Assessing Osteolytic Lesion Size on Sequential CT Scans Is a Reliable Study Endpoint for Bone Remineralization in Newly Diagnosed Multiple Myeloma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Imaging Parameters
2.3. Radiological Assessment of Treatment Response
2.4. Statistics
3. Results
3.1. Patient Characteristics
3.2. Quantitative Lesion Characterization before and after Induction Therapy
3.3. Qualitative Criteria of Therapy Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| E-KRd | elotuzumab, carfilzomib, lenalidomide, and dexamethasone |
| IMWG | International Myeloma Working Group |
| MM | multiple myeloma |
| (s)CR | (stringent) complete response |
| SLAMF-7 | signaling lymphocytic activation molecule family member 7 |
| VGPR | very good partial response |
References
- Röllig, C.; Knop, S.; Bornhäuser, M. Multiple myeloma. Lancet 2015, 385, 2197–2208. [Google Scholar] [CrossRef]
- Cancer Today Website. Available online: http://gco.iarc.fr/today/home (accessed on 27 July 2023).
- Kosmala, A.; Weng, A.M.; Krauss, B.; Knop, S.; Bley, T.A.; Petritsch, B. Dual-energy CT of the bone marrow in multiple myeloma: Diagnostic accuracy for quantitative differentiation of infiltration patterns. Eur. Radiol. 2018, 28, 5083–5090. [Google Scholar] [CrossRef]
- Moulopoulos, L.A.; Koutoulidis, V.; Hillengass, J.; Zamagni, E.; Aquerreta, J.D.; Roche, C.L.; Lentzsch, S.; Moreau, P.; Cavo, M.; Miguel, J.S.; et al. Recommendations for acquisition, interpretation and reporting of whole body low dose CT in patients with multiple myeloma and other plasma cell disorders: A report of the IMWG Bone Working Group. Blood Cancer J. 2018, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Koutoulidis, V.; Fontara, S.; Terpos, E.; Zagouri, F.; Matsaridis, D.; Christoulas, D.; Panourgias, E.; Kastritis, E.; Dimopoulos, M.A.; Moulopoulos, L.A. Quantitative Diffusion-weighted Imaging of the Bone Marrow: An Adjunct Tool for the Diagnosis of a Diffuse MR Imaging Pattern in Patients with Multiple Myeloma. Radiology 2017, 282, 484–493. [Google Scholar] [CrossRef]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.-V.; Lonial, S.; Joao, C.; Anderson, K.C.; García-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Koutoulidis, V.; Terpos, E.; Papanikolaou, N.; Fontara, S.; Seimenis, I.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Bourgioti, C.; Santinha, J.; Moreira, J.M.; et al. Comparison of MRI Features of Fat Fraction and ADC for Early Treatment Response Assessment in Participants with Multiple Myeloma. Radiology 2022, 304, 137–144. