Influence of De Novo Malignancies on Long-Term Survival after Lung Transplantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion/Exclusion Criteria
2.3. Pre-Transplant Assessment
2.4. Donor Selection and Surgical Procedure
2.5. Recipient Post-Transplant Assessment and Management
2.6. Immunosuppression
2.7. Management of Infections
2.8. Data Collection
2.9. Statistical Analysis
2.9.1. Univariable Analysis
2.9.2. Multivariable Analysis
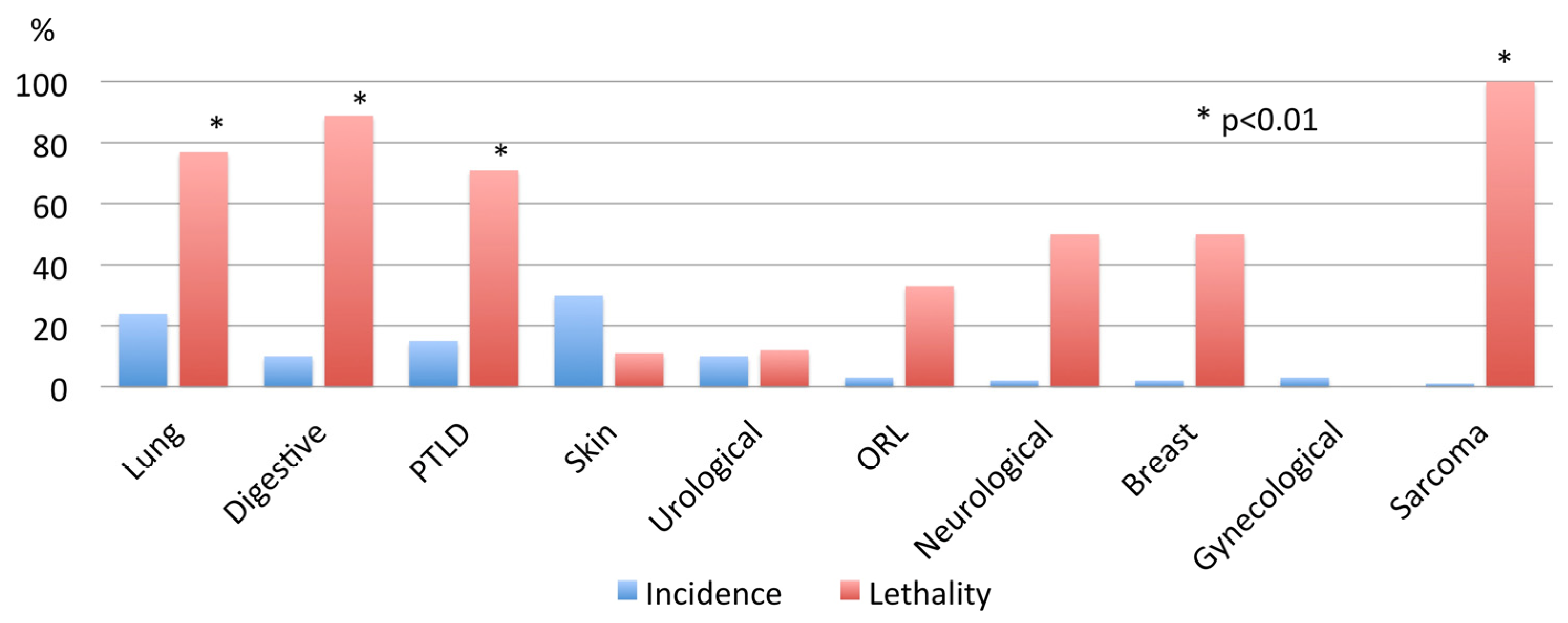
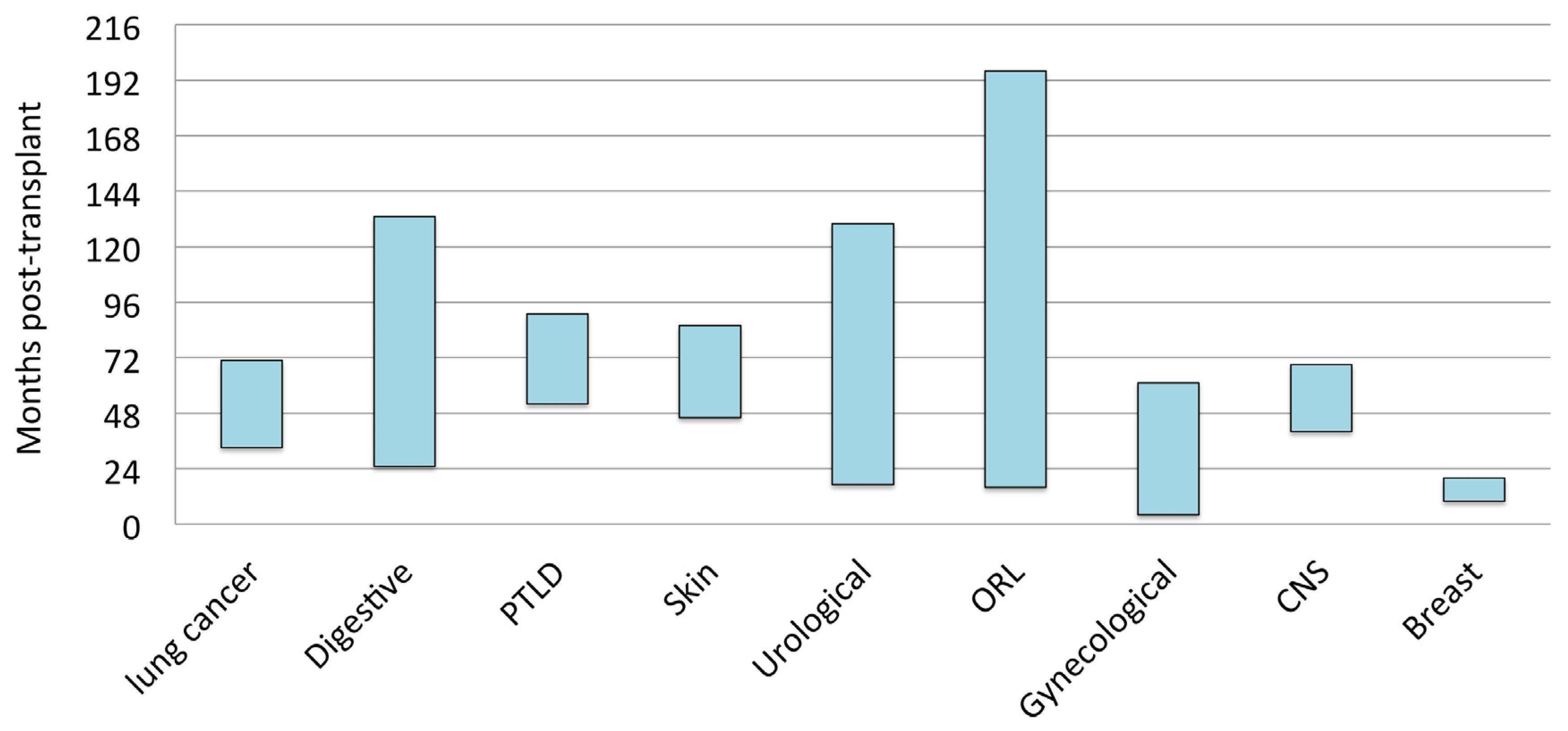
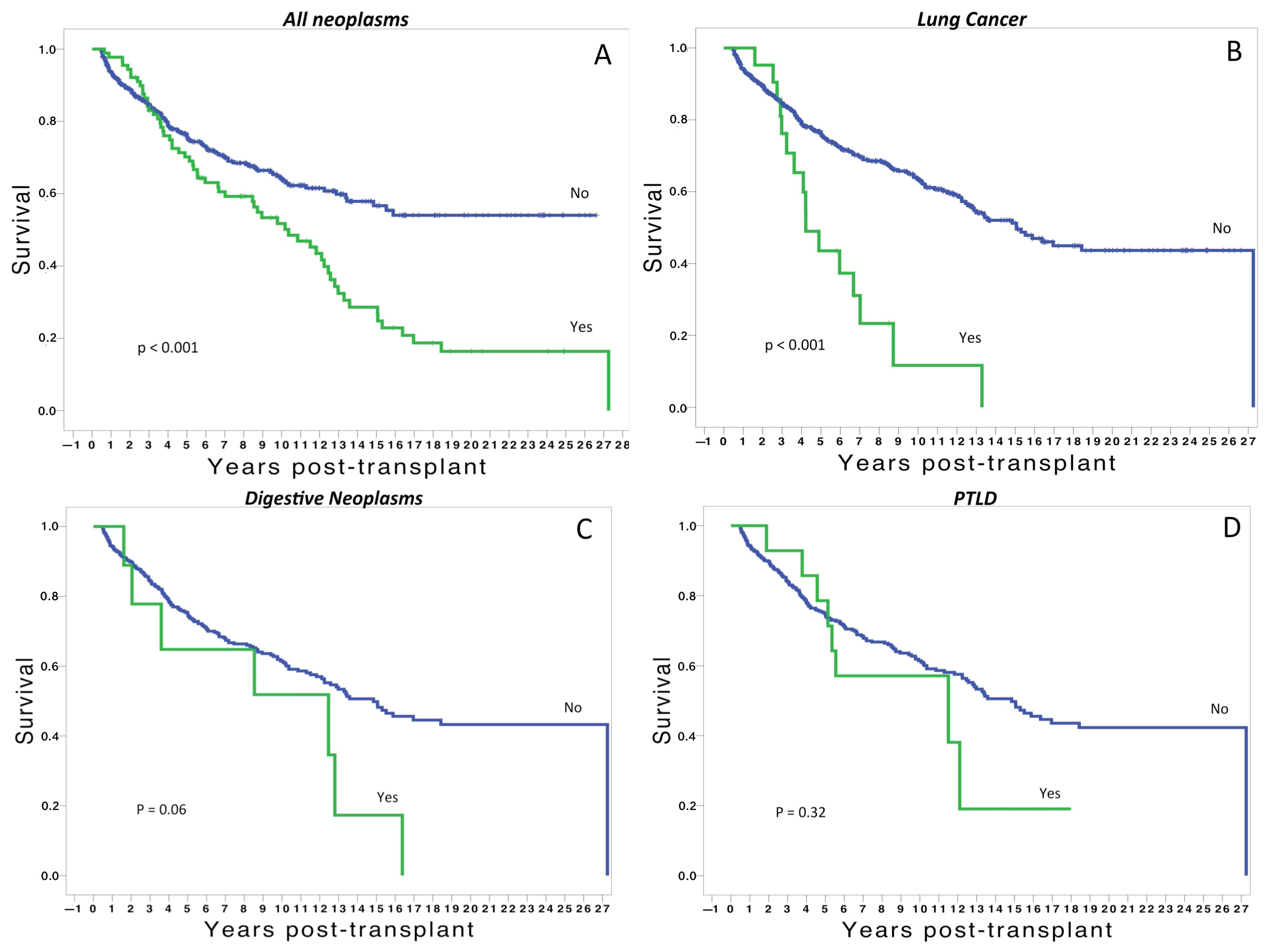
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F., Jr.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [PubMed]
- Chambers, D.C.; Zuckermann, A.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Potena, L.; Sadavarte, A.; Singh, T.P.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult lung transplantation report—2020; focus on deceased donor characteristics. J. Heart Lung Transplant. 2020, 39, 1016–1027. [Google Scholar] [PubMed]
- Lashari, B.H.; Vender, R.J.; Fleitas-Sosa, D.C.; Sinha, T.; Criner, G.J. Lung cancer in recipients after lung transplant: Single-centre experience and literature review. BMJ Open Respir. Res. 2022, 9, e001194. [Google Scholar] [PubMed]
- Minai, O.A.; Shah, S.; Mazzone, P.; Budev, M.M.; Sahoo, D.; Murthy, S.; Mason, D.; Petterson, G.; Metha, A.C. Bronchogenic carcinoma after lung transplantation: Characteristics and outcomes. J. Thorac. Oncol. 2008, 3, 1404–1409. [Google Scholar]
- Magruder, J.T.; Crawford, T.C.; Grimm, J.C.; Kim, B.; Shah, A.S.; Bush, E.L.; Higgins, R.S.; Merlo, C.A. Risk factors for de novo malignancy following lung transplantation. Am. J. Transplant. 2017, 17, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Morimoto, T.; Maluccio, M.; Asano, T.; Morimoto, K.; Langman, M.; Shimbo, T.; Suthanthiran, M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature 1999, 397, 530–534. [Google Scholar]
