Gastric Intestinal Metaplasia: Challenges and the Opportunity for Precision Prevention
Abstract
:Simple Summary
Abstract
1. Introduction
2. Background
2.1. Global Burden of Gastric Cancer
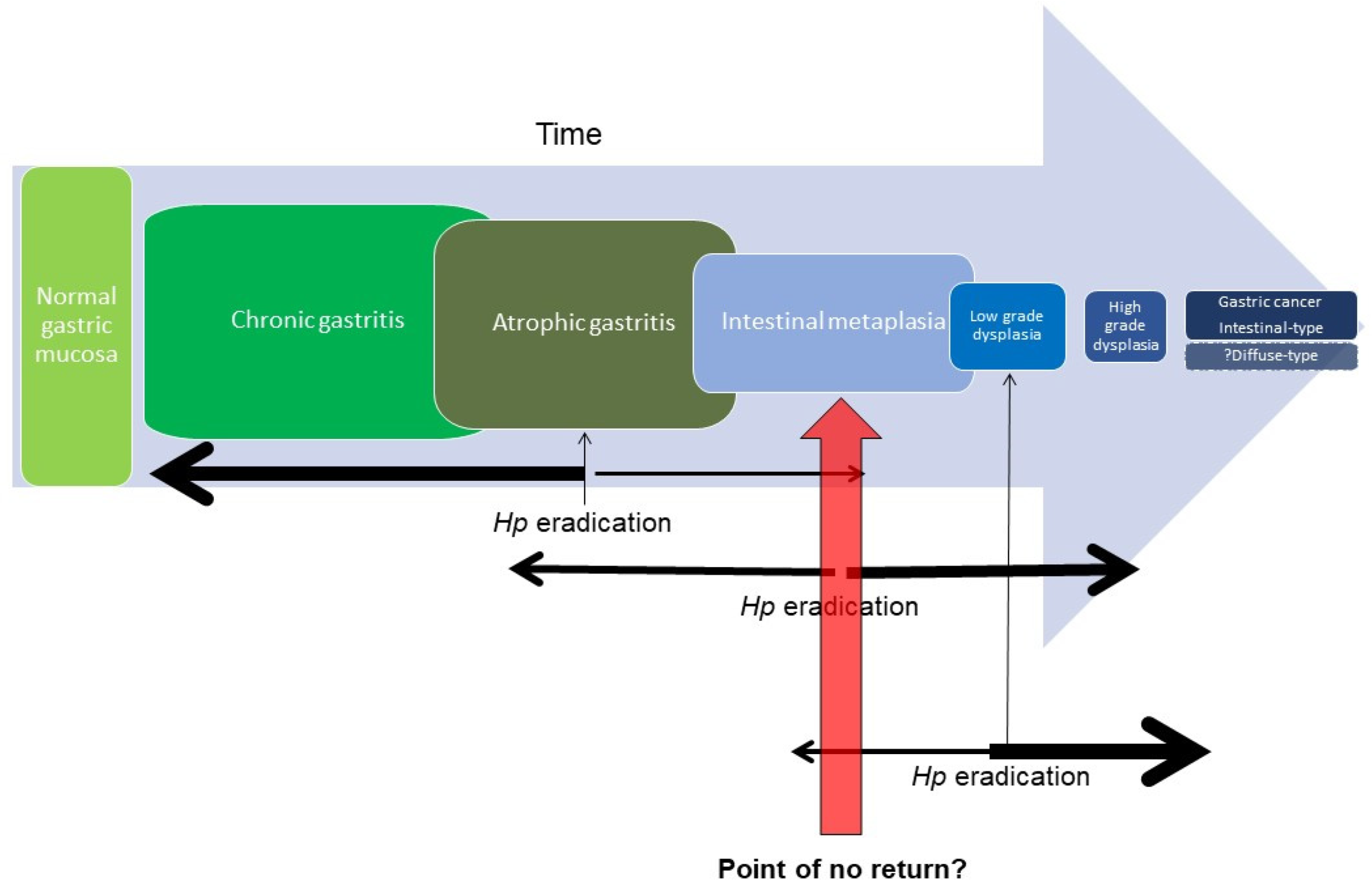
2.2. Gastric Intestinal Metaplasia as a Premalignant State
3. Predictors of Progression of Gastric IM
3.1. Clinical Features
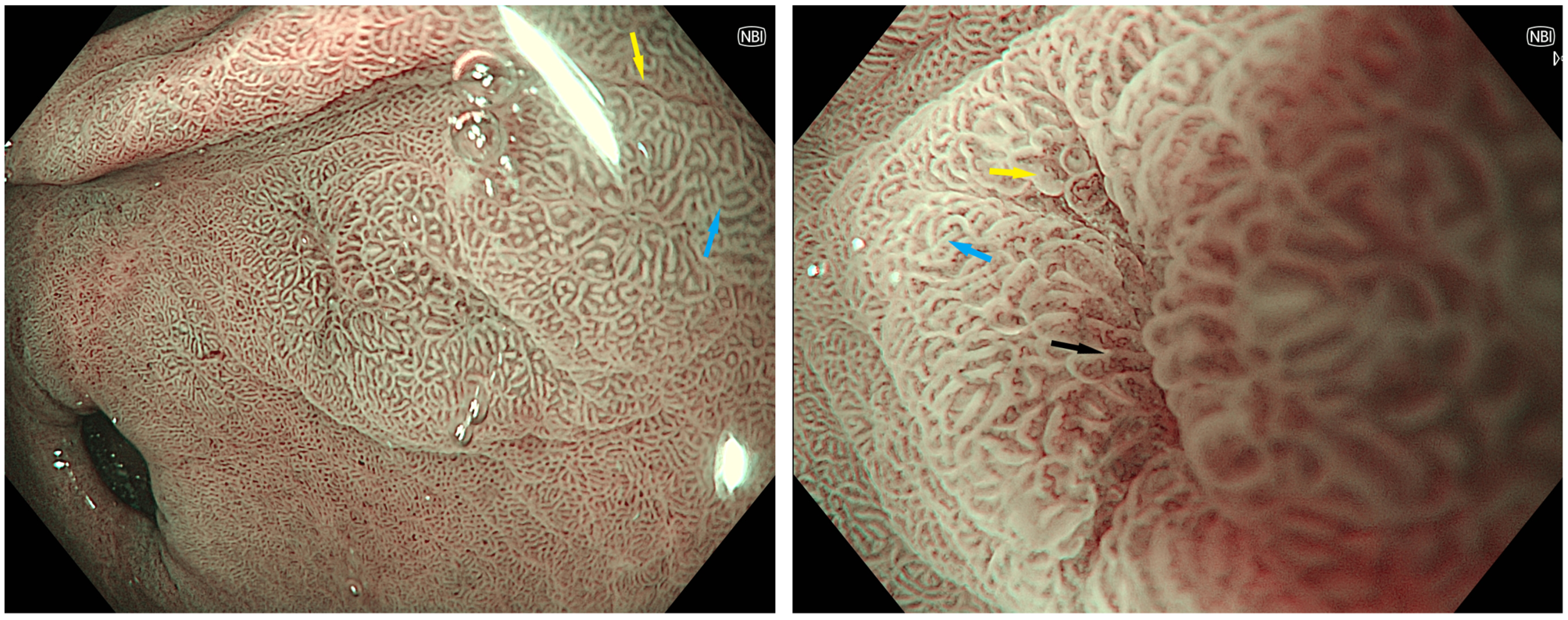
3.2. Endoscopic Features
| Study | Participants | Study Design | Endoscope | Magnification | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Uedo et al. (2006) [75] | 107 Japan | Prospective | GIF-Q240Z | 80× (optical) | 89% (83–96%) | 93% (88–97%) |
| An et al. (2012) [62] | 47 South Korea | Prospective | GIF-H260Z | 85× (optical) | 72.1% | 96.0% |
| Savarino et al. (2013) [76] | 100 Italy | Prospective | GIF-Q160Z | 115× (optical) | 80% (67–92%) | 96% (93–99%) |
| Ang et al. (2015) [65] | 458 Asia Pacific | Prospective | GIF-290 and 190 | Unclear | 92.3% (80.6–97.5%) | 94.3% (85.3–98.3%) |
| Drasovean et al. (2018) [77] | 59 Romania | Prospective | HQ-190 | 150× (optical) | 80.43% (70.9–88%) | 80% (69.9–87.9%) |
| Sobrino-Cossio et al. (2018) [69] | 338 Mexico | Retrospective | H-180 | 1.5× (digital) | 85% (76.7–91.4%) | 98% (96.7–98.9%) |
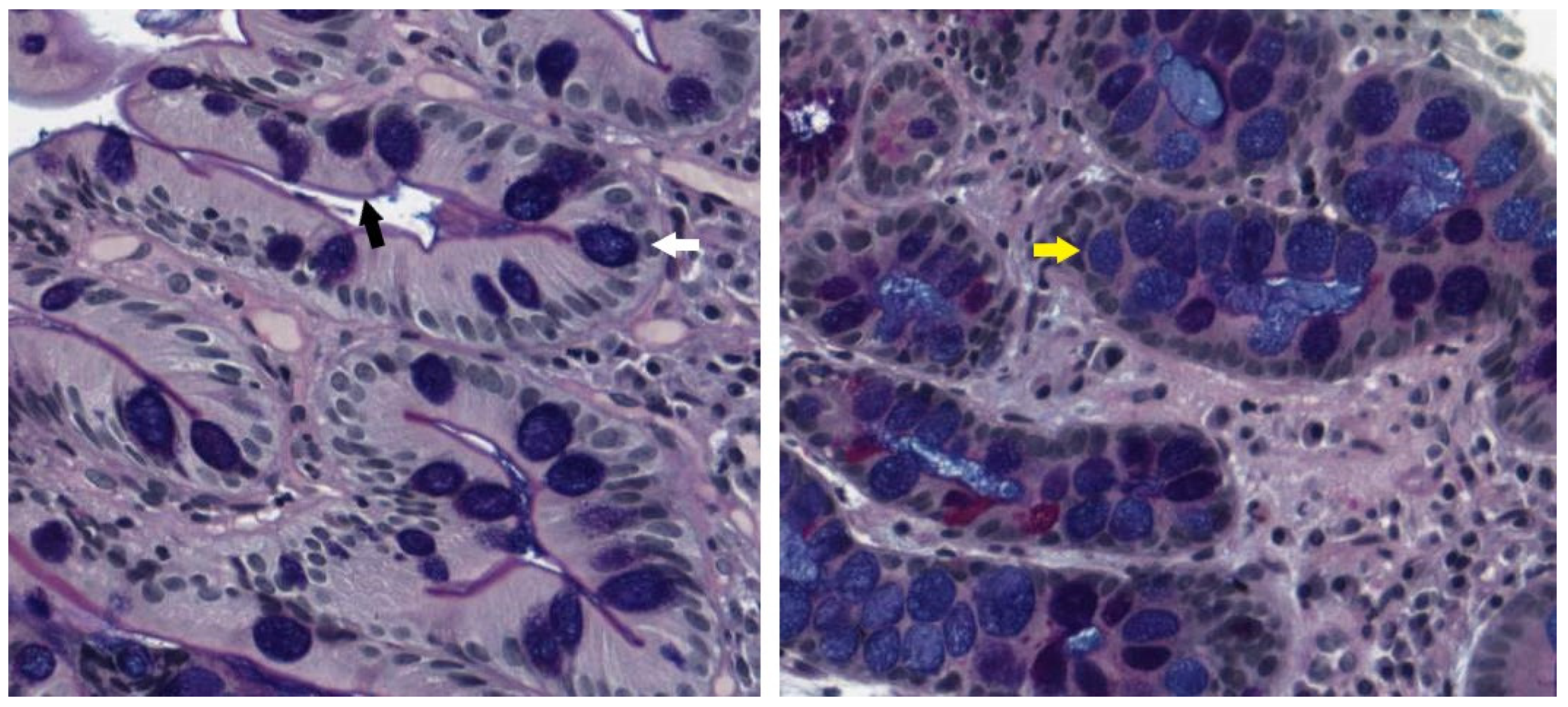
3.3. Histologic Features
3.4. Current Guidelines
4. Future Directions
4.1. Approaches to Population-Level Screening and Surveillance
4.2. Artificial Intelligence and Machine Learning
4.3. Biomarkers
4.4. Genomic, Epigenomic and Molecular Markers Uncover the Pathogenesis of IM and Stratify Risk
4.5. Microbiome and the Immune Landscape
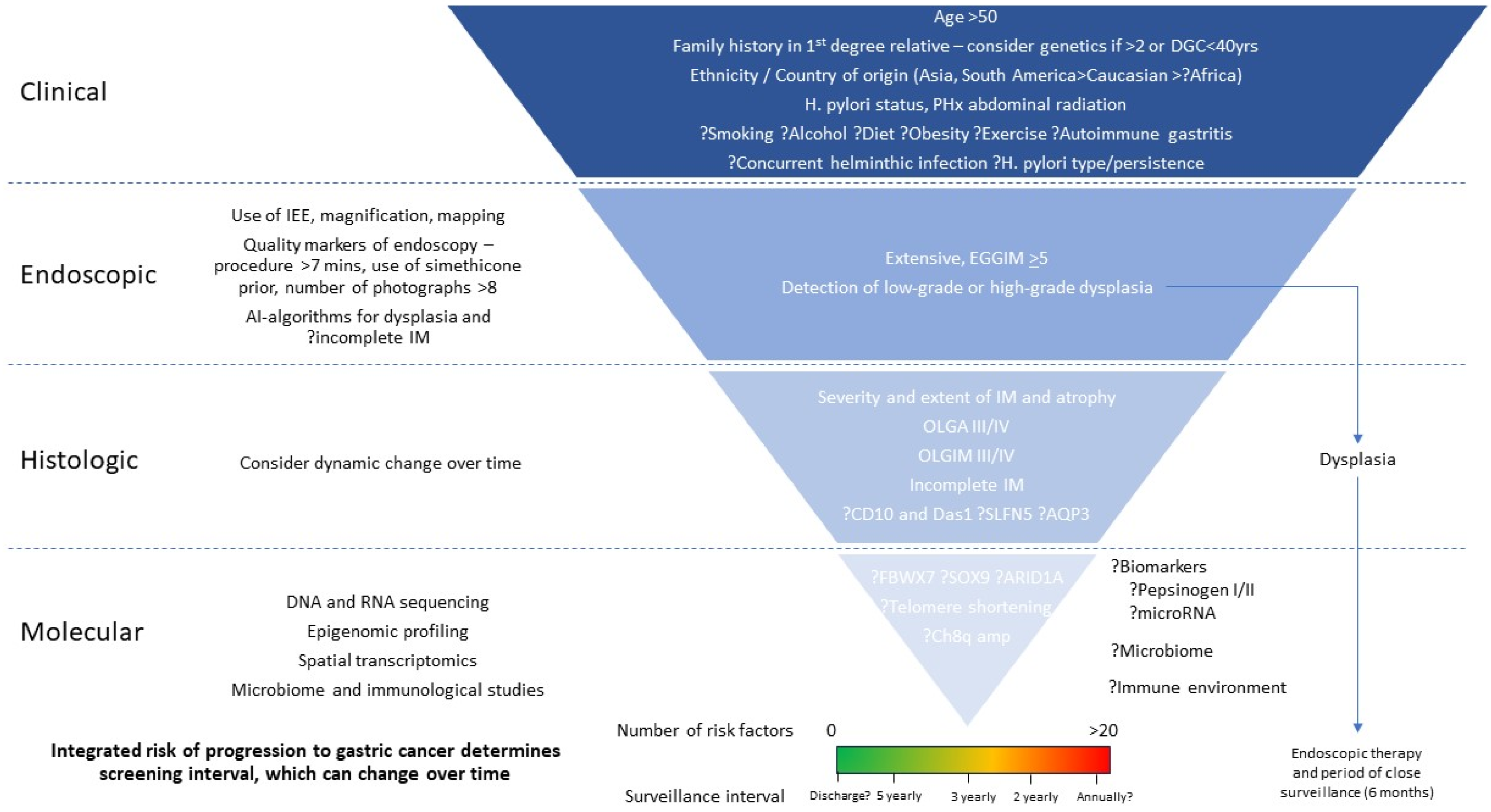
4.6. Utilising Integrated Variables to Increase Precision of Risk Prediction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: A population-based modelling study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.V.; Harper, A.S.; Sung, H.; Jemal, A.; Fidler-Benaoudia, M.M. Emerging cancer incidence trends in Canada: The growing burden of young adult cancers. Cancer 2020, 126, 4553–4562. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer. Polychlorinated Dibenzo-Para-Dioxins and Polychlorinated Dibenzofurans. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1997. [Google Scholar]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [Green Version]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.G.; Beck, P.; Dangler, C.A.; Whary, M.T.; Wang, T.C.; Shi, H.N.; Nagler-Anderson, C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 2000, 6, 536–542. [Google Scholar] [CrossRef]
- Holcombe, C. Helicobacter pylori: The African enigma. Gut 1992, 33, 429–431. [Google Scholar] [CrossRef] [Green Version]
- Kidd, M.; Louw, J.A.; Marks, I.N. Helicobacter pylori in Africa: Observations on an ‘enigma within an enigma’. J. Gastroenterol. Hepatol. 1999, 14, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Mukaisho, K.; Nakayama, T.; Hagiwara, T.; Hattori, T.; Sugihara, H. Two distinct etiologies of gastric cardia adenocarcinoma: Interactions among pH, Helicobacter pylori, and bile acids. Front. Microbiol. 2015, 6, 412. [Google Scholar] [CrossRef] [Green Version]
- Berlth, F.; Bollschweiler, E.; Drebber, U.; Hoelscher, A.H.; Moenig, S. Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value. World J. Gastroenterol. 2014, 20, 5679–5684. [Google Scholar] [CrossRef]
- Qiu, M.-Z.; Cai, M.-Y.; Zhang, D.-S.; Wang, Z.-Q.; Wang, D.-S.; Li, Y.-H.; Xu, R.-H. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J. Transl. Med. 2013, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Fang, W.L.; Wang, R.F.; Liu, C.A.; Yang, M.H.; Lo, S.S.; Wu, C.W.; Li, A.F.; Shyr, Y.M.; Huang, K.H. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol. Oncol. Res. 2016, 22, 197–202. [Google Scholar] [CrossRef]
- Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [CrossRef] [Green Version]
- Hu, H.-M.; Tsai, H.-J.; Ku, H.-Y.; Lo, S.-S.; Shan, Y.-S.; Chang, H.-C.; Chao, Y.; Chen, J.-S.; Chen, S.-C.; Chiang, C.-J.; et al. Survival outcomes of management in metastatic gastric adenocarcinoma patients. Sci. Rep. 2021, 11, 23142. [Google Scholar] [CrossRef]