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Hillengass, J.; Usmani, S.; Zamagni, E.; Lentzsch, S.; Davies, F.E.; Raje, N.; Sezer, O.; Zweegman, S.; Shah, J.; et al. Role of Magnetic Resonance Imaging in the Management of Patients with Multiple Myeloma: A Consensus Statement. J. Clin. Oncol. 2015, 33, 657–664. [Google Scholar] [CrossRef]
- Terpos, E.; Zamagni, E.; Lentzsch, S.; Drake, M.T.; García-Sanz, R.; Abildgaard, N.; Ntanasis-Stathopoulos, I.; Schjesvold, F.; de la Rubia, J.; Kyriakou, C.; et al. Treatment of multiple myeloma-related bone dis-ease: Recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet Oncol. 2021, 22, e119–e130. [Google Scholar] [CrossRef]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; García-Sanz, R.; Durie, B.; Legieć, W.; Krejčí, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Morgan, G.J.; Child, J.A.; Gregory, W.M.; Szubert, A.J.; Cocks, K.; Bell, S.E.; Navarro-Coy, N.; Drayson, M.T.; Owen, R.G.; Feyler, S.; et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in pa-tients with newly diagnosed multiple myeloma (MRC Myeloma IX): Secondary outcomes from a ran-domised controlled trial. Lancet Oncol. 2011, 12, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Cavo, M.; Tacchetti, P.; Patriarca, F.; Petrucci, M.T.; Pantani, L.; Galli, M.; Di Raimondo, F.; Crippa, C.; Zamagni, E.; Palumbo, A.; et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet 2010, 376, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Heath, D.J.; Rahemtulla, A.; Zervas, K.; Chantry, A.; Anagnostopoulos, A.; Pouli, A.; Katodritou, E.; Verrou, E.; Vervessou, E.C.; et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br. J. Haematol. 2006, 135, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Weisel, K.; Grandjean, C.; Oehrlein, K.; Zago, M.; Spira, D.; Horger, M. Increasing Bone Sclerosis During Bortezomib Therapy in Multiple Myeloma Patients: Results of a Reduced-Dose Whole-Body MDCT Study. Am. J. Roentgenol. 2014, 202, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Lath, D.; Hudson, K.; Walkley, B.; Down, J.M.; Owen, R.; Evans, H.R.; Paton-Hough, J.; Reilly, G.C.; Lawson, M.A.; et al. TGFβ Inhibition Stimulates Collagen Maturation to Enhance Bone Repair and Fracture Resistance in a Murine Myeloma Model. J. Bone Miner. Res. 2019, 34, 2311–2326. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Weisel, K.; San-Miguel, J.; Shpilberg, O.; Grosicki, S.; Špička, I.; Walter-Croneck, A.; et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020, 10, 91. [Google Scholar] [CrossRef]