- Rossi, A.P.; Klein, C.L. Posttransplant malignancy. Surg. Clin. N. Am. 2019, 99, 49–64. [Google Scholar] [CrossRef]
- Paya, C.V.; Fung, J.J.; Nalesnik, M.A.; Kieff, E.; Green, M.; Gores, G.; Habermann, T.M.; Wiesner, P.H.; Swinnen, J.L.; Woodle, E.S.; et al. Epstein-Barr virus-induced posttransplant lymphoproliferative disorders. ASTS/ASTP EBV-PTLD Task Force and The Mayo Clinic Organized International Consensus Development Meeting. Transplantation 1999, 68, 1517–1525. [Google Scholar]
- Shtraichman, O.; Ahya, V.N. Malignancy after lung transplantation. Ann. Transl. Med. 2020, 8, 416–426. [Google Scholar] [CrossRef]
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379. [Google Scholar]
- Orens, J.B.; Boehler, A.; de Perrot, M.; Estenne, M.; Glanville, A.R.; Keshavjee, S.; Kotloff, R.; Morton, J.; Studer, S.M.; Van Raemdonck, D.; et al. A review of lung transplant donor acceptability criteria. J. Heart Lung Transplant. 2003, 22, 1183–1200. [Google Scholar] [CrossRef]
- Sundaresan, S.; Trachiotis, G.D.; Aoe, M.; Patterson, G.A.; Cooper, J.D. Donor lung procurement: Assessment and operative technique. Ann. Thorac. Surg. 1993, 56, 1409–1413. [Google Scholar]
- Nosotti, M.; Tarsia, P.; Morlacchi, L.C. Infections after lung transplantation. J. Thorac. Dis. 2018, 10, 3849–3868. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300, Erratum in CA Cancer J. Clin. 2011, 61, 133–134. [Google Scholar]
- Hosseini, M.; Salvatore, M. Is pulmonary fibrosis a precancerous disease? Eur. J. Radiol. 2023, 160, 110723. [Google Scholar]
- Rashtak, S.; Dierkhising, R.A.; Kremers, W.K.; Peters, S.G.; Cassivi, S.D.; Otley, C.C. Incidence and risk factors for skin cancer following lung transplantation. J. Am. Acad. Dermatol. 2015, 72, 92–98. [Google Scholar]
