Correlation between [68Ga]Ga-FAPI-46 PET Imaging and HIF-1α Immunohistochemical Analysis in Cervical Cancer: Proof-of-Concept
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. [68Ga]Ga-FAPI PET/CT Procedure
2.3. Image Analysis
2.4. Immunohistochemistry with HIF-1
2.5. Statistical Analyses
3. Results
3.1. Demographic Data
3.2. PET/CT Findings
3.3. Immunohistochemistry Findings
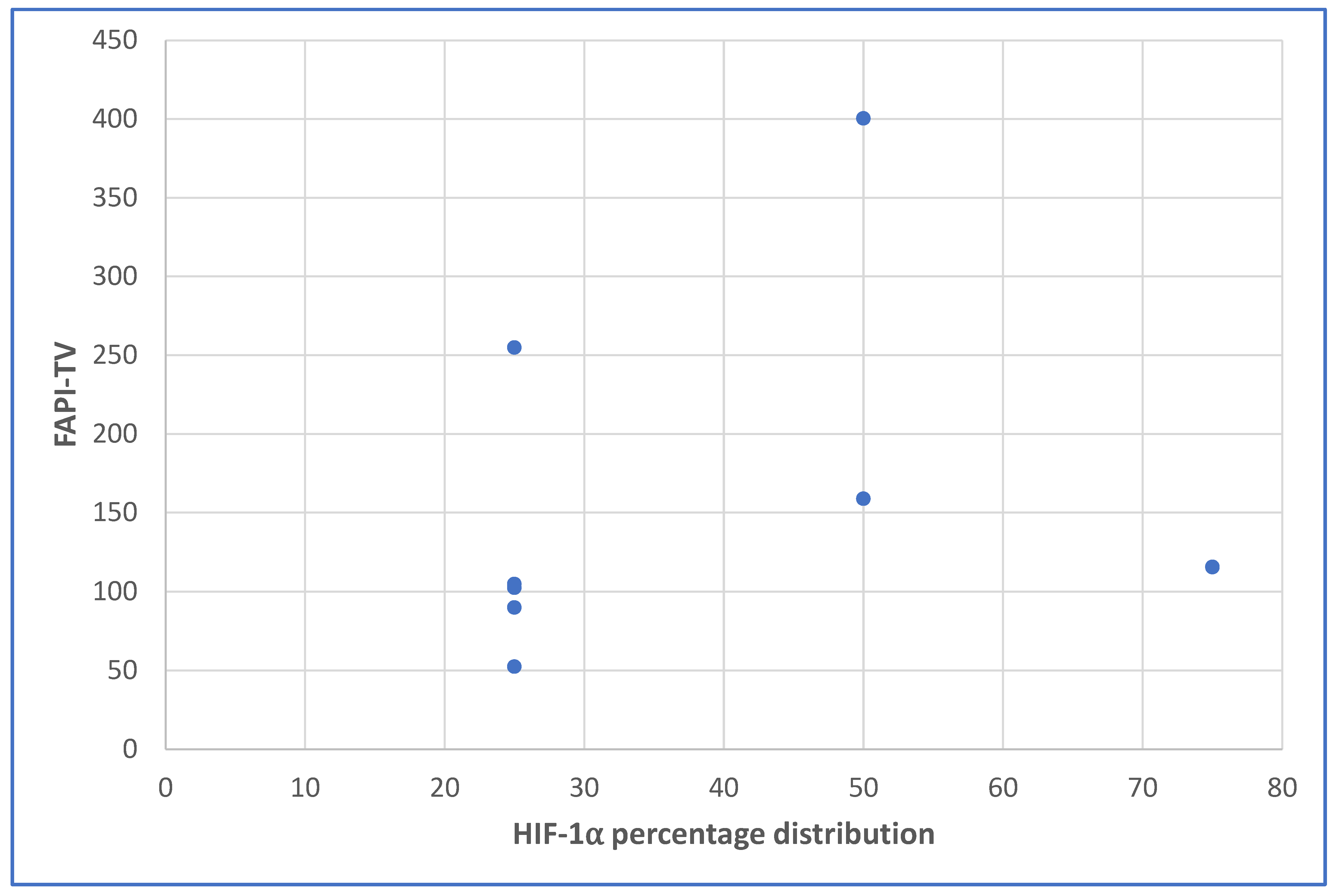
3.4. Relationships between the Different Variables Studied
3.5. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Wanandi, S.I.; Ningsih, S.S.; Asikin, H.; Hosea, R.; Neolaka, G.M.G. Metabolic Interplay between Tumour Cells and Cancer-Associated Fibroblasts (CAFs) under Hypoxia versus Normoxia. Malays. J. Med. Sci. 2018, 25, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Fullár, A.; Dudás, J.; Oláh, L.; Hollósi, P.; Papp, Z.; Sobel, G.; Karászi, K.; Paku, S.; Baghy, K.; Kovalszky, I. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC Cancer 2015, 15, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.H.; Chung, J.-Y.; Kim, J.-H.; Kim, B.-G.; Hewitt, S.M. Expression of fibroblast growth factor receptor family members is associated with prognosis in early stage cervical cancer patients. J. Transl. Med. 2016, 14, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.-J.; Yang, Y.; Wei, W.-F.; Wu, X.-G.; Yan, R.-M.; Zhou, C.-F.; Chen, X.-J.; Wu, S.; Wang, W.; Fan, L.-S. Tumor-secreted exosomal Wnt2B activates fibroblasts to promote cervical cancer progression. Oncogenesis 2021, 10, 30. [Google Scholar] [CrossRef]
- Murata, T.; Mizushima, H.; Chinen, I.; Moribe, H.; Yagi, S.; Hoffman, R.M.; Kimura, T.; Yoshino, K.; Ueda, Y.; Enomoto, T.; et al. HB-EGF and PDGF Mediate Reciprocal Interactions of Carcinoma Cells with Cancer-Associated Fibroblasts to Support Progression of Uterine Cervical Cancers. Cancer Res. 2011, 71, 6633–6642. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Choi, S.; Yoo, S.; Lee, M.; Kim, I.S. Cancer-Associated Fibroblasts in the Hypoxic Tumor Microenvironment. Cancers 2022, 14, 3321. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [Green Version]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Kugeratski, F.G.; Atkinson, S.J.; Neilson, L.J.; Lilla, S.; Knight, J.R.P.; Serneels, J.; Juin, A.; Ismail, S.; Bryant, D.M.; Markert, E.K.; et al. Hypoxic cancer-associated fibroblasts increase NCBP2-AS2/HIAR to promote endothelial sprouting through enhanced VEGF signaling. Sci. Signal. 2019, 12, eaan8247. [Google Scholar] [CrossRef] [Green Version]