- Derman, B.A.; Kansagra, A.; Zonder, J.; Stefka, A.T.; Grinblatt, D.L.; Anderson, L.D.J.; Gurbuxani, S.; Narula, S.; Rayani, S.; Major, A.; et al. Elotuzumab and Weekly Carfilzomib, Lenalidomide, and Dexame-thasone in Patients with Newly Diagnosed Multiple Myeloma without Transplant Intent: A Phase 2 Measurable Residual Disease-Adapted Study. JAMA Oncol. 2022, 8, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Sieren, M.M.; Brenne, F.; Hering, A.; Kienapfel, H.; Gebauer, N.; Oechtering, T.H.; Fürschke, A.; Wegner, F.; Stahlberg, E.; Heldmann, S.; et al. Rapid study assessment in follow-up whole-body computed tomography in patients with multiple myeloma using a dedicated bone subtraction software. Eur. Radiol. 2020, 30, 3198–3209. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report from International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Mohan, M.; Samant, R.S.; Yoon, D.; Buros, A.F.; Branca, A.; Montgomery, C.O.; Nicholas, R.; Suva, L.J.; Morello, R.; Thanendrarajan, S.; et al. Extensive Remineralization of Large Pelvic Lytic Lesions Following Total Therapy Treatment in Patients with Multiple Myeloma. J. Bone Miner. Res. 2017, 32, 1261–1266. [Google Scholar] [CrossRef] [Green Version]
- Takasu, M.; Tani, C.; Kaichi, Y.; Sakoda, Y.; Kiguchi, M.; Date, S.; Kuroda, Y.; Sakai, A.; Awai, K. A lon-gitudinal computed tomography study of lenalidomide and bortezomib treatment for multiple myeloma: Trabecular microarchitecture and biomechanics assessed using multidetector computed tomography. Clin. Lymphoma Myeloma Leuk. 2014, 14, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Christoulas, D.; Kokkoris, P.; Anargyrou, K.; Gavriatopoulou, M.; Migkou, M.; Tsionos, K.; Dimopoulos, M.A. Increased bone mineral density in a subset of patients with relapsed multiple myeloma who received the combination of bortezomib, dexamethasone and zoledronic acid. Ann. Oncol. 2010, 21, 1561–1562. [Google Scholar] [CrossRef] [PubMed]
- Du, J.S.; Yen, C.H.; Hsu, C.M.; Hsiao, H.H. Management of Myeloma Bone Lesions. Int. J. Mol. Sci. 2021, 22, 3389. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Katodritou, E.; Kyrtsonis, M.C.; Douka, V.; Spanoudakis, E.; Papatheo-dorou, A.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Gavriatopoulou, M.; et al. Carfilzomib Im-proves Bone Metabolism in Patients with Advanced Relapsed/Refractory Multiple Myeloma: Results of the CarMMa Study. Cancers 2021, 13, 1257. [Google Scholar] [CrossRef]