- Grager, N.; Leffler, M.; Gottlieb, J.; Fuge, J.; Warnecke, G.; Gutzmer, R.; Stazger, I. Risk factors for developing nonmelanoma skin cancer after lung transplantation. J. Skin Cancer 2019, 2019, 7089482. [Google Scholar] [CrossRef]
- Hamandi, B.; Fegbeutel, C.; Silveira, F.P.; Verschuuren, E.A.; Younus, M.; Mo, J.; Yan, J.; Ussetti, P.; Chin-Hong, P.V.; Solé, A.; et al. Voriconazole and squamous cell carcinoma after lung transplantation: A multicenter study. Am. J. Transplant. 2018, 18, 113–124. [Google Scholar]
- Cheng, J.; Moore, C.A.; Iasella, C.J.; Glanville, A.R.; Morrell, M.R.; Smith, R.B.; McDyer, J.F.; Ensor, C.R. Systematic review and meta-analysis of post-transplant lymphoproliferative disorder in lung transplant recipients. Clin. Transplant. 2018, 32, e13235. [Google Scholar]
- Leyssens, A.; Dierickx, D.; Verbeken, E.K.; Tousseyn, T.; Verleden, S.E.; Vanaudenaerde, B.M.; Dupont, L.J.; Yserbyt, J.; Verleden, G.M.; Van Raemdonck, D.E.; et al. Post-transplant lymphoproliferative disease in lung transplantation: A nested case-control study. Clin. Transplant. 2017, 31, e12983. [Google Scholar] [CrossRef]
- Reshef, R.; Vardhanabhuti, S.; Luskin, M.R.; Heitjan, D.F.; Hadjiliadis, D.; Goral, S.; Krok, K.L.; Goldberg, L.R.; Porter, D.L.; Stadtmauer, E.A.; et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder (bigstar). Am. J. Transplant. 2011, 11, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Triplette, M.; Crothers, K.; Mahale, P.; Yanik, E.L.; Valapour, M.; Lynch, C.F.; Schabath, M.B.; Castenson, D.; Engels, E.A. Risk of lung cancer in lung transplant recipients in the United States. Am. J. Transplant. 