- Ziani, L.; Buart, S.; Chouaib, S.; Thiery, J. Hypoxia increases melanoma-associated fibroblasts immunosuppressive potential and inhibitory effect on T cell-mediated cytotoxicity. Oncoimmunology 2021, 10, 1950953. [Google Scholar] [CrossRef] [PubMed]
- Simkova, A.; Busek, P.; Sedo, A.; Konvalinka, J. Molecular recognition of fibroblast activation protein for diagnostic and therapeutic applications. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140409. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Rohrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jager, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Dendl, K.; Koerber, S.A.; Kratochwil, C.; Cardinale, J.; Finck, R.; Dabir, M.; Novruzov, E.; Watabe, T.; Kramer, V.; Choyke, P.L.; et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers 2021, 13, 4946. [Google Scholar] [CrossRef]
- Gu, B.; Liu, X.; Wang, S.; Xu, X.; Liu, X.; Hu, S.; Yan, W.; Luo, Z.; Song, S. Head-to-head evaluation of [18F]FDG and [68Ga]Ga-DOTA-FAPI-04 PET/CT in recurrent soft tissue sarcoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2889–2901. [Google Scholar] [CrossRef]
- Wegen, S.; van Heek, L.; Linde, P.; Claus, K.; Akuamoa-Boateng, D.; Baues, C.; Sharma, S.J.; Schomacker, K.; Fischer, T.; Roth, K.S.; et al. Head-to-Head Comparison of [68Ga]Ga-FAPI-46-PET/CT and [18F]F-FDG-PET/CT for Radiotherapy Planning in Head and Neck Cancer. Mol. Imaging Biol. 2022, 24, 986–994. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, L.; Feng, Y.; Wang, L.; Chen, Y. Comparison of 68 Ga-FAPI-04 and fluorine-18-fluorodeoxyglucose PET/computed tomography in the detection of ovarian malignancies. Nucl. Med. Commun. 2023, 44, 194–203. [Google Scholar] [CrossRef]
- Liu, S.; Feng, Z.; Xu, X.; Ge, H.; Ju, X.; Wu, X.; Song, S. Head-to-head comparison of [18F]-FDG and [68 Ga]-DOTA-FAPI-04 PET/CT for radiological evaluation of platinum-sensitive recurrent ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1521–1531. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Li, Y. [68Ga]Ga-FAPI-04 PET MRI/CT in the evaluation of gastric carcinomas compared with [18F]-FDG PET MRI/CT: A meta-analysis. Eur. J. Med. Res. 2023, 28, 34. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, W.; Ren, S.; Kong, Y.; Huang, Q.; Zhao, J.; Guan, Y.; Jia, H.; Chen, J.; Lu, L.; et al. 68Ga-FAPI-04 Versus 18F-FDG PET/CT in the Detection of Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 693640. [Google Scholar] [CrossRef]
- Novruzov, E.; Dendl, K.; Ndlovu, H.; Choyke, P.L.; Dabir, M.; Beu, M.; Novruzov, F.; Mehdi, E.; Guliyev, F.; Koerber, S.A.; et al. Head-to-head Intra-individual Comparison of [68Ga]-FAPI and [18F]-FDG PET/CT in Patients with Bladder Cancer. Mol. Imaging Biol. 2022, 24, 651–658. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Q.; Yang, H.; Peng, L.; Zhang, W.; Li, F. Fibroblast Activation Protein-Targeted PET/CT with 68Ga-FAPI for Imaging IgG4-Related Disease: Comparison to 18F-FDG PET/CT. J. Nucl. Med. 2021, 62, 266–271. [Google Scholar] [CrossRef]
- Wegen, S.; Roth, K.S.; Weindler, J.; Claus, K.; Linde, P.; Trommer, M.; Akuamoa-Boateng, D.; van Heek, L.; Baues, C.; Schomig-Markiefka, B.; et al. First Clinical Experience With [68Ga]Ga-FAPI-46-PET/CT Versus [18F]F-FDG PET/CT for Nodal Staging in Cervical Cancer. Clin. Nucl. Med. 2023, 48, 150–155. [Google Scholar] [CrossRef]
- Mokoala, K.M.G.; Lawal, I.O.; Maserumule, L.C.; Hlongwa, K.N.; Ndlovu, H.; Reed, J.; Bida, M.; Maes, A.; van de Wiele, C.; Mahapane, J.; et al. A Prospective Investigation of Tumor Hypoxia Imaging with 68Ga-Nitroimidazole PET/CT in Patients with Carcinoma of the Cervix Uteri and Comparison with 18F-FDG PET/CT: Correlation with Immunohistochemistry. J. Clin. Med. 2022, 11, 962. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Lappano, R.; Santolla, M.F.; Marsico, S.; Caruso, A.; Maggiolini, M. HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs). Breast Cancer Res. 2013, 15, R64. [Google Scholar] [CrossRef]
- Hockel, M.; Knoop, C.; Schlenger, K.; Vorndran, B.; Knapstein, P.G.; Vaupel, P. Intratumoral pO2 histography as predictive assay in advanced cancer of the uterine cervix. Adv. Exp. Med. Biol. 1994, 345, 445–450. [Google Scholar] [PubMed]
- Hockel, M.; Schlenger, K.; Knoop, C.; Vaupel, P. Oxygenation of carcinomas of the uterine cervix: Evaluation by computerized O2 tension measurements. Cancer Res. 1991, 51, 6098–6102. [Google Scholar] [PubMed]
- Fiori, M.E.; Di Franco, S.; Villanova, L.; Bianca, P.; Stassi, G.; De Maria, R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol. Cancer 2019, 18, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the antitumor immune response by cancer-associated fibroblasts. Front. Immunol. 2018, 9, 414. [Google Scholar] [CrossRef]
- Kwa, M.Q.; Herum, K.M.; Brakebusch, C. Cancer-associated fibroblasts: How do they contribute to metastasis? Clin. Exp. Metastasis 2019, 36, 71–86. [Google Scholar] [CrossRef]