- Sano, T.; Katai, H.; Sasako, M.; Maruyama, K. The management of early gastric cancer. Surg. Oncol. 2000, 9, 17–22. [Google Scholar] [CrossRef]
- Asaka, M.; Mabe, K. Strategies for eliminating death from gastric cancer in Japan. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.M.; Kim, Y.H. Current approaches to gastric cancer in Korea. Gastrointest. Cancer Res. 2008, 2, 137–144. [Google Scholar] [PubMed]
- Arnold, M.; Morgan, E.; Bardot, A.; Rutherford, M.J.; Ferlay, J.; Little, A.; Møller, B.; Bucher, O.; De, P.; Woods, R.R.; et al. International variation in oesophageal and gastric cancer survival 2012–2014: Differences by histological subtype and stage at diagnosis (an ICBP SURVMARK-2 population-based study). Gut 2022, 71, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Chen, S.; Hu, J.; Guo, Q.; Liu, R.; Zheng, H.; Jin, Z.; Yuan, Y.; Xi, Y.; et al. Endoscopic Screening in Asian Countries Is Associated With Reduced Gastric Cancer Mortality: A Meta-analysis and Systematic Review. Gastroenterology 2018, 155, 347–354.e9. [Google Scholar] [CrossRef] [Green Version]
- Park, H.A.; Nam, S.Y.; Lee, S.K.; Kim, S.G.; Shim, K.N.; Park, S.M.; Lee, S.Y.; Han, H.S.; Shin, Y.M.; Kim, K.M.; et al. The Korean guideline for gastric cancer screening. J. Korean Med. Assoc. 2015, 58, 373–384. [Google Scholar] [CrossRef]
- Hamashima, C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn. J. Clin. Oncol. 2018, 48, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Haenszel, W.; Cuello, C.; Tannenbaum, S.; Archer, M. A model for gastric cancer epidemiology. Lancet 1975, 2, 58–60. [Google Scholar] [CrossRef]
- Correa, P. The biological model of gastric carcinogenesis. IARC Sci. Publ. 2004, 157, 301–310. [Google Scholar]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kook, M.C.; Seong, M.W.; Park, S.R.; Lee, J.S.; Kim, Y.W.; Ryu, K.W.; et al. Helicobacter pylori Seropositivity Is Associated with Gastric Cancer Regardless of Tumor Subtype in Korea. Gut Liver 2010, 4, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, C.Y.; Kim, N.; Lee, J.; Lee, J.Y.; Hwang, Y.J.; Lee, H.S.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, J.W.; et al. Usefulness of OLGA and OLGIM system not only for intestinal type but also for diffuse type of gastric cancer, and no interaction among the gastric cancer risk factors. Helicobacter 2018, 23, e12542. [Google Scholar] [CrossRef] [PubMed]
- Altayar, O.; Davitkov, P.; Shah, S.C.; Gawron, A.J.; Morgan, D.R.; Turner, K.; Mustafa, R.A. AGA Technical Review on Gastric Intestinal Metaplasia-Epidemiology and Risk Factors. Gastroenterology 2020, 158, 732–744.e16. [Google Scholar] [CrossRef]
- Koulis, A.; Buckle, A.; Boussioutas, A. Premalignant lesions and gastric cancer: Current understanding. World J. Gastrointest. Oncol. 2019, 11, 665–678. [Google Scholar] [CrossRef]
- Sugiyama, T.; Awakawa, T.; Hayashi, S.; Hisano, K.; Yabana, T.; Kurokawa, I.; Yachi, A. The effect of the immune response toHelicobacter pyloriin the development of intestinal metaplasia. Eur. J. Gastroenterol. Hepatol. 1994, 6, S89–S92. [Google Scholar] [PubMed]
- Gobert, A.P.; Wilson, K.T. Induction and Regulation of the Innate Immune Response in Helicobacter pylori Infection. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1347–1363. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Zhu, J.; Yu, X.; Feng, T.; Ji, H.; Li, Y.; Zhang, W.; Hu, B. Immune Response in H. pylori-Associated Gastritis and Gastric Cancer. Gastroenterol. Res. Pract. 2020, 2020, 9342563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oertli, M.; Engler, D.B.; Kohler, E.; Koch, M.; Meyer, T.F.; Müller, A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic Gastritis and Colitis. J. Immunol. 2011, 187, 3578–3586. [Google Scholar] [CrossRef] [Green Version]
- Sobala, G.M.; O’Connor, H.J.; Dewar, E.P.; King, R.F.; Axon, A.T.; Dixon, M.F. Bile reflux and intestinal metaplasia in gastric mucosa. J. Clin. Pathol. 1993, 46, 235–240. [Google Scholar] [CrossRef]