2019, 19, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Belli, E.V.; Landolfo, K.; Keller, C.; Thomas, M.; Odell, J. Lung cancer following lung transplant: Single institution 10 year experience. Lung Cancer 2013, 81, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Alvarez, E.; Moreno, P.; Poveda, D.; Ruiz, E.; Fernandez, A.M.; Salvatierra, A.; Alvarez, A. The influence of the native lung on early outcomes and survival after single lung transplantation. PLoS ONE 2021, 16, e0249758. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.M.; Poveda, D.S.; Ruiz, R.; Alvarez, E.; González, F.J.; Moreno, P.; Salvatierra, A.; Álvarez, A. Incidence of carcinoma in the native lung after single lung transplantation. Transplant. Proc. 2022, 54, 57–58. [Google Scholar] [CrossRef]
- Olland, A.M.; Falcoz, P.-E.; Massard, G. Malignancies after lung transplantation. J. Thorac. Dis. 2018, 10, 3132–3140. [Google Scholar] [CrossRef]
- Dickson, R.P.; Davis, R.D.; Rea, J.B.; Palmer, S.M. High frequency of bronchogenic carcinoma after single-lung transplantation. J. Heart Lung Transplant. 2006, 25, 1297–1301. [Google Scholar] [CrossRef]
- Brand, T.; Haithcock, B. Lung cancer and lung transplantation. Thorac. Surg. Clin. 2018, 28, 15–18. [Google Scholar] [CrossRef]
- Tian, T.; Li, X.; Zhang, J. mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef]
- Van Raemdonck, D.; Vos, R.; Yserbyt, J.; Decaluwe, H.; De Leyn, P.; Verleden, G.M. Lung cancer: A rare indication for, but frequent complication after lung transplantation. J. Thorac. Dis. 2016, 8, S915–S924. [Google Scholar]
| Malignancies NO (n = 640) YES (n =91) | p | |||||
|---|---|---|---|---|---|---|
| 95%CI | 95%CI | |||||
| Recipient age (years) | 46 ± 16 | 51 ± 13 | 0.01 | |||
| Donor age (years) | 42 ± 17 | 38 ±16 | 0.03 | |||
| Recipient gender | n (%) | |||||
| Male | 434 (68) | 64–72 | 74 (81) | 73–89 | ||
