Early-Stage HCC Percutaneous Locoregional Management: East versus West Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
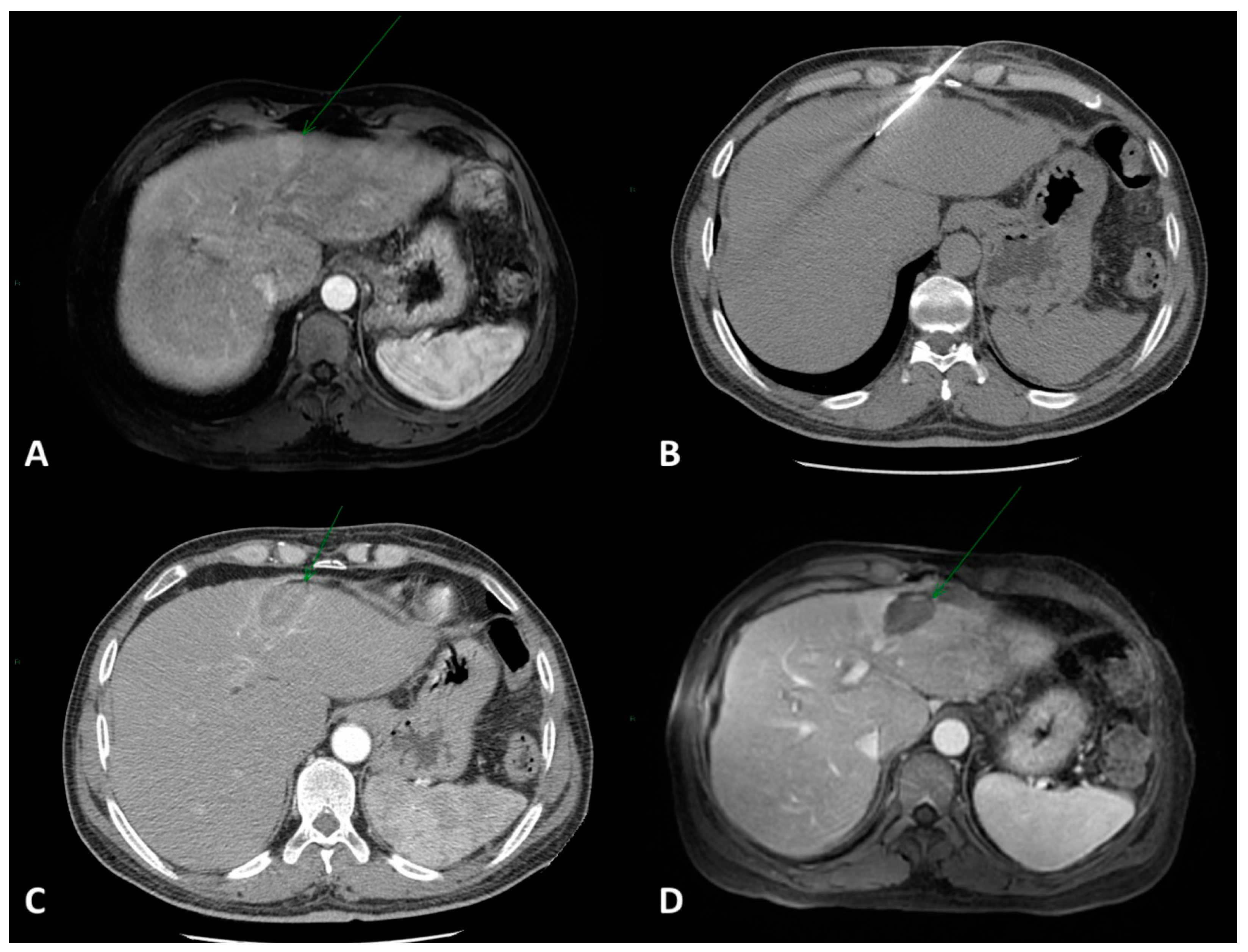
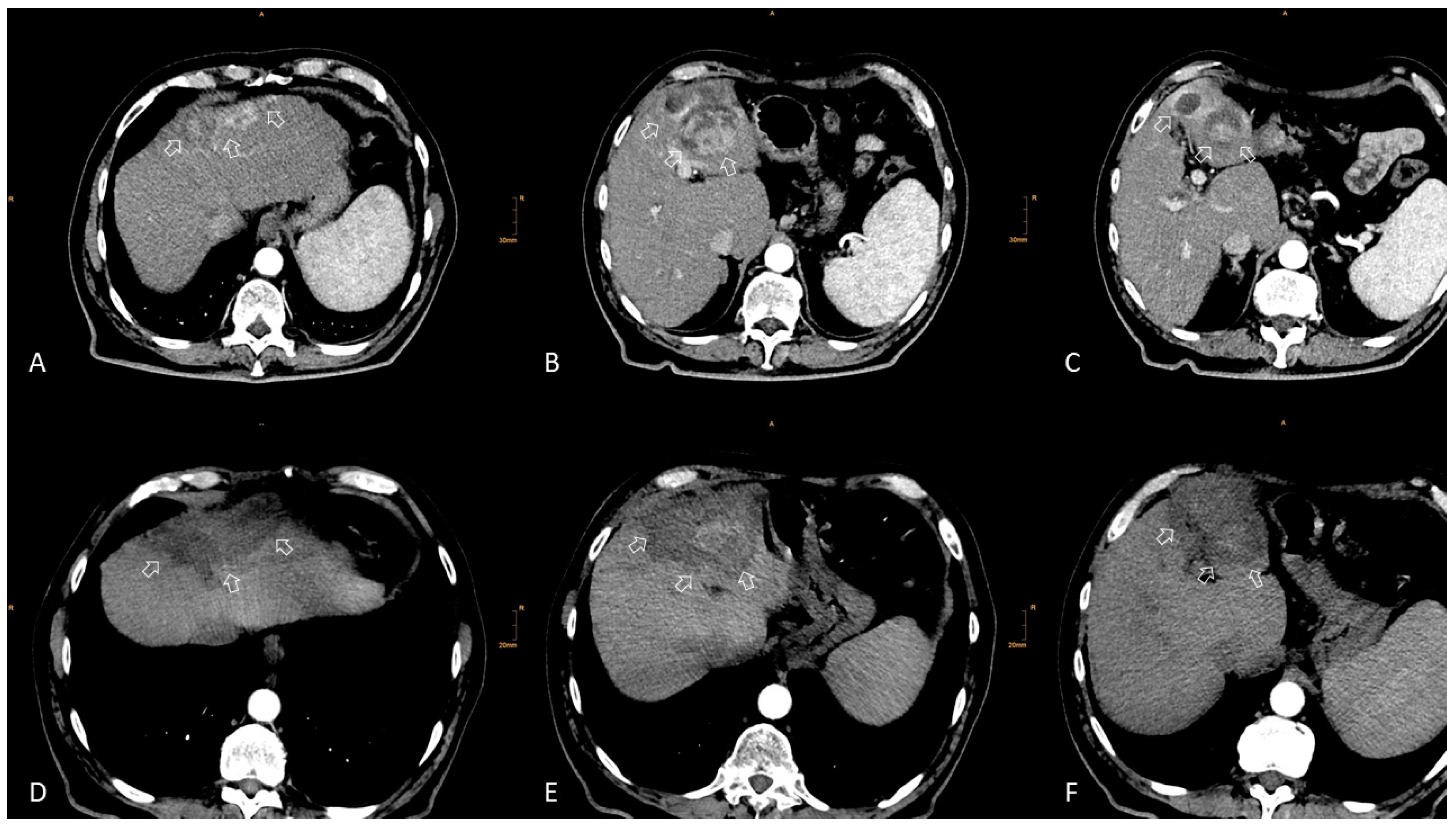
2. Local Ablation
2.1. Eastern Perspective
2.2. Western Perspective
3. TACE
3.1. Eastern Perspective
3.2. Western Perspective
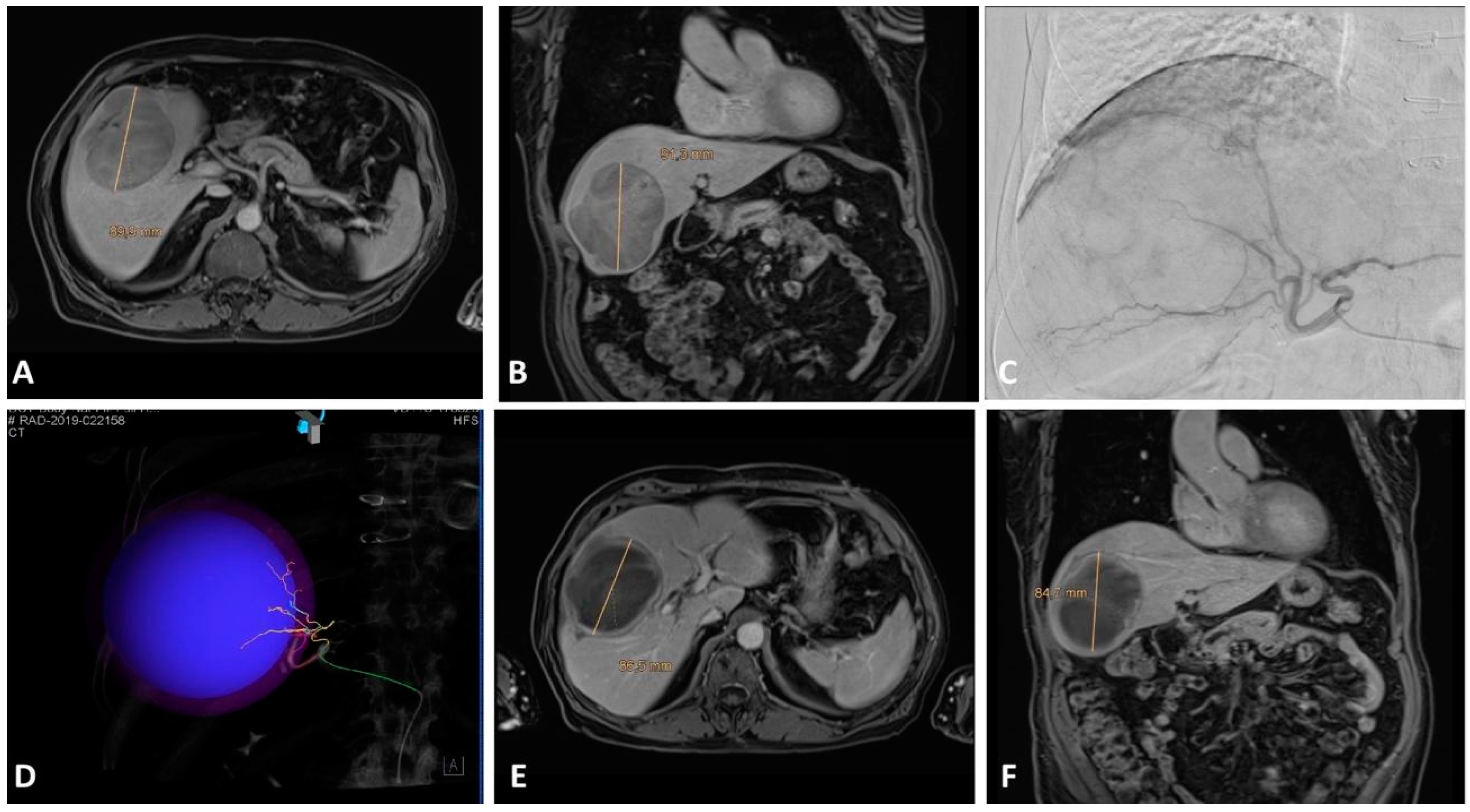
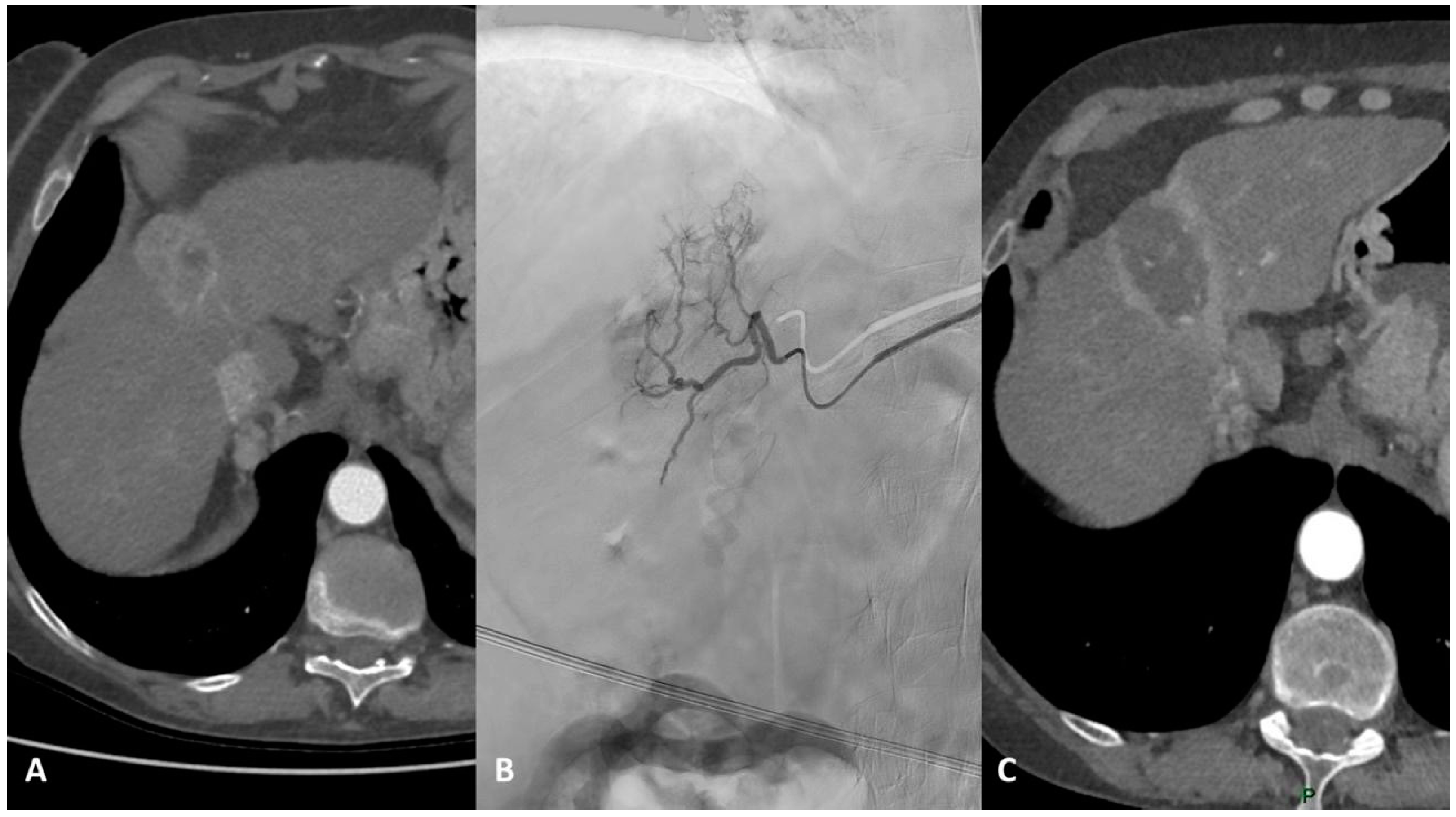
4. TARE
4.1. Eastern Perspective
4.2. Western Perspective
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [PubMed]
- Makary, M.S.; Ramsell, S.; Miller, E.; Beal, E.W.; Dowell, J.D. Hepatocellular carcinoma locoregional therapies: Outcomes and future horizons. World J. Gastroenterol. 2021, 27, 7462–7479. [Google Scholar] [CrossRef]
- Gnutzmann, D.; Kortes, N.; Sumkauskaite, M.; Schmitz, A.; Weiss, K.H.; Radeleff, B. Transvascular therapy of Hepatocellular Carcinoma (HCC), status and developments. Minim. Invasive Ther. Allied Technol. 2018, 27, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; de Baere, T.; Martin, R.C.; Nutting, C.W.; Narayanan, G. Image-Guided Ablation of Malignant Liver Tumors: Recommendations for Clinical Validation of Novel Thermal and Non-Thermal Technologies—A Western Perspective. Liver Cancer 2015, 4, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lim, H.K.; Rhim, H.; Lee, M.W.; Choi, D.; Lee, W.J.; Paik, S.W.; Koh, K.C.; Lee, J.H.; Choi, M.S.; et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: Analysis of prognostic factors. J. Hepatol. 2013, 58, 89–97. [Google Scholar] [CrossRef]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577; quiz 578. [Google Scholar] [CrossRef] [Green Version]