- Shao, L.; Li, P.; Ye, J.; Chen, J.; Han, Y.; Cai, J.; Lu, X. Risk of gastric cancer among patients with gastric intestinal metaplasia. Int. J. Cancer 2018, 143, 1671–1677. [Google Scholar] [CrossRef]
- Neumann, W.L.; Coss, E.; Rugge, M.; Genta, R.M. Autoimmune atrophic gastritis—Pathogenesis, pathology and management. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 529–541. [Google Scholar] [CrossRef]
- Busuttil, R.A.; Boussioutas, A. Intestinal metaplasia: A premalignant lesion involved in gastric carcinogenesis. J. Gastroenterol. Hepatol. 2009, 24, 193–201. [Google Scholar] [CrossRef]
- Chen, H.N.; Wang, Z.; Li, X.; Zhou, Z.G. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: Evidence from a meta-analysis. Gastric Cancer 2016, 19, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Gonzalez, L.; Graham, T.A.; Rodriguez-Justo, M.; Leedham, S.J.; Novelli, M.R.; Gay, L.J.; Ventayol-Garcia, T.; Green, A.; Mitchell, I.; Stoker, D.L.; et al. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology 2011, 140, e1251–e1256. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.-J.; Kim, N.; Lee, H.S.; Lee, J.B.; Choi, Y.J.; Yoon, H.; Shin, C.M.; Park, Y.S.; Lee, D.H. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication—A prospective study for up to 10 years. Aliment. Pharmacol. Ther. 2018, 47, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Sung, J.J.; Lin, S.; Jin, Z.; Ding, S.; Huang, X.; Xia, Z.; Guo, H.; Liu, J.; Chao, W. A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chin. Med. J. 2003, 116, 11–14. [Google Scholar]
- Leung, W.K.; Lin, S.R.; Ching, J.Y.; To, K.F.; Ng, E.K.; Chan, F.K.; Lau, J.Y.; Sung, J.J. Factors predicting progression of gastric intestinal metaplasia: Results of a randomised trial on Helicobacter pylori eradication. Gut 2004, 53, 1244–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mera, R.M.; Bravo, L.E.; Camargo, M.C.; Bravo, J.C.; Delgado, A.G.; Romero-Gallo, J.; Yepez, M.C.; Realpe, J.L.; Schneider, B.G.; Morgan, D.R.; et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 2018, 67, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Bautista, M.C.; Jiang, S.F.; Daryani, P.; Brackett, M.; Armstrong, M.A.; Hung, Y.Y.; Postlethwaite, D.; Ladabaum, U. Risks and Predictors of Gastric Adenocarcinoma in Patients with Gastric Intestinal Metaplasia and Dysplasia: A Population-Based Study. Am. J. Gastroenterol. 2016, 111, 1104–1113. [Google Scholar] [CrossRef]
- Rugge, M.; Farinati, F.; Baffa, R.; Sonego, F.; Di Mario, F.; Leandro, G.; Valiante, F. Gastric epithelial dysplasia in the natural history of gastric cancer: A multicenter prospective follow-up study. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastroenterology 1994, 107, 1288–1296. [Google Scholar] [CrossRef]
- de Vries, A.C.; van Grieken, N.C.; Looman, C.W.; Casparie, M.K.; de Vries, E.; Meijer, G.A.; Kuipers, E.J. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Song, H.; Ekheden, I.G.; Zheng, Z.; Ericsson, J.; Nyrén, O.; Ye, W. Incidence of gastric cancer among patients with gastric precancerous lesions: Observational cohort study in a low risk Western population. BMJ Br. Med. J. 2015, 351, h3867. [Google Scholar] [CrossRef] [Green Version]
- Vannella, L.; Lahner, E.; Osborn, J.; Bordi, C.; Miglione, M.; Delle Fave, G.; Annibale, B. Risk factors for progression to gastric neoplastic lesions in patients with atrophic gastritis. Aliment. Pharmacol. Ther. 2010, 31, 1042–1050. [Google Scholar] [CrossRef]
- Gawron, A.J.; Shah, S.C.; Altayar, O.; Davitkov, P.; Morgan, D.; Turner, K.; Mustafa, R.A. AGA Technical Review on Gastric Intestinal Metaplasia—Natural History and Clinical Outcomes. Gastroenterology 2020, 158, 705–731.e5. [Google Scholar] [CrossRef]
- Pabla, B.S.; Shah, S.C.; Corral, J.E.; Morgan, D.R. Increased Incidence and Mortality of Gastric Cancer in Immigrant Populations from High to Low Regions of Incidence: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 347–359. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.J.; Zhu, F.; Srivastava, S.; Tsao, S.K.; Khor, C.; Ho, K.Y.; Fock, K.M.; Lim, W.C.; Ang, T.L.; Chow, W.C.; et al. Severity of gastric intestinal metaplasia predicts the risk of gastric cancer: A prospective multicentre cohort study (GCEP). Gut 2022, 71, 854–863. [Google Scholar] [CrossRef]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Ushiku, T.; Fukayama, M.; Koike, K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest. Endosc. 2016, 84, 618–624. [Google Scholar] [CrossRef]