| Female | 205 (32) | 28–36 | 17 (19) | 11–27 | 0.005 | |
| Donor gender | n (%) | |||||
| Male | 291 (49) | 44–53 | 60 (66) | 56–76 | ||
| Female | 293 (50) | 46–54 | 31 (34) | 24–44 | 0.001 | |
| Extended donor | n (%) | 212 (33) | 30–39 | 24 (26) | 17–35 | 0.06 |
| Indication for LT | n (%) | |||||
| Emphysema | 226 (35) | 31–39 | 50 (55) | 45–65 | ||
| Cystic Fibrosis | 138 (21) | 18–24 | 11 (12) | 6–18 | ||
| Pulmonary Fibrosis | 144 (22) | 19–25 | 23 (25) | 16–34 | ||
| Bronchiectasis | 21 (4) | 3–5 | 2 (2) | 0–4 | ||
| Other | 110 (18) | 15–21 | 5 (6) | 1–11 | 0.003 | |
| Donor smoking | n (%) | 108 (17) | 14–20 | 9 (10) | 4–16 | 0.62 |
| EBV D/R | n (%) | |||||
| −/− | 15 (3) | 2–4 | 7 (8) | 3–13 | ||
| −/+ | 62 (10) | 8–12 | 3 (3) | 0–6 | ||
| +/− | 54 (8) | 6–10 | 3 (3) | 0–6 | ||
| +/+ | 173 (27) | 24–30 | 32 (35) | 25–45 | ||
| ?/− | 44 (7) | 5–9 | 9 (10) | 4–16 | ||
| ?/+ | 292 (45) | 41–49 | 37 (41) | 31–51 | 0.03 | |
| Initial IS | n (%) | |||||
| CS + AZA + Steroids | 113 (17) | 14–20 | 31 (34) | 24–44 | ||
| FK+ AZA+ Steroids | 32 (5) | 4–6 | 0 | |||
| CS + MMF + Steroids | 123 (19) | 16–22 | 23 (25) | 16–34 | ||
| FK + MMF + Steroids | 372 (58) | 54–62 | 37 (41) | 31–51 | <0.001 | |
| Calcineurin inhib. based IS | n (%) | |||||
| CS | 236 (37) | 33–41 | 54 (59) | 49–69 | ||
| FK | 404 (63) | 59–67 | 37 (41) | 31–51 | <0.001 | |
| Changes in IS | n (%) | |||||
| Yes/No | 168/472 | 68/23 | <0.001 | |||
| FK to CS | 117 (18) | 15–21 | 38 (42) | 32–52 | 0.07 | |
| MMF to AZA | 51 (8) | 6–10 | 30 (33) | 23–43 | 0.01 | |
| CMV infection/disease n (%) | ||||||
| <1 month post-LT | 21 (3) | 2–4 | 11 (12) | 6–18 | 0.07 | |
| 2–3 months post-LT | 29 (5) | 4–6 | 16 (17) | 9–25 | 0.09 | |
| >3 months post-LT | 48 (8) | 6–10 | 23 (25) | 16–34 | 0.06 | |
| Total AR episodes (n) | 1.0 ± 1.14 | 1.1–1.3 | 1.1 ± 1.05 | 0.9–1.3 | 0.55 | |
| AB0 group | n (%) | |||||
| 0 | 276 (38) | 239 (37) | 33–41 | 37 (40) | 30–50 | |
| A | 354 (48) | 310 (49) | 45–53 | 44 (48) | 38–58 | |
| B | 75 (10) | 67 (10) | 8–12 | 8 (9) | 3–15 | |
| AB | 25 (3) | 24 (4) | 3–5 | 2 (3) | 0–6 | 0.02 |
| Type of LT | n (%) | 44–52 47–55 | 55–75 25–45 | |||
| Single LT | 308 (48) | 59 (65) | ||||
| Double LT | 327 (51) | 32 (35) | ||||
| Combined liver + Double LT | 5 (0.7) | 0 | 0.01 | |||
| OR | 95%CI | p | |
|---|---|---|---|
| Cyclosporine-based immunosuppression | 1.8 | 1.3–2.4 | <0.001 |
| De novo lung cancer | 2.6 | 1.5–4.4 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, E.; Moreno, P.; Gonzalez, F.J.; Fernandez, A.M.; Cantador, B.; Parraga, J.L.; Salvatierra, A.; Alvarez, A. Influence of De Novo Malignancies on Long-Term Survival after Lung Transplantation. Cancers 2023, 15, 4011. https://doi.org/10.3390/cancers15154011
Ruiz E, Moreno P, Gonzalez FJ, Fernandez AM, Cantador B, Parraga JL, Salvatierra A, Alvarez A. Influence of De Novo Malignancies on Long-Term Survival after Lung Transplantation. Cancers. 2023; 15(15):4011. https://doi.org/10.3390/cancers15154011
Chicago/Turabian StyleRuiz, Eloisa, Paula Moreno, Francisco Javier Gonzalez, Alba Maria Fernandez, Benito Cantador, Juan Luis Parraga, Angel Salvatierra, and Antonio Alvarez. 2023. "Influence of De Novo Malignancies on Long-Term Survival after Lung Transplantation" Cancers 15, no. 15: 4011. https://doi.org/10.3390/cancers15154011
APA StyleRuiz, E., Moreno, P., Gonzalez, F. J., Fernandez, A. M., Cantador, B., Parraga, J. L., Salvatierra, A., & Alvarez, A. (2023). Influence of De Novo Malignancies on Long-Term Survival after Lung Transplantation. Cancers, 15(15), 4011. https://doi.org/10.3390/cancers15154011