- Ju, M.R.; Yopp, A.C. Surgical resection of early stage hepatocellular carcinoma: Balancing tumor biology with the host liver. Chin. Clin. Oncol. 2021, 10, 5. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, Y.S.; Kim, B.K.; Jung, Y.K.; Kim, S.U.; Park, J.Y.; Kim, J.H.; An, H.; Kim, D.Y.; Yim, H.J.; et al. Change in the Recurrence Pattern and Predictors over Time after Complete Cure of Hepatocellular Carcinoma. Gut Liver 2021, 15, 420–429. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J. Radiol. 2022, 23, 1126–1240. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.M. Recent Advances in the Image-Guided Tumor Ablation of Liver Malignancies: Radiofrequency Ablation with Multiple Electrodes, Real-Time Multimodality Fusion Imaging, and New Energy Sources. Korean J. Radiol. 2018, 19, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, J.M.; Lee, D.H.; Yoon, J.H.; Kim, Y.J.; Lee, J.H.; Yu, S.J.; Cho, E.J. Radiofrequency Ablation Using a Separable Clustered Electrode for the Treatment of Hepatocellular Carcinomas: A Randomized Controlled Trial of a Dual-Switching Monopolar Mode Versus a Single-Switching Monopolar Mode. Korean J. Radiol. 2021, 22, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Hocquelet, A.; Aubé, C.; Rode, A.; Cartier, V.; Sutter, O.; Manichon, A.F.; Boursier, J.; N’kontchou, G.; Merle, P.; Blanc, J.F.; et al. Comparison of no-touch multi-bipolar vs. monopolar radiofrequency ablation for small HCC. J. Hepatol. 2017, 66, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, M.W.; Kim, P.N.; Lee, Y.J.; Park, H.S.; Lee, J.M. Outcome of No-Touch Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Multicenter Clinical Trial. Radiology 2021, 301, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.S.; Choi, J.W.; Yoon, J.H.; Lee, D.H.; Kim, Y.J.; Lee, J.H.; Yu, S.J.; Cho, E.J.; Yoon, J.H.; Lee, J.M. No-Touch vs. Conventional Radiofrequency Ablation Using Twin Internally Cooled Wet Electrodes for Small Hepatocellular Carcinomas: A Randomized Prospective Comparative Study. Korean J. Radiol. 2021, 22, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Öcal, O.; Rössler, D.; Ricke, J.; Seidensticker, M. Advances in Diagnostic and Interventional Radiology in Hepatocellular Carcinoma. Dig. Dis. 2022, 40, 458–467. [Google Scholar] [CrossRef]