- Menon, S.; Trudgill, N. How commonly is upper gastrointestinal cancer missed at endoscopy? A meta-analysis. Endosc. Int. Open 2014, 2, E46–E50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teh, J.L.; Tan, J.R.; Lau, L.J.; Saxena, N.; Salim, A.; Tay, A.; Shabbir, A.; Chung, S.; Hartman, M.; So, J.B. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin. Gastroenterol. Hepatol. 2015, 13, 480–487.e2. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.S.; Rehman, S.; Chedgy, F.; Singh, K.K. Improving the mucosal visualization at gastroscopy: A systematic review and meta-analysis of randomized, controlled trials reporting the role of Simethicone ± N-acetylcysteine. Transl. Gastroenterol. Hepatol. 2018, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Y.; Zhang, X.; Ma, C.; Li, A.; Yu, H.; Zhang, W.; Zhang, H.; Yang, T.; Miao, X.; et al. Simethicone administration improves gastric cleanness for esophagogastroduodenoscopy: A randomized clinical trial. Trials 2021, 22, 555. [Google Scholar] [CrossRef]
- Basford, P.J.; Brown, J.; Gadeke, L.; Fogg, C.; Haysom-Newport, B.; Ogollah, R.; Bhattacharyya, R.; Longcroft-Wheaton, G.; Thursby-Pelham, F.; Neale, J.R.; et al. A randomized controlled trial of pre-procedure simethicone and N-acetylcysteine to improve mucosal visibility during gastroscopy-NICEVIS. Endosc. Int. Open 2016, 4, E1197–E1202. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Kwek, A.B.; Law, N.M.; Ong, J.P.; Tan, J.Y.; Harichander Thurairajah, P.; Ang, D.S.; Ang, T.L. Efficacy of small-volume simethicone given at least 30 min before gastroscopy. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 572–578. [Google Scholar] [CrossRef]
- Chiu, P.W.Y.; Uedo, N.; Singh, R.; Gotoda, T.; Ng, E.K.W.; Yao, K.; Ang, T.L.; Ho, S.H.; Kikuchi, D.; Yao, F.; et al. An Asian consensus on standards of diagnostic upper endoscopy for neoplasia. Gut 2019, 68, 186–197. [Google Scholar] [CrossRef] [Green Version]
- An, J.K.; Song, G.A.; Kim, G.H.; Park, D.Y.; Shin, N.R.; Lee, B.E.; Woo, H.Y.; Ryu, D.Y.; Kim, D.U.; Heo, J. Marginal turbid band and light blue crest, signs observed in magnifying narrow-band imaging endoscopy, are indicative of gastric intestinal metaplasia. BMC Gastroenterol. 2012, 12, 169. [Google Scholar] [CrossRef] [Green Version]
- Bhat, Y.M.; Abu Dayyeh, B.K.; Chauhan, S.S.; Gottlieb, K.T.; Hwang, J.H.; Komanduri, S.; Konda, V.; Lo, S.K.; Manfredi, M.A.; Maple, J.T.; et al. High-definition and high-magnification endoscopes. Gastrointest. Endosc. 2014, 80, 919–927. [Google Scholar] [CrossRef]
- Panteris, V.; Nikolopoulou, S.; Lountou, A.; Triantafillidis, J.K. Diagnostic capabilities of high-definition white light endoscopy for the diagnosis of gastric intestinal metaplasia and correlation with histologic and clinical data. Eur. J. Gastroenterol. Hepatol. 2014, 26, 594–601. [Google Scholar] [CrossRef]
- Ang, T.L.; Pittayanon, R.; Lau, J.Y.; Rerknimitr, R.; Ho, S.H.; Singh, R.; Kwek, A.B.; Ang, D.S.; Chiu, P.W.; Luk, S.; et al. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1473–1478. [Google Scholar] [CrossRef]
- Buxbaum, J.L.; Hormozdi, D.; Dinis-Ribeiro, M.; Lane, C.; Dias-Silva, D.; Sahakian, A.; Jayaram, P.; Pimentel-Nunes, P.; Shue, D.; Pepper, M.; et al. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: A prospective blinded trial. Gastrointest. Endosc. 2017, 86, 857–865. [Google Scholar] [CrossRef]