- Adams, M.C.; Turkington, T.G.; Wilson, J.M.; Wong, T.Z. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am. J. Roentgenol. 2010, 195, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Lodge, M.A.; Chaudhry, M.A.; Wahl, R.L. Noise considerations for PET quantification using maximum and peak standardized uptake value. J. Nucl. Med. 2012, 53, 1041–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, I.A.; Huser, D.M.; Burger, C.; von Schulthess, G.K.; Buck, A. Repeatability of FDG quantification in tumor imaging: Averaged SUVs are superior to SUVmax. Nucl. Med. Biol. 2012, 39, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Choi, J.Y.; Moon, S.H.; Bae, D.S.; Park, S.B.; Choe, Y.S.; Lee, K.H.; Kim, B.T. Prognostic significance of volume-based metabolic parameters in uterine cervical cancer determined using 18F-fluorodeoxyglucose positron emission tomography. Int. J. Gynecol. Cancer 2012, 22, 1226–1233. [Google Scholar] [CrossRef]
- Im, H.J.; Bradshaw, T.; Solaiyappan, M.; Cho, S.Y. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One is Better? Nucl. Med. Mol. Imaging 2018, 52, 5–15. [Google Scholar] [CrossRef]
- Zschaeck, S.; Li, Y.; Lin, Q.; Beck, M.; Amthauer, H.; Bauersachs, L.; Hajiyianni, M.; Rogasch, J.; Ehrhardt, V.H.; Kalinauskaite, G.; et al. Prognostic value of baseline [18F]-fluorodeoxyglucose positron emission tomography parameters MTV, TLG and asphericity in an international multicenter cohort of nasopharyngeal carcinoma patients. PLoS ONE 2020, 15, e0236841. [Google Scholar] [CrossRef]
- Ntekim, A.; Campbell, O.; Rothenbacher, D. Optimal management of cervical cancer in HIV-positive patients: A systematic review. Cancer Med. 2015, 4, 1381–1393. [Google Scholar] [CrossRef]
- Maiman, M.; Fruchter, R.G.; Serur, E.; Remy, J.C.; Feuer, G.; Boyce, J. Human immunodeficiency virus infection and cervical neoplasia. Gynecol. Oncol. 1990, 38, 377–382. [Google Scholar] [CrossRef]
- Lawal, I.O.; Nyakale, N.E.; Harry, L.M.; Modiselle, M.R.; Ankrah, A.O.; Msomi, A.P.; Mokgoro, N.P.; Boshomane, T.G.; de Wiele, C.V.; Sathekge, M.M. The role of F-18 FDG PET/CT in evaluating the impact of HIV infection on tumor burden and therapy outcome in patients with Hodgkin lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2025–2033. [Google Scholar] [CrossRef]
- Mokoala, K.M.G.; Lawal, I.O.; Lengana, T.; Popoola, G.O.; Boshomane, T.M.G.; Mokgoro, N.P.; Vorster, M.; Sathekge, M.M. The Association of Tumor Burden by 18F-FDG PET/CT and Survival in Vulvar Carcinoma. Clin. Nucl. Med. 2021, 46, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.O.; Ankrah, A.O.; Mokoala, K.M.G.; Popoola, G.O.; Kaoma, C.A.; Maes, A.; Mokgoro, N.P.; Van de Wiele, C.; Sathekge, M.M. Prognostic Value of Pre-treatment F-18 FDG PET Metabolic Metrics in Patients with Locally Advanced Carcinoma of the Anus with and without HIV Infection. Nuklearmedizin 2018, 57, 190–197. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Finck, R.; Mokoala, K.M.G.; Staudinger, F.; Schillings, L.; Heger, U.; Röhrich, M.; Kratochwil, C.; Sathekge, M.; et al. 68Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4089–4100. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.; Freudlsperger, C.; Plinkert, P.; Debus, J. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- Dendl, K.; Koerber, S.A.; Tamburini, K.; Mori, Y.; Cardinale, J.; Haberkorn, U.; Giesel, F.L. Advancement and Future Perspective of FAPI PET/CT In Gynecological Malignancies. Semin. Nucl. Med. 2022, 52, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, W.; Qin, C.; Song, Y.; Liu, F.; Hu, F.; Lan, X. Uterine Uptake of 68Ga-FAPI-04 in Uterine Pathology and Physiology. Clin. Nucl. Med. 2022, 47, 7–13. [Google Scholar] [CrossRef]
- Cysouw, M.C.F.; Jansen, B.H.E.; Yaqub, M.; Voortman, J.; Vis, A.N.; van Moorselaar, R.J.A.; Hoekstra, O.S.; Boellaard, R.; Oprea-Lager, D.E. Letter to the Editor re: Semiquantitative Parameters in PSMA-Targeted PET Imaging with [18F]DCFPyL: Impact of Tumor Burden on Normal Organ Uptake. Mol. Imaging Biol. 2020, 22, 15–17. [Google Scholar] [CrossRef]
- Ortega, C.; Wong, R.K.; Schaefferkoetter, J.; Veit-Haibach, P.; Myrehaug, S.; Juergens, R.; Laidley, D.; Anconina, R.; Liu, Z.A.; Metser, U. Quantitative 68Ga-DOTATATE PET/CT parameters for the prediction of therapy response in patients with progressive metastatic neuroendocrine tumors treated with 177Lu-DOTATATE. J. Nucl. Med. 2021, 62, 1406–1414. [Google Scholar] [CrossRef]




| Patient No. | Age (Years) | Histological Subtype | FAPI-TV | Immunohistochemistry Staining Intensity of HIF-1alpha | Immunohistochemistry Staining Percentage Distribution | Metastases |
|---|---|---|---|---|---|---|
| 1 | 63 | Papillary serous ca | 414.66 | N/A | LN & Bone | |
| 2 | 62 | SCC | 102.47 | 1 | 25% | LN, lung & liver |
| 3 | 35 | SCC | 89.95 | 2 | 25% | LN |
| 4 | 42 | SCC | 158.90 | 2 | 50% | LN & Bone |
| 5 | 38 | SCC | 254.92 | 1 | 25% | LN |
| 6 | 39 | SCC | 52.39 | 1 | 25% | LN |
| 7 | 37 | SCC | 400.35 | 2 | 50% | LN & Bone |
| 8 | 36 | SCC | 262.50 | N/A | LN | |
| 9 | 50 | SCC | 115.54 | 3 | 75% | LN, liver, spleen & bone |
| 10 | 68 | Adenocarcinoma | 104.88 | 1 | 25% | LN & liver |
| [18F]F-FDG PET/CT | [68Ga]Ga-FAPI PET/CT | |||||
|---|---|---|---|---|---|---|
| Patient No. | SUVmax | SUVmean | MTV | SUVmax | SUVmean | FAPI-TV |
| 2 | 13.16 | 5.39 | 110.23 | 10.46 | 5.29 | 102.47 |
| 3 | 19.47 | 6.6 | 111.51 | 29.02 | 4.79 | 89.95 |
| 4 | 14.4 | 6.21 | 158.91 | 15.64 | 6.29 | 158.90 |
| 5 | 16.41 | 5.89 | 204.32 | 19.32 | 5.54 | 254.92 |
| 6 | 18.21 | 7.52 | 88.13 | 29.21 | 9.93 | 52.39 |
| 7 | 19.43 | 8.54 | 359.09 | 23.48 | 7.83 | 400.35 |
| 9 | 25.09 | 6.6 | 140.75 | 22.79 | 5.93 | 115.53 |
| 10 | 16.95 | 6.28 | 111.93 | 22.47 | 5.25 | 104.88 |
| Intensity of HIF-1α on Staining | Percentage Distribution of HIF-1α Staining | |||
|---|---|---|---|---|
| Variable | r | p Value | r | p Value |
| Age | −0.287 | 0.491 | 0.014 | 0.974 |
| Primary SUVmax | −0.300 | 0.470 | 0.027 | 0.948 |
| Primary SUVmean | −0.117 | 0.782 | 0.220 | 0.601 |
| Primary FAPI-TV | 0.300 | 0.470 | 0.550 | 0.158 |
| Visceral metastasis | −0.062 | 0.885 | 0.065 | 0.878 |
| Skeletal metastasis | 0.690 | 0.058 | 0.800 | 0.017 |
| Patient No. | FAPI-TV | Immunohistochemistry Staining Intensity of HIF-1α | Immunohistochemistry Percentage Distribution | Metastases | Alive (Yes/No) |
|---|---|---|---|---|---|
| 2 | 102.47 | 1 | 25% | LN, Lung & liver | No |
| 3 | 89.95 | 2 | 25% | LN | Yes |
| 4 | 158.90 | 2 | 50% | LN & Bone | No |
| 5 | 254.92 | 1 | 25% | LN | No |
| 6 | 52.39 | 1 | 25% | LN | Yes |
| 7 | 400.35 | 2 | 50% | LN & Bone | No |
| 9 | 115.54 | 3 | 75% | LN, Liver, Spleen & Bone | No |
| 10 | 104.88 | 1 | 25% | LN & Liver | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokoala, K.M.G.; Lawal, I.O.; Maserumule, L.C.; Bida, M.; Maes, A.; Ndlovu, H.; Reed, J.; Mahapane, J.; Davis, C.; Van de Wiele, C.; et al. Correlation between [68Ga]Ga-FAPI-46 PET Imaging and HIF-1α Immunohistochemical Analysis in Cervical Cancer: Proof-of-Concept. Cancers 2023, 15, 3953. https://doi.org/10.3390/cancers15153953
Mokoala KMG, Lawal IO, Maserumule LC, Bida M, Maes A, Ndlovu H, Reed J, Mahapane J, Davis C, Van de Wiele C, et al. Correlation between [68Ga]Ga-FAPI-46 PET Imaging and HIF-1α Immunohistochemical Analysis in Cervical Cancer: Proof-of-Concept. Cancers. 2023; 15(15):3953. https://doi.org/10.3390/cancers15153953
Chicago/Turabian StyleMokoala, Kgomotso M. G., Ismaheel O. Lawal, Letjie C. Maserumule, Meshack Bida, Alex Maes, Honest Ndlovu, Janet Reed, Johncy Mahapane, Cindy Davis, Christophe Van de Wiele, and et al. 2023. "Correlation between [68Ga]Ga-FAPI-46 PET Imaging and HIF-1α Immunohistochemical Analysis in Cervical Cancer: Proof-of-Concept" Cancers 15, no. 15: 3953. https://doi.org/10.3390/cancers15153953
APA StyleMokoala, K. M. G., Lawal, I. O., Maserumule, L. C., Bida, M., Maes, A., Ndlovu, H., Reed, J., Mahapane, J., Davis, C., Van de Wiele, C., Popoola, G., Giesel, F. L., Vorster, M., & Sathekge, M. M. (2023). Correlation between [68Ga]Ga-FAPI-46 PET Imaging and HIF-1α Immunohistochemical Analysis in Cervical Cancer: Proof-of-Concept. Cancers, 15(15), 3953. https://doi.org/10.3390/cancers15153953