- Vietti Violi, N.; Duran, R.; Guiu, B.; Cercueil, J.P.; Aubé, C.; Digklia, A.; Pache, I.; Deltenre, P.; Knebel, J.F.; Denys, A. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: A randomised controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 3, 317–325. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.C.; Wang, K. Radiofrequency ablation versus microwave ablation for early stage hepatocellular carcinoma: A PRISMA-compliant systematic review and meta-analysis. Medicine 2020, 99, e22703. [Google Scholar] [CrossRef]
- Bale, R.; Widmann, G.; Haidu, M. Stereotactic radiofrequency ablation. Cardiovasc. Intervent Radiol. 2011, 34, 852–856. [Google Scholar] [CrossRef]
- Schullian, P.; Johnston, E.W.; Putzer, D.; Eberle, G.; Laimer, G.; Bale, R. Safety and efficacy of stereotactic radiofrequency ablation for very large (≥8 cm) primary and metastatic liver tumors. Sci. Rep. 2020, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Schullian, P.; Putzer, D.; Eberle, G.; Laimer, G.; Bale, R. Simultaneous Stereotactic Radiofrequency Ablation of Multiple (≥4) Liver Tumors: Feasibility, Safety, and Efficacy. J. Vasc. Interv. Radiol. 2020, 31, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Bale, R.; Schullian, P.; Eberle, G.; Putzer, D.; Zoller, H.; Schneeberger, S.; Manzl, C.; Moser, P.; Oberhuber, G. Stereotactic Radiofrequency Ablation of Hepatocellular Carcinoma: A Histopathological Study in Explanted Livers. Hepatology 2019, 70, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Schullian, P.; Laimer, G.; Putzer, D.; Levy, E.; Braunwarth, E.; Stättner, S.; Bale, R. Stereotactic radiofrequency ablation as first-line treatment of recurrent HCC following hepatic resection. Eur. J. Surg. Oncol. 2020, 46, 1503–1509. [Google Scholar] [CrossRef]