- Rokkas, T.; Ekmektzoglou, K. Current role of narrow band imaging in diagnosing gastric intestinal metaplasia: A systematic review and meta-analysis of its diagnostic accuracy. Ann. Gastroenterol. 2023, 36, 149–156. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, M.; Esposito, G.; Libânio, D.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Image-enhanced endoscopy for gastric preneoplastic conditions and neoplastic lesions: A systematic review and meta-analysis. Endoscopy 2020, 52, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Sobrino-Cossío, S.; Abdo Francis, J.M.; Emura, F.; Galvis-García, E.S.; Márquez Rocha, M.L.; Mateos-Pérez, G.; González-Sánchez, C.B.; Uedo, N. Efficacy of narrow-band imaging for detecting intestinal metaplasia in adult patients with symptoms of dyspepsia. Rev. Gastroenterol. Mex. (Engl. Ed.) 2018, 83, 245–252. [Google Scholar] [CrossRef]
- Dohi, O.; Majima, A.; Naito, Y.; Yoshida, T.; Ishida, T.; Azuma, Y.; Kitae, H.; Matsumura, S.; Mizuno, N.; Yoshida, N.; et al. Can image-enhanced endoscopy improve the diagnosis of Kyoto classification of gastritis in the clinical setting? Dig. Endosc. 2020, 32, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.K.; Liu, D.; Sun, L.M. Diagnostic performance of confocal laser endomicroscopy for optical diagnosis of gastric intestinal metaplasia: A meta-analysis. BMC Gastroenterol. 2016, 16, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.; Wang, J.; Zheng, W.; Ho, K.Y.; Teh, M.; Yeoh, K.G.; Huang, Z. Rapid Fiber-optic Raman Spectroscopy for Real-Time In Vivo Detection of Gastric Intestinal Metaplasia during Clinical Gastroscopy. Cancer Prev. Res. 2016, 9, 476–483. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Lim, L.G.; Yeoh, K.G. Advanced endoscopic imaging in gastric neoplasia and preneoplasia. BMJ Open Gastroenterol. 2017, 4, e000105. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Yin, Z.; Wang, S.; Wang, J.; Bai, B.; Qiu, Z.; Zhao, Q. Meta-analysis: The diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. J. Gastroenterol. Hepatol. 2016, 31, 1539–1545. [Google Scholar] [CrossRef]
- Uedo, N.; Ishihara, R.; Iishi, H.; Yamamoto, S.; Yamamoto, S.; Yamada, T.; Imanaka, K.; Takeuchi, Y.; Higashino, K.; Ishiguro, S.; et al. A new method of diagnosing gastric intestinal metaplasia: Narrow-band imaging with magnifying endoscopy. Endoscopy 2006, 38, 819–824. [Google Scholar] [CrossRef]
- Savarino, E.; Corbo, M.; Dulbecco, P.; Gemignani, L.; Giambruno, E.; Mastracci, L.; Grillo, F.; Savarino, V. Narrow-band imaging with magnifying endoscopy is accurate for detecting gastric intestinal metaplasia. World J. Gastroenterol. 2013, 19, 2668–2675. [Google Scholar] [CrossRef]
- Draşovean, S.C.; Boeriu, A.M.; Akabah, P.S.; Mocan, S.L.; Pascarenco, O.D.; Dobru, E.D. Optical biopsy strategy for the assessment of atrophic gastritis, intestinal metaplasia, and dysplasia. Rom. J. Morphol. Embryol. 2018, 59, 505–512. [Google Scholar]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P.; the Participants in the International Workshop on the Histopathology of Gastritis, Houston. Classification and Grading of Gastritis: The Updated Sydney System. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Xirouchakis, E.; Laoudi, F.; Tsartsali, L.; Spiliadi, C.; Georgopoulos, S.D. Screening for gastric premalignant lesions with narrow band imaging, white light and updated Sydney protocol or both? Dig. Dis. Sci. 2013, 58, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, M.; Libânio, D.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Where should gastric biopsies be performed when areas of intestinal metaplasia are observed? Endosc. Int. Open 2019, 7, E1636–E1639. [Google Scholar] [CrossRef]
- Faknak, N.; Pittayanon, R.; Tiankanon, K.; Lerttanatum, N.; Sanpavat, A.; Klaikaew, N.; Rerknimitr, R. Performance status of targeted biopsy alone versus Sydney protocol by non-NBI expert gastroenterologist in gastric intestinal metaplasia diagnosis. Endosc. Int. Open 2022, 10, E273–E279. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Libânio, D.; Lage, J.; Abrantes, D.; Coimbra, M.; Esposito, G.; Hormozdi, D.; Pepper, M.; Drasovean, S.; White, J.R.; et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016, 48, 723–730. [Google Scholar] [CrossRef]
- Fang, S.; Fu, Y.; Du, S.; Wang, L.; Qing, X.; Luo, X.; Song, G.; Yang, Y.; Wei, W. The role of the endoscopic grading of gastric intestinal metaplasia in assessing gastric cancer risk: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 1018248. [Google Scholar] [CrossRef]
- Rugge, M.; Genta, R.M. Staging and grading of chronic gastritis. Hum. Pathol. 2005, 36, 228–233. [Google Scholar] [CrossRef]
- Capelle, L.G.; de Vries, A.C.; Haringsma, J.; Ter Borg, F.; de Vries, R.A.; Bruno, M.J.; van Dekken, H.; Meijer, J.; van Grieken, N.C.; Kuipers, E.J. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc. 2010, 71, 1150–1158. [Google Scholar] [CrossRef]
- Marcos, P.; Brito-Gonçalves, G.; Libânio, D.; Pita, I.; Castro, R.; Sá, I.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Endoscopic grading of gastric intestinal metaplasia on risk assessment for early gastric neoplasia: Can we replace histology assessment also in the West? Gut 2020, 69, 1762–1768. [Google Scholar] [CrossRef]
- Shah, S.C.; Gawron, A.J.; Mustafa, R.A.; Piazuelo, M.B. Histologic Subtyping of Gastric Intestinal Metaplasia: Overview and Considerations for Clinical Practice. Gastroenterology 2020, 158, 745–750. [Google Scholar] [CrossRef]
- Reis, C.A.; David, L.; Correa, P.; Carneiro, F.; de Bolós, C.; Garcia, E.; Mandel, U.; Clausen, H.; Sobrinho-Simões, M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999, 59, 1003–1007. [Google Scholar]
- Ikeda, Y.; Nishikura, K.; Watanabe, H.; Watanabe, G.; Ajioka, Y.; Hatakeyama, K. Histopathological differences in the development of small intestinal metaplasia between antrum and body of stomach. Pathol. Res. Pract. 2005, 201, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Yang, Y.; Fang, S.; Guo, S.; Xu, C.; Zhang, P.; Wei, W. Gastric Cancer Risk of Intestinal Metaplasia Subtypes: A Systematic Review and Meta-Analysis of Cohort Studies. Clin. Transl. Gastroenterol. 2021, 12, e00402. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Sanz-Anquela, J.M.; Gisbert, J.P.; Correa, P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int. J. Cancer 2013, 133, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; Pietro, M.D.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Li, D.; El Serag, H.B.; Davitkov, P.; Altayar, O.; Sultan, S.; Falck-Ytter, Y.; Mustafa, R.A. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology 2020, 158, 693–702. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef] [Green Version]
- Lansdorp-Vogelaar, I.; Meester, R.G.S.; Laszkowska, M.; Escudero, F.A.; Ward, Z.J.; Yeh, J.M. Cost-effectiveness of prevention and early detection of gastric cancer in Western countries. Best Pract. Res. Clin. Gastroenterol. 2021, 50–51, 101735. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.W.L.; Khoo, M.J.W.; Leong, X.H.; Lim, T.Z.; Shabbir, A.; Yeoh, K.G.; Koh, C.J.; So, J.B.Y. Opportunistic upper endoscopy during colonoscopy as a screening strategy for countries with intermediate gastric cancer risk. J. Gastroenterol. Hepatol. 2021, 36, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.Y.; So, J.B.; Yeoh, K.G. Endoscopic screening for gastric cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Zhou, J.; Naidoo, N.; Yang, W.Y.; Lin, X.C.; Wang, P.; Ding, J.Q.; Wu, C.B.; Zhou, H.J. Determining the cost-effectiveness of endoscopic surveillance for gastric cancer in patients with precancerous lesions. Asia Pac. J. Clin. Oncol. 2016, 12, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Maitra, I.; Date, R.S.; Martin, F.L. Towards screening Barrett’s oesophagus: Current guidelines, imaging modalities and future developments. Clin. J. Gastroenterol. 2020, 13, 635–649. [Google Scholar] [CrossRef]
- Kligman, E.; Ali, H.; Chen, E.; Peng, F.; Szafron, D.; Staggers, K.; Tan, M.C.; Patel, K.; Othman, M.O. Ethnicity Is an Important Consideration in Screening for Gastric Intestinal Metaplasia. Dig. Dis. Sci. 2022, 67, 4509–4517. [Google Scholar] [CrossRef]
- Rugge, M.; Meggio, A.; Pravadelli, C.; Barbareschi, M.; Fassan, M.; Gentilini, M.; Zorzi, M.; Pretis, G.; Graham, D.Y.; Genta, R.M. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: A 5-year prospective study of 1755 patients. Gut 2019, 68, 11–17. [Google Scholar] [CrossRef]