- Terpos, E.; Kastritis, E.; Ntanasis-Stathopoulos, I.; Christoulas, D.; Papatheodorou, A.; Eleuthera-kis-Papaiakovou, E.; Kanellias, N.; Fotiou, D.; Ziogas, D.C.; Migkou, M.; et al. Consolidation therapy with the combination of bortezomib and lenalidomide (VR) without dexamethasone in multiple mye-loma patients after transplant: Effects on survival and bone outcomes in the absence of bisphosphonates. Am. J. Hematol. 2019, 94, 400–407. [Google Scholar] [CrossRef]
- Mohan, M.; Kumar, M.; Samant, R.; Van Hemert, R.J.; Tian, E.; Desai, S.; van Rhee, F.; Thanendrarajan, S.; Schinke, C.; Suva, L.J.; et al. Bone remineralization of lytic lesions in multiple myeloma—The Ar-kansas experience. Bone 2021, 146, 115876. [Google Scholar] [CrossRef]
- Seefried, L.; Genest, F.; Strömsdörfer, J.; Engelmann, B.; Lapa, C.; Jakob, F.; Baumann, F.T.; Sperlich, B.; Jundt, F. Impact of whole-body vibration exercise on physical performance and bone turnover in patients with monoclonal gammopathy of undetermined significance. J. Bone Oncol. 2020, 25, 100323. [Google Scholar] [CrossRef]
- Wefelnberg, M.M.; Niels, T.; Holtick, U.; Jundt, F.; Scheid, C.; Baumann, F.T. Clinical exercise therapy program with multiple myeloma patients: Impacts on feasibility, adherence and efficacy. Support. Care Cancer 2022, 30, 9615–9623. [Google Scholar] [CrossRef]
- Costa, F.; Toscani, D.; Chillemi, A.; Quarona, V.; Bolzoni, M.; Marchica, V.; Vescovini, R.; Mancini, C.; Martella, E.; Campanini, N.; et al. Expression of CD38 in myeloma bone niche: A rational basis for the use of anti-CD38 immunotherapy to inhibit osteoclast formation. Oncotarget 2017, 8, 56598–56611. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Kastritis, E.; Hatjiharissi, E.; Katodritou, E.; Eleutherakis-Papaiakovou, E.; Verrou, E.; Gavriatopoulou, M.; Leonidakis, A.; Manousou, K.; et al. Daratumumab Improves Bone Turnover in Relapsed/Refractory Multiple Myeloma; Phase 2 Study “REBUILD”. Cancers 2022, 14, 2768. [Google Scholar] [CrossRef] [PubMed]


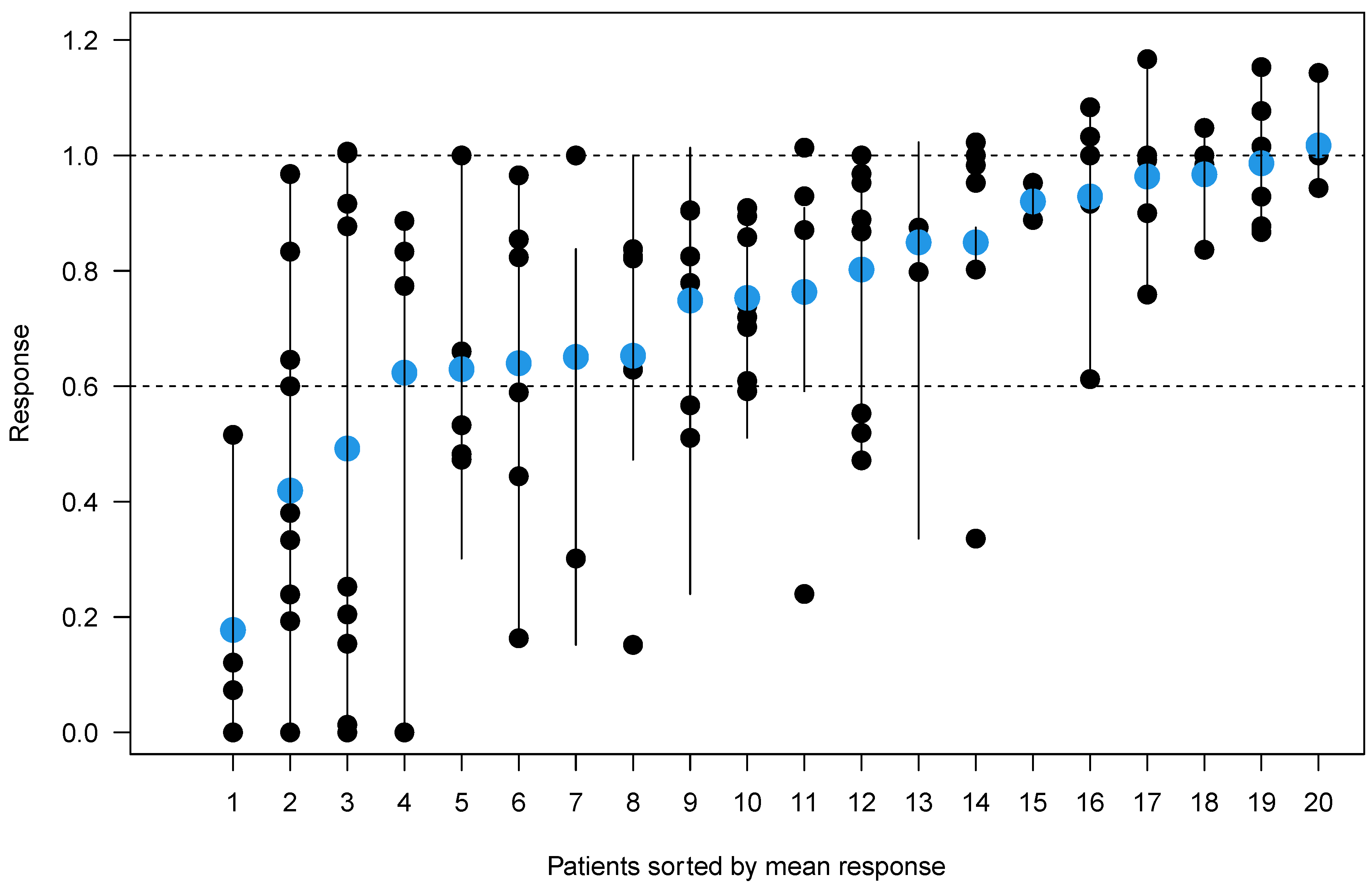
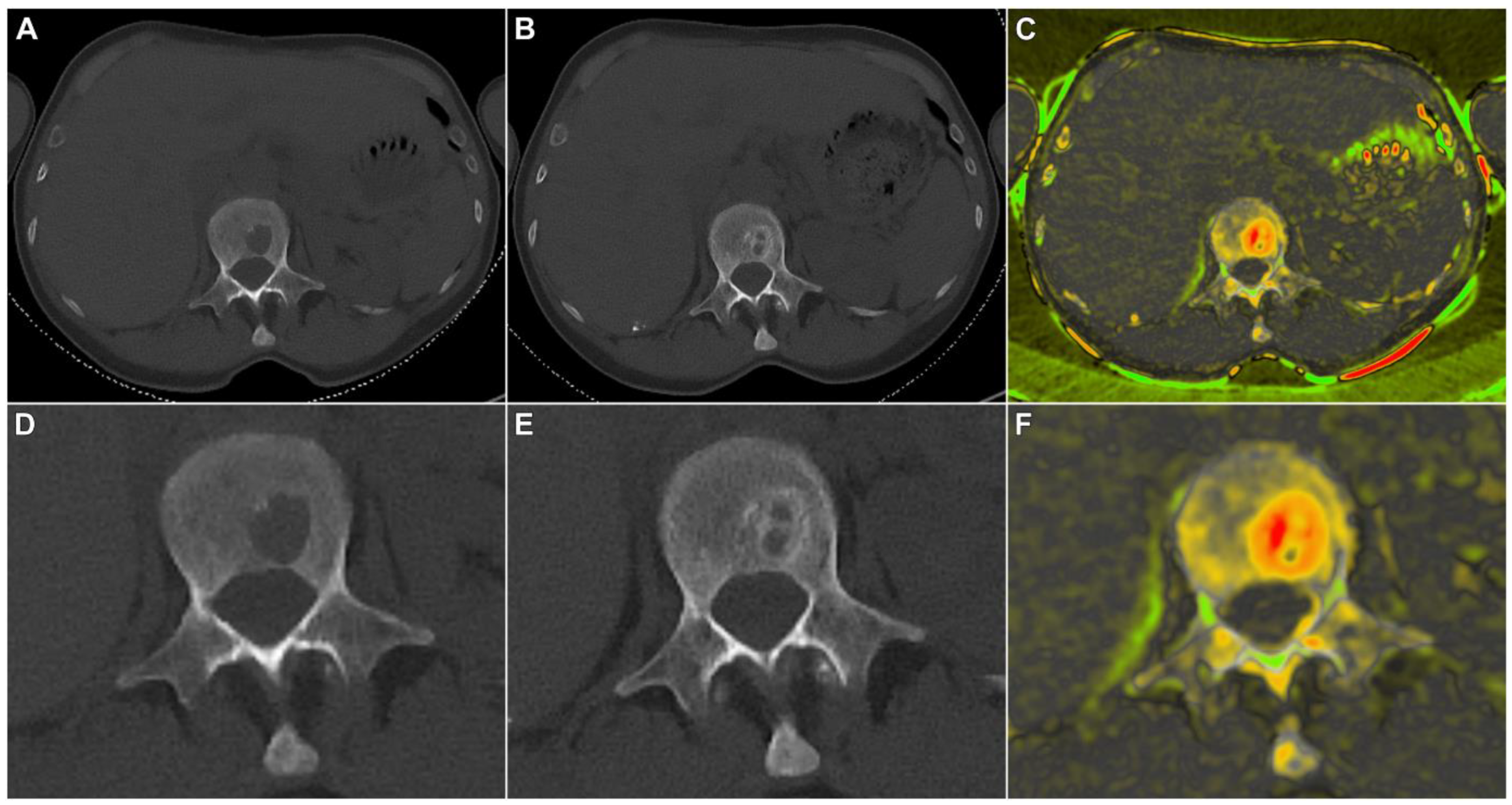


| Patient | Age | Sex | Diagnosis | R-ISS | E-KRd Cycles | Bone Therapy (q4w) | Interval between CT Scans (days) | Bone Marrow Infiltration at Baseline (%) | Bone Marrow Infiltration after Treatment (%) | Hematologic Response at End of E-KRd |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | m | IgG lambda | II | 6 | Denosumab | 209 | 90 | <10 | VGPR |
| 2 | 61 | m | IgG kappa | I | 6 | Zoledronic acid | 204 | 90 | 3 | VGPR |
| 3 | 54 | m | IgG lambda | II | 6 | Zoledronic acid | 245 | 20 | 0 | VGPR |
| 4 | 69 | f | IgG kappa | I | 6 | Zoledronic acid | 207 | 50 | 0 | VGPR |
| 5 | 58 | m | IgG kappa | II | 6 | Zoledronic acid | 218 | 70 | <10 | VGPR |
| 6 | 59 | f | IgG kappa | I | 6 | Zoledronic acid | 214 | 30 | 0 | VGPR |
| 7 | 48 | m | IgG lambda | I | 6 | Zoledronic acid | 294 | 0 | 0 | VGPR |
| 8 | 58 | f | IgG kappa | I | 6 | Zoledronic acid | 202 | 70 | 0 | VGPR |
| 9 | 57 | m | IgG kappa | I | 6 | Zoledronic acid | 198 | 8 | 0 | CR |
| 10 | 58 | m | IgG kappa | II | 6 | Zoledronic acid | 230 | 70 | 0 | VGPR |
| 11 | 62 | f | IgG kappa | I | 6 | Denosumab * | 204 | 15 | 0 | VGPR |
| 12 | 56 | m | IgA kappa | II | 6 | Zoledronic acid | 205 | 90 | 0 | VGPR |
| 13 | 57 | m | IgG kappa | II | 6 | Zoledronic acid | 280 | 90 | 0 | VGPR |
| 14 | 63 | m | IgG lambda | I | 6 | Zoledronic acid | 209 | 20 | <10 | VGPR |
| 15 | 40 | m | IgA kappa | I | 6 | Zoledronic acid | 229 | 30 | 0 | CR |
| 16 | 58 | f | IgA kappa | I | 6 | Zoledronic acid | 211 | 80 | 10 | VGPR |
| 17 | 69 | m | IgA lambda | I | 6 | Pamidronic ** | 207 | 15 | 0 | CR |
| 18 | 41 | f | IgG kappa | II | 6 | Zoledronic acid | 242 | 45 | 0 | sCR |
| 19 | 68 | m | IgG kappa | III | 6 | Zoledronic acid | 217 | 60 | <10 | VGPR |
| 20 | 66 | f | IgG lambda | II | 6 | Zoledronic acid | 196 | 50 | 5 | VGPR |
| Patient | Number of Lesions | Sum of Lesion Sizes at Baseline (mm2) | Sum of Lesion Sizes after Treatment (mm2) | Sum of Response Ratios | Mean Response (%) |
|---|---|---|---|---|---|
| 1 | 6 | 1108 | 818 | 0.74 | −25 |
| 2 | 4 | 912 | 811 | 0.89 | −3 |
| 3 | 9 | 1877 | 1506 | 0.80 | −20 |
| 4 | 5 | 933 | 526 | 0.56 | −37 |
| 5 | 8 | 2238 | 1706 | 0.76 | −25 |
| 6 | 5 | 542 | 530 | 0.98 | +2 |
| 7 | 10 | 1136 | 498 | 0.44 | −58 |
| 8 | 5 | 510 | 496 | 0.97 | −4 |
| 9 | 4 | 621 | 249 | 0.4 | −82 |
| 10 | 4 | 492 | 402 | 0.82 | −38 |
| 11 | 9 | 1320 | 910 | 0.69 | −51 |
| 12 | 3 | 808 | 692 | 0.86 | −15 |
| 13 | 6 | 2040 | 1726 | 0.85 | −15 |
| 14 | 2 | 104 | 56 | 0.54 | −35 |
| 15 | 6 | 640 | 632 | 0.99 | −1 |
| 16 | 4 | 477 | 433 | 0.91 | −24 |
| 17 | 5 | 745 | 710 | 0.95 | −7 |
| 18 | 2 | 212 | 198 | 0.93 | −8 |
| 19 | 5 | 2291 | 1659 | 0.72 | −35 |
| 20 | 6 | 766 | 554 | 0.72 | −36 |