- Mahnken, A.H. Leitliniengerechte Anwendung der Thermoablation beim hepatozellulären Karzinom [Guideline-based thermal ablation of hepatocellular carcinoma]. Radiologe 2022, 62, 219–224. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Li, R.; Wang, C.; Wang, J.; Xie, X.; Li, Y.; Li, S.; Mao, X.; Liang, P. Sorafenib as adjuvant therapy following radiofrequency ablation for recurrent hepatocellular carcinoma within Milan criteria: A multicenter analysis. J. Gastroenterol. 2022, 57, 684–694. [Google Scholar] [CrossRef]
- Seidensticker, M.; Öcal, O.; Schütte, K.; Malfertheiner, P.; Berg, T.; Loewe, C.; Klümpen, H.J.; van Delden, O.; Ümütlü, M.R.; Ben Khaled, N.; et al. Impact of adjuvant sorafenib treatment after local ablation for HCC in the phase II SORAMIC trial. JHEP Rep. 2023, 5, 100699. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Abd El Aziz, M.A.; Cincione, I.; Cea, U.V.; Germini, A.; Granieri, S.; Cotsoglou, C.; Sacco, R. Angiotensin Receptor 1 Blockers Prolong Time to Recurrence after Radiofrequency Ablation in Hepatocellular Carcinoma patients: A Retrospective Study. Biomedicines 2020, 8, 399. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Pusceddu, S.; Giacomelli, L.; Ambrosi, A.; Sacco, R. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 3796. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Rhim, H. Thermal ablation for hepatocellular carcinoma: What’s new in 2019. Chin. Clin. Oncol. 2019, 8, 58. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, B.H.; Park, J.W. Overview of Asian clinical practice guidelines for the management of hepatocellular carcinoma: An Asian perspective comparison. Clin. Mol. Hepatol. 2023, 29, 252–262. [Google Scholar] [CrossRef]