- Yan, T.; Wong, P.K.; Choi, I.C.; Vong, C.M.; Yu, H.H. Intelligent diagnosis of gastric intestinal metaplasia based on convolutional neural network and limited number of endoscopic images. Comput. Biol. Med. 2020, 126, 104026. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, W.; Wu, L.; Zhang, J.; Wang, J.; Mu, G.; Huang, X.; Li, Y.; Yuan, J.; Zeng, Z.; et al. Artificial intelligence in the diagnosis of gastric precancerous conditions by image-enhanced endoscopy: A multicenter, diagnostic study (with video). Gastrointest. Endosc. 2021, 94, 540–548.e4. [Google Scholar] [CrossRef]
- Dilaghi, E.; Lahner, E.; Annibale, B.; Esposito, G. Systematic review and meta-analysis: Artificial intelligence for the diagnosis of gastric precancerous lesions and Helicobacter pylori infection. Dig. Liver Dis. 2022, 54, 1630–1638. [Google Scholar] [CrossRef]
- Mohan, B.P.; Khan, S.R.; Kassab, L.L.; Ponnada, S.; Mohy-Ud-Din, N.; Chandan, S.; Dulai, P.S.; Kochhar, G.S. Convolutional neural networks in the computer-aided diagnosis of Helicobacter pylori infection and non-causal comparison to physician endoscopists: A systematic review with meta-analysis. Ann. Gastroenterol. 2021, 34, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wen, J.; Dong, X.; He, R.; Gao, C.; Zhang, W.; Zhang, Z.; Shen, L. Identification of AQP3 and CD24 as biomarkers for carcinogenesis of gastric intestinal metaplasia. Oncotarget 2017, 8, 63382–63391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Yang, X.; Zhou, Y.; Zhang, W.; Wang, Y.; Wen, J.; Zhang, Z.; Shen, L. Potential role of aquaporin 3 in gastric intestinal metaplasia. Oncotarget 2015, 6, 38926–38933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koulis, A.; Di Costanzo, N.; Mitchell, C.; Lade, S.; Goode, D.; Busuttil, R.A.; Boussioutas, A. CD10 and Das1: A biomarker study using immunohistochemistry to subtype gastric intestinal metaplasia. BMC Gastroenterol. 2022, 22, 197. [Google Scholar] [CrossRef]
- Samloff, I.M.; Varis, K.; Ihamaki, T.; Siurala, M.; Rotter, J.I. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology 1982, 83 Pt 2, 204–209. [Google Scholar] [CrossRef]
- De Vries, A.C.; Kuipers, E.J. Epidemiology of premalignant gastric lesions: Implications for the development of screening and surveillance strategies. Helicobacter 2007, 12 (Suppl. S2), 22–31. [Google Scholar] [CrossRef]
- Tu, H.; Sun, L.; Dong, X.; Gong, Y.; Xu, Q.; Jing, J.; Bostick, R.M.; Wu, X.; Yuan, Y. A Serological Biopsy Using Five Stomach-Specific Circulating Biomarkers for Gastric Cancer Risk Assessment: A Multi-Phase Study. Off. J. Am. Coll. Gastroenterol. 2017, 112, 704–715. [Google Scholar] [CrossRef]
- Huang, R.J.; Park, S.; Shen, J.; Longacre, T.; Ji, H.; Hwang, J.H. Pepsinogens and Gastrin Demonstrate Low Discrimination for Gastric Precancerous Lesions in a Multi-Ethnic United States Cohort. Clin. Gastroenterol. Hepatol. 2022, 20, 950–952.e3. [Google Scholar] [CrossRef]
- Huang, Y.K.; Yu, J.C.; Kang, W.M.; Ma, Z.Q.; Ye, X.; Tian, S.B.; Yan, C. Significance of Serum Pepsinogens as a Biomarker for Gastric Cancer and Atrophic Gastritis Screening: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0142080. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.K.; Ramnarayanan, K.; Zhu, F.; Srivastava, S.; Xu, C.; Tan, A.L.K.; Lee, M.; Tay, S.; Das, K.; Xing, M.; et al. Genomic and Epigenomic Profiling of High-Risk Intestinal Metaplasia Reveals Molecular Determinants of Progression to Gastric Cancer. Cancer Cell 2018, 33, 137–150.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.K.; Ma, H.; Uchihara, T.; Sheng, T.; Chong, R.H.H.; Zhu, F.; Srivastava, S.; Tay, S.T.; Sundar, R.; Tan, A.L.K.; et al. Spatiotemporal Genomic Profiling of Intestinal Metaplasia Reveals Clonal Dynamics of Gastric Cancer Progression. bioRxiv 2023. [Google Scholar] [CrossRef]
- Schmidt, P.H.; Lee, J.R.; Joshi, V.; Playford, R.J.; Poulsom, R.; Wright, N.A.; Goldenring, J.R. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab. Investig. 1999, 79, 639–646. [Google Scholar] [PubMed]