| Reader Analysis | Lesions Described by Reader 1 (n = 103) | Lesions Described by Reader 2 (n = 99) | Lesions Described by Reader 1 and 2 (n = 94) | Inter-Reader Reliability | |||
|---|---|---|---|---|---|---|---|
| CT Scan | Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | Krippendorff’s α |
| Cortical destruction | 35% (36/103) | 17% (17/103) | 37% (37/99) | 12% (12/99) | 35% (33/94) | 13% (12/94) | 0.85 (0.76–0.93) |
| Pathologic fracture | 6% (6/103) | 9% (9/103) | 5% (5/99) | 8% (8/99) | 5% (5/94) | 9% (8/94) | 0.92 (0.81–1) |
| Rim sclerosis | 14% (14/103) | 72% (74/103) | 9% (9/99) | 56% (55/99) | 10% (9/94) | 54% (51/94) | 0.74 (0.64–0.83) |
| Trabecular sclerosis | 0% (0/103) | 33% (34/103) | 1% (1/99) | 39% (39/99) | 0% (0/94) | 31% (29/94) | 0.81 (0.68–0.91) |
| Lesion Level Response | No Trabecular Sclerosis | Trabecular Sclerosis | Sum | p Value |
|---|---|---|---|---|
| Therapy response ≤20% | 58 (54%) | 5 (5%) | 63 (58%) | |
| Therapy response >20% | 21 (19%) | 24 (22%) | 45 (42%) | |
| Sum | 79 (73%) | 29 (27%) | 108 (100%) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grunz, J.-P.; Kunz, A.S.; Baumann, F.T.; Hasenclever, D.; Sieren, M.M.; Heldmann, S.; Bley, T.A.; Einsele, H.; Knop, S.; Jundt, F. Assessing Osteolytic Lesion Size on Sequential CT Scans Is a Reliable Study Endpoint for Bone Remineralization in Newly Diagnosed Multiple Myeloma. Cancers 2023, 15, 4008. https://doi.org/10.3390/cancers15154008
Grunz J-P, Kunz AS, Baumann FT, Hasenclever D, Sieren MM, Heldmann S, Bley TA, Einsele H, Knop S, Jundt F. Assessing Osteolytic Lesion Size on Sequential CT Scans Is a Reliable Study Endpoint for Bone Remineralization in Newly Diagnosed Multiple Myeloma. Cancers. 2023; 15(15):4008. https://doi.org/10.3390/cancers15154008
Chicago/Turabian StyleGrunz, Jan-Peter, Andreas Steven Kunz, Freerk T. Baumann, Dirk Hasenclever, Malte Maria Sieren, Stefan Heldmann, Thorsten Alexander Bley, Hermann Einsele, Stefan Knop, and Franziska Jundt. 2023. "Assessing Osteolytic Lesion Size on Sequential CT Scans Is a Reliable Study Endpoint for Bone Remineralization in Newly Diagnosed Multiple Myeloma" Cancers 15, no. 15: 4008. https://doi.org/10.3390/cancers15154008
APA StyleGrunz, J.-P., Kunz, A. S., Baumann, F. T., Hasenclever, D., Sieren, M. M., Heldmann, S., Bley, T. A., Einsele, H., Knop, S., & Jundt, F. (2023). Assessing Osteolytic Lesion Size on Sequential CT Scans Is a Reliable Study Endpoint for Bone Remineralization in Newly Diagnosed Multiple Myeloma. Cancers, 15(15), 4008. https://doi.org/10.3390/cancers15154008