- Chang, Y.; Jeong, S.W.; Young Jang, J.; Jae Kim, Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 8165. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Sinn, D.H.; Choi, G.S.; Kim, J.M.; Joh, J.W.; Kang, T.W.; Hyun, D.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; et al. Comparison of outcome between liver resection, radiofrequency ablation, and transarterial therapy for multiple small hepatocellular carcinoma within the Milan criteria. Ann. Surg. Treat. Res. 2020, 99, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, C.J.; Seong, N.J.; Jeong, S.H.; Kim, J.W. Comparison of Combined Therapy Using Conventional Chemoembolization and Radiofrequency Ablation Versus Conventional Chemoembolization for Ultrasound-Invisible Early-Stage Hepatocellular Carcinoma (Barcelona Clinic Liver Cancer Stage 0 or A). Korean J. Radiol. 2018, 19, 1130–1139. [Google Scholar] [CrossRef]
- Lee, I.J.; Lee, J.H.; Lee, Y.B.; Kim, Y.J.; Yoon, J.H.; Yin, Y.H.; Lee, M.; Hur, S.; Kim, H.C.; Jae, H.J.; et al. Effectiveness of drug-eluting bead transarterial chemoembolization versus conventional transarterial chemoembolization for small hepatocellular carcinoma in Child-Pugh class A patients. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866072. [Google Scholar] [CrossRef]
- Lee, I.J.; Chung, J.W.; Yin, Y.H.; Kim, H.C.; Kim, Y.I.; Jae, H.J.; Park, H.J. Cone-Beam Computed Tomography (CBCT) Hepatic Arteriography in Chemoembolization for Hepatocellular Carcinoma: Performance Depicting Tumors and Tumor Feeders. Cardiovasc. Intervent Radiol. 2015, 38, 1218–1230. [Google Scholar] [CrossRef]
- Bzeizi, K.I.; Arabi, M.; Jamshidi, N.; Albenmousa, A.; Sanai, F.M.; Al-Hamoudi, W.; Alghamdi, S.; Broering, D.; Alqahtani, S.A. Conventional Transarterial Chemoembolization Versus Drug-Eluting Beads in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 6172. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, D.Y. Conventional vs. drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J. Hepatol. 2017, 9, 808–814. [Google Scholar] [CrossRef]