- Link, A.; Kupcinskas, J. MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: Current insights and future perspectives. World J. Gastroenterol. 2018, 24, 3313–3329. [Google Scholar] [CrossRef]
- Jonaitis, P.; Kupcinskas, L.; Kupcinskas, J. Molecular Alterations in Gastric Intestinal Metaplasia. Int. J. Mol. Sci. 2021, 22, 5758. [Google Scholar] [CrossRef]
- Li, H.; Wu, Q.; Li, T.; Liu, C.; Xue, L.; Ding, J.; Shi, Y.; Fan, D. The miR-17-92 cluster as a potential biomarker for the early diagnosis of gastric cancer: Evidence and literature review. Oncotarget 2017, 8, 45060–45071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Wang, J.; Liao, B.; Cheng, L.; Ren, B. The interactions between oral-gut axis microbiota and Helicobacter pylori. Front. Cell Infect. Microbiol. 2022, 12, 914418. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Coker, O.O.; Chu, E.; Szeto, C.H.; Luk, S.T.Y.; Lau, H.C.H.; Yu, J. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020, 69, 1572–1581. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, C.; Cao, W.; Zhang, Z. Alterations of Gastric Microbiota in Gastric Cancer and Precancerous Stages. Front. Cell Infect. Microbiol. 2021, 11, 559148. [Google Scholar] [CrossRef]
- Kadeerhan, G.; Gerhard, M.; Gao, J.J.; Mejías-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; Liu, W.D.; et al. Microbiota alteration at different stages in gastric lesion progression: A population-based study in Linqu, China. Am. J. Cancer Res. 2021, 11, 561–575. [Google Scholar]
- Guo, L.; Liu, Z.; Zhang, Y.; Quan, Q.; Huang, L.; Xu, Y.; Cao, L.; Zhang, X. Association of increased B7 protein expression by infiltrating immune cells with progression of gastric carcinogenesis. Medicine 2019, 98, e14663. [Google Scholar] [CrossRef]
- Cheng, H.H.; Tseng, G.Y.; Yang, H.B.; Wang, H.J.; Lin, H.J.; Wang, W.C. Increased numbers of Foxp3-positive regulatory T cells in gastritis, peptic ulcer and gastric adenocarcinoma. World J. Gastroenterol. 2012, 18, 34–43. [Google Scholar] [CrossRef]
- Ying, L.; Yan, F.; Meng, Q.; Yuan, X.; Yu, L.; Williams, B.R.G.; Chan, D.W.; Shi, L.; Tu, Y.; Ni, P.; et al. Understanding immune phenotypes in human gastric disease tissues by multiplexed immunohistochemistry. J. Transl. Med. 2017, 15, 206. [Google Scholar] [CrossRef]
- Song, B.; Li, T.; Zhang, Y.; Yang, Q.; Pei, B.; Liu, Y.; Wang, J.; Dong, G.; Sun, Q.; Fan, S.; et al. Identification and verification of ferroptosis-related genes in gastric intestinal metaplasia. Front. Genet. 2023, 14, 1152414. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, R.; Vinagre, I.D.F.; Vilar, E.S.A.; Fecury, A.A.; Martins, L.C. Helicobacter pylori infection and immune profile of patients with different gastroduodenal diseases. Arq. Gastroenterol. 2018, 55, 122–127. [Google Scholar] [CrossRef]
- Tan, M.P.; Pedersen, J.; Zhan, Y.; Lew, A.M.; Pearse, M.J.; Wijburg, O.L.; Strugnell, R.A. CD8+ T cells are associated with severe gastritis in Helicobacter pylori-infected mice in the absence of CD4+ T cells. Infect. Immun. 2008, 76, 1289–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, M.; Haghbin, H.; Sayeh, W.; Alfatlawi, H.; Gangwani, M.K.; Sohail, A.H.; Zahdeh, T.; Weissman, S.; Kamal, F.; Lee-Smith, W.; et al. Comparison of Artificial Intelligence With Other Interventions to Improve Adenoma Detection Rate for Colonoscopy: A Network Meta-analysis. J. Clin. Gastroenterol. 2022. [Google Scholar] [CrossRef] [PubMed]
| CORPUS (BODY) | |||||
| Atrophy Score | No atrophy (0) | Mild atrophy (1) | Moderate atrophy (2) | Severe atrophy (3) | |
| ANTRUM (INCLUDING INCISURA) | No atrophy (0) | Stage 0 | Stage I | Stage II | Stage II |
| Mild atrophy (1) | Stage I | Stage I | Stage II | Stage III | |
| Moderate atrophy (2) | Stage II | Stage II | Stage III | Stage IV | |
| Severe atrophy (3) | Stage III | Stage III | Stage IV | Stage IV | |
| CORPUS (BODY) | |||||
| IM Score | No IM (0) | Mild IM (1) | Moderate IM (2) | Severe IM (3) | |
| ANTRUM (INCLUDING INCISURA) | No IM (0) | Stage 0 | Stage I | Stage II | Stage II |
| Mild IM (1) | Stage I | Stage I | Stage II | Stage III | |
| Moderate IