Carboplatin Against Anaplastic Thyroid Carcinoma. Thyroid. 2014, 24, 232–240. [Google Scholar] [CrossRef]
- Mooney, C.J.; Nagaiah, G.; Fu, P.; Wasman, J.K.; Cooney, M.M.; Savvides, P.S.; Bokar, J.A.; Dowlati, A.; Wang, D.; Agarwala, S.S.; et al. A Phase II Trial of Fosbretabulin in Advanced Anaplastic Thyroid Carcinoma and Correlation of Baseline Serum-Soluble Intracellular Adhesion Molecule-1 with Outcome. Thyroid 2009, 19, 233–240. [Google Scholar] [CrossRef]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr.-Relat. Cancer 2014, 21, T273–T283. [Google Scholar] [CrossRef]
- Bertoni, A.P.S.; Manfroi, P.D.A.; Tomedi, J.; Assis-Brasil, B.M.; Meyer, E.L.D.S.; Furlanetto, T.W. The gene expression of GPER1 is low in fresh samples of papillary thyroid carcinoma (PTC), and in silico analysis. Mol. Cell. Endocrinol. 2021, 535, 111397. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, W.; Li, J.; Zhang, H.; Shan, Z.; Teng, W. Differential expression patterns and clinical significance of estrogen receptor-α and β in papillary thyroid carcinoma. BMC Cancer 2014, 14, 383. [Google Scholar] [CrossRef]
- Vannucchi, G.; De Leo, S.; Perrino, M.; Rossi, S.; Tosi, D.; Cirello, V.; Colombo, C.; Bulfamante, G.; Vicentini, L.; Fugazzola, L. Impact of estrogen and progesterone receptor expression on the clinical and molecular features of papillary thyroid cancer. Eur. J. Endocrinol. 2015, 173, 29–36. [Google Scholar] [CrossRef]
- Kang, M.-H.; Choi, H.; Oshima, M.; Cheong, J.-H.; Kim, S.; Lee, J.H.; Park, Y.S.; Choi, H.-S.; Kweon, M.-N.; Pack, C.-G.; et al. Estrogen-related receptor gamma functions as a tumor suppressor in gastric cancer. Nat. Commun. 2018, 9, 1920. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chin, J.; Im, C.Y.; Yoo, E.K.; Woo, S.; Hwang, H.J.; Cho, J.-H.; Seo, K.-A.; Song, J.; Hwang, H.; et al. Synthesis and biological evaluation of novel 4-hydroxytamoxifen analogs as estrogen-related receptor gamma inverse agonists. Eur. J. Med. Chem. 2016, 120, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Riggins, R.B.; Lan, J.P.-J.; Klimach, U.; Zwart, A.; Cavalli, L.R.; Haddad, B.R.; Chen, L.; Gong, T.; Xuan, J.; Ethier, S.P.; et al. ERRγ Mediates Tamoxifen Resistance in Novel Models of Invasive Lobular Breast Cancer. Cancer Res. 2008, 68, 8908–8917. [Google Scholar] [CrossRef]
- Audet-Walsh, E.; Yee, T.; McGuirk, S.; Vernier, M.; Ouellet, C.; St-Pierre, J.; Giguère, V. Androgen-Dependent Repression of ERRγ Reprograms Metabolism in Prostate Cancer. Cancer Res. 2017, 77, 378–389. [Google Scholar] [CrossRef]
- Singh, T.D.; Jeong, S.Y.; Lee, S.-W.; Ha, J.-H.; Lee, I.-K.; Kim, S.H.; Kim, J.; Cho, S.J.; Ahn, B.-C.; Lee, J.; et al. Inverse Agonist of Estrogen-Related Receptor γ Enhances Sodium Iodide Symporter Function Through Mitogen-Activated Protein Kinase Signaling in Anaplastic Thyroid Cancer Cells. J. Nucl. Med. 2015, 56, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.D.; Song, J.; Kim, J.; Chin, J.; Ji, H.D.; Lee, J.-E.; Lee, S.B.; Yoon, H.; Yu, J.H.; Kim, S.K.; et al. A Novel Orally Active Inverse Agonist of Estrogen-related Receptor Gamma (ERRγ), DN200434, A Booster of NIS in Anaplastic Thyroid Cancer. Clin. Cancer Res. 2019, 25, 5069–5081. [Google Scholar] [CrossRef]
- Gulwani, D.; Upadhyay, P.; Goel, R.; Sarangthem, V.; Singh, T.D. Unfolding of Imminent Bio-Signatures in the Prognosis of Thyroid Cancer; The Emergence of Estrogen Related Receptor Gamma (ERRγ) as a Hurricane. Asian Pac. J. Cancer Prev. 2023, 24, 375–387. [Google Scholar] [CrossRef]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid Function and Human Reproductive Health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.A.; Qadir, A.; Wani, M.A.; Wani, M.M. Spectrum of EGFR mutation and its relation with high-risk predictors in thyroid cancer in Kashmiri population: 2 years prospective study at a tertiary care hospital. J. Egypt. Natl. Cancer Inst. 2022, 34, 43. [Google Scholar] [CrossRef] [PubMed]
- Schiff, B.A.; McMurphy, A.B.; Jasser, S.A.; Younes, M.N.; Doan, D.; Yigitbasi, O.G.; Kim, S.; Zhou, G.; Mandal, M.; Bekele, B.N.; et al. Epidermal Growth Factor Receptor (EGFR) Is Overexpressed in Anaplastic Thyroid Cancer, and the EGFR Inhibitor Gefitinib Inhibits the Growth of Anaplastic Thyroid Cancer. Clin. Cancer Res. 2004, 10, 8594–8602. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-R.; Huang, J.-K.; Yin, Q.-F.; Shi, X.-M.; Tang, J.-C.; Hao, L.-L.; Li, P.-F.; Zhu, J.; Wang, Y.-X. Clinicopathological significance of epidermal growth factor receptor expression in papillary thyroid carcinoma: A meta-analysis. Minerva Endocrinol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.M.; Andrade, J.; Shupnik, M.A. Novel actions of estrogen to promote proliferation: Integration of cytoplasmic and nuclear pathways. Steroids 2009, 74, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Puli, O.R.; Danysh, B.P.; McBeath, E.; Sinha, D.K.; Hoang, N.M.; Powell, R.T.; Danysh, H.E.; Cabanillas, M.E.; Cote, G.J.; Hofmann, M.-C. The Transcription Factor ETV5 Mediates BRAFV600E-Induced Proliferation and TWIST1 Expression in Papillary Thyroid Cancer Cells. Neoplasia 2018, 20, 1121–1134. [Google Scholar] [CrossRef]
- Meng, D.; Li, Z.; Ma, X.; Wu, L.; Fu, L.; Qin, G. ETV5 overexpression contributes to tumor growth and progression of thyroid cancer through PIK3CA. Life Sci. 2020, 253, 117693. [Google Scholar] [CrossRef]
- Somasundaram, A.; Rothenberger, N.J.; Stabile, L.P. The Impact of Estrogen in the Tumor Microenvironment. Tumor Microenviron. Mol. Play. Part B 2020, 1277, 33–52. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef]
- Chakraborty, B.; Byemerwa, J.; Krebs, T.; Lim, F.; Chang, C.-Y.; McDonnell, D.P. Estrogen Receptor Signaling in the Immune System. Endocr. Rev. 2023, 44, 117–141. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Morales-Montor, J. Immune Tumor Microenvironment in Breast Cancer and the Participation of Estrogen and Its Receptors in Cancer Physiopathology. Front. Immunol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Wang, J.; Jin, H.; Song, X.; Yan, J.; Kang, Y.; Zhao, L.; An, X.; Du, X.; Chen, X.; et al. Induction of regulatory T cells by physiological level estrogen. J. Cell. Physiol. 2007, 214, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Faria, C.C.; Peixoto, M.S.; Carvalho, D.; Fortunato, R.S. The Emerging Role of Estrogens in Thyroid Redox Homeostasis and Carcinogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 2514312. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, W.; Li, L.; Zhuang, G.; Luo, G.; Zhou, H.; Hao, W.; Liu, Y.; Guo, F.; Tian, M.; et al. Identification and validation of eight estrogen-related genes for predicting prognosis of papillary thyroid cancer. Aging 2023, 15, 1668. [Google Scholar] [CrossRef]
- Lazarus, J.H. Thyroid function in pregnancy. Br. Med. Bull. 2010, 97, 137–148. [Google Scholar] [CrossRef]
- Angell, T.E.; Alexander, E.K. Thyroid Nodules and Thyroid Cancer in the Pregnant Woman. Endocrinol. Metab. Clin. N. Am. 2019, 48, 557–567. [Google Scholar] [CrossRef]
- Dehghan, M.H.; Ashrafi, M.R.; Hedayati, M.; Shivaee, S.; Rajabi, S. Oral Contraceptive Steroids Promote Papillary Thyroid Cancer Metastasis by Targeting Angiogenesis and Epithelial-Mesenchymal Transition. Int. J. Mol. Cell. Med. 2021, 10, 218–226. [Google Scholar] [CrossRef]
- Hedayati, M.; Rajabi, S.; Nikzamir, A. Papillary Thyroid Cancer-Promoting Activities of Combined Oral Contraceptive Components. Galen Med. J. 2020, 9, e1648. [Google Scholar] [CrossRef]
- Messuti, I.; Corvisieri, S.; Bardesono, F.; Rapa, I.; Giorcelli, J.; Pellerito, R.; Volante, M.; Orlandi, F. Impact of pregnancy on prognosis of differentiated thyroid cancer: Clinical and molecular features. Eur. J. Endocrinol. 2014, 170, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, B.H.; Lee, H.; Nam, H.; Park, S.; Jang, M.H.; Kim, J.M.; Kim, E.H.; Jeon, Y.K.; Kim, S.S.; et al. Thyroid cancer after hysterectomy and oophorectomy: A nationwide cohort study. Eur. J. Endocrinol. 2021, 184, 143–151. [Google Scholar] [CrossRef]