- Li, H.; Wu, F.; Duan, M.; Zhang, G. Drug-eluting bead transarterial chemoembolization (TACE) vs. conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: A comparison of efficacy and safety. Medicine 2019, 98, e15314. [Google Scholar] [CrossRef] [PubMed]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc. Intervent Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Golfieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; Gasparini, D.; et al. Randomised controlled trial of doxorubicin-eluting beads vs. conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Burrel, M.; Reig, M.; Forner, A.; Barrufet, M.; de Lope, C.R.; Tremosini, S.; Ayuso, C.; Llovet, J.M.; Real, M.I.; Bruix, J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J. Hepatol. 2012, 56, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Byrne, T.J.; Rakela, J. Loco-regional therapies for patients with hepatocellular carcinoma awaiting liver transplantation: Selecting an optimal therapy. World J. Transplant. 2016, 6, 306–313. [Google Scholar] [CrossRef]
- Chapman, W.C.; Majella Doyle, M.B.; Stuart, J.E.; Vachharajani, N.; Crippin, J.S.; Anderson, C.D.; Lowell, J.A.; Shenoy, S.; Darcy, M.D.; Brown, D.B. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann. Surg. 2008, 248, 617–625. [Google Scholar] [CrossRef]
- Affonso, B.B.; Galastri, F.L.; da Motta Leal Filho, J.M.; Nasser, F.; Falsarella, P.M.; Cavalcante, R.N.; de Almeida, M.D.; Felga, G.E.G.; Valle, L.G.M.; Wolosker, N. Long-term outcomes of hepatocellular carcinoma that underwent chemoembolization for bridging or downstaging. World J. Gastroenterol. 2019, 25, 5687–5701. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Guo, R.P.; Lai, E.C.; Zhang, Y.J.; Lau, W.Y.; Chen, M.S.; Shi, M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: A prospective comparative study. Ann. Surg. Oncol. 2011, 18, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Miller, F.H.; Lopes Vendrami, C.; Gabr, A.; Horowitz, J.M.; Kelahan, L.C.; Riaz, A.; Salem, R.; Lewandowski, R.J. Evolution of Radioembolization in Treatment of Hepatocellular Carcinoma: A Pictorial Review. Radiographics 2021, 41, 1802–1818. [Google Scholar] [CrossRef]
- Torimura, T.; Iwamoto, H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2022, 42, 2042–2054. [Google Scholar] [CrossRef]
- Yim, S.Y.; Chun, H.S.; Lee, J.S.; Lim, J.H.; Kim, T.H.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Kim, G.M.; et al. Transarterial Radioembolization for Unresectable Hepatocellular Carcinoma: Real-Life Efficacy and Safety Analysis of Korean Patients. Cancers 2022, 14, 385. [Google Scholar] [CrossRef]
- Liebl, M.; Pedersoli, F.; Zimmermann, M.; Schulze-Hagen, M.; Truhn, D.; Sieben, P.; von Stillfried, S.; Tschinaev, A.; Heinzel, A.; Kuhl, C.K.; et al. Induction of Contralateral Hepatic Hypertrophy by Unilobar Yttrium-90 Transarterial Radioembolization versus Portal Vein Embolization: An Animal Study. J. Vasc. Interv. Radiol. 2021, 32, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Padia, S.A.; Kwan, S.W.; Roudsari, B.; Monsky, W.L.; Coveler, A.; Harris, W.P. Superselective yttrium-90 radioembolization for hepatocellular carcinoma yields high response rates with minimal toxicity. J. Vasc. Interv. Radiol. 2014, 25, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, V.; Miura, K.; Kuemmerli, C.; Capel, A.; Eshmuminov, D.; Ferreras, D.; Baroja-Mazo, A.; Cascales-Campos, P.; Jiménez-Mascuñán, M.I.; Pons, J.A.; et al. Selecting the Appropriate Downstaging and Bridging Therapies for Hepatocellular Carcinoma: What Is the Role of Transarterial Radioembolization? A Pooled Analysis. Cancers 2023, 15, 2122. [Google Scholar] [CrossRef]