- Schonfeld, S.; Ron, E.; Kitahara, C.; Brenner, A.; Park, Y.; Sigurdson, A.; Schatzkin, A.; de González, A.B. Hormonal and reproductive factors and risk of postmenopausal thyroid cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol. 2011, 35, e85–e90. [Google Scholar] [CrossRef]
- Vannucchi, G.; Perrino, M.; Rossi, S.; Colombo, C.; Vicentini, L.; Dazzi, D.; Beck-Peccoz, P.; Fugazzola, L. Clinical and molecular features of differentiated thyroid cancer diagnosed during pregnancy. Eur. J. Endocrinol. 2010, 162, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Mair, K.M.; Gaw, R.; MacLean, M.R. Obesity, estrogens and adipose tissue dysfunction—Implications for pulmonary arterial hypertension. Pulm. Circ. 2020, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.; Subbaramaiah, K.; Iyengar, N.M.; Zhou, X.K.; Chen, I.-C.; Bhardwaj, P.; Gucalp, A.; Morrow, M.; Hudis, C.A.; Dannenberg, A.J.; et al. Leptin regulation of the p53-HIF1α/PKM2-aromatase axis in breast adipose stromal cells: A novel mechanism for the obesity–breast cancer link. Int. J. Obes. 2017, 42, 711–720. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Li, L.-R.; Song, J.-L.; Liu, H.-Q.; Chen, C. Metabolic syndrome and thyroid Cancer: Risk, prognosis, and mechanism. Discov. Oncol. 2023, 14, 23. [Google Scholar] [CrossRef]
- Colombo, C.; Giancola, N.; Fugazzola, L. Personalized treatment for differentiated thyroid cancer: Current data and new perspectives. Minerva Endocrinol. 2021, 46, 62–89. [Google Scholar] [CrossRef] [PubMed]
| Trial | Protocol | Expected Date |
|---|---|---|
| NCT04171622 | Lenvatinib and Pembrolizumab for the Treatment of Stage IVB Locally Advanced and Unresectable or Stage IVC Metastatic Anaplastic Thyroid Cancer | August 2023 |
| NCT04675710 | Pembrolizumab, Dabrafenib, and Trametinib Before Surgery for the Treatment of BRAF-Mutated Anaplastic Thyroid Cancer | June 2024 |
| NCT05059470 | IMRT Followed by Pembrolizumab in the Adjuvant Setting in Anaplastic Cancer of the Thyroid (IMPAACT): Phase II Trial Adjuvant Pembrolizumab After IMRT in ATC | October 2023 |
| NCT02628067 | Study of Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-3475-158/KEYNOTE-158) | June 2026 |
| NCT03360890 | Pembrolizumab With Chemotherapy for Poorly Chemo-responsive Thyroid and Salivary Gland Tumors (iPRIME) | September 2024 |
| NCT04061980 | Encorafenib and Binimetinib With or Without Nivolumab in Treating Patients With Metastatic Radioiodine Refractory BRAFV600 Mutant Thyroid Cancer | June 2023 |
| NCT05453799 | Vudalimab for the Treatment of Locally Advanced or Metastatic Anaplastic Thyroid Cancer or Hurthle Cell Thyroid Cancer | July 2024 |
| NCT05659186 | PD-1 Inhibitor and Anlotinib Combined With Multimodal Radiotherapy in Recurrent or Metastatic Anaplastic Thyroid Cancer | December 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denaro, N.; Romanò, R.; Alfieri, S.; Dolci, A.; Licitra, L.; Nuzzolese, I.; Ghidini, M.; Bareggi, C.; Bertaglia, V.; Solinas, C.; et al. The Tumor Microenvironment and the Estrogen Loop in Thyroid Cancer. Cancers 2023, 15, 2458. https://doi.org/10.3390/cancers15092458
Denaro N, Romanò R, Alfieri S, Dolci A, Licitra L, Nuzzolese I, Ghidini M, Bareggi C, Bertaglia V, Solinas C, et al. The Tumor Microenvironment and the Estrogen Loop in Thyroid Cancer. Cancers. 2023; 15(9):2458. https://doi.org/10.3390/cancers15092458
Chicago/Turabian StyleDenaro, Nerina, Rebecca Romanò, Salvatore Alfieri, Alessia Dolci, Lisa Licitra, Imperia Nuzzolese, Michele Ghidini, Claudia Bareggi, Valentina Bertaglia, Cinzia Solinas, and et al. 2023. "The Tumor Microenvironment and the Estrogen Loop in Thyroid Cancer" Cancers 15, no. 9: 2458. https://doi.org/10.3390/cancers15092458
APA StyleDenaro, N., Romanò, R., Alfieri, S., Dolci, A., Licitra, L., Nuzzolese, I., Ghidini, M., Bareggi, C., Bertaglia, V., Solinas, C., & Garrone, O. (2023). The Tumor Microenvironment and the Estrogen Loop in Thyroid Cancer. Cancers, 15(9), 2458. https://doi.org/10.3390/cancers15092458