- Ljuboja, D.; Ahmed, M.; Ali, A.; Perez, E.; Subrize, M.W.; Kaplan, R.S.; Sarwar, A. Time-Driven Activity-Based Costing in Interventional Oncology: Cost Measurement and Cost Variability for Hepatocellular Carcinoma Therapies. J. Am. Coll. Radiol. 2021, 18, 1095–1105. [Google Scholar] [CrossRef]
- Gabr, A.; Ranganathan, S.; Mouli, S.K.; Riaz, A.; Gates, V.L.; Kulik, L.; Ganger, D.; Maddur, H.; Moore, C.; Hohlastos, E.; et al. Streamlining radioembolization in UNOS T1/T2 hepatocellular carcinoma by eliminating lung shunt estimation. J. Hepatol. 2020, 72, 1151–1158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iezzi, R.; Posa, A.; Contegiacomo, A.; Lee, I.J.; Bale, R.; Tanzilli, A.; Tenore, L.; Giuliante, F.; Gasbarrini, A.; Goldberg, S.N.; et al. Early-Stage HCC Percutaneous Locoregional Management: East versus West Perspectives. Cancers 2023, 15, 3988. https://doi.org/10.3390/cancers15153988
Iezzi R, Posa A, Contegiacomo A, Lee IJ, Bale R, Tanzilli A, Tenore L, Giuliante F, Gasbarrini A, Goldberg SN, et al. Early-Stage HCC Percutaneous Locoregional Management: East versus West Perspectives. Cancers. 2023; 15(15):3988. https://doi.org/10.3390/cancers15153988
Chicago/Turabian StyleIezzi, Roberto, Alessandro Posa, Andrea Contegiacomo, In Joon Lee, Reto Bale, Alessandro Tanzilli, Lorenzo Tenore, Felice Giuliante, Antonio Gasbarrini, Shraga Nahum Goldberg, and et al. 2023. "Early-Stage HCC Percutaneous Locoregional Management: East versus West Perspectives" Cancers 15, no. 15: 3988. https://doi.org/10.3390/cancers15153988
APA StyleIezzi, R., Posa, A., Contegiacomo, A., Lee, I. J., Bale, R., Tanzilli, A., Tenore, L., Giuliante, F., Gasbarrini, A., Goldberg, S. N., Jakobs, T., Pompili, M., Bargellini, I., Sala, E., & Kim, H.-C. (2023). Early-Stage HCC Percutaneous Locoregional Management: East versus West Perspectives. Cancers, 15(15), 3988. https://doi.org/10.3390/cancers15153988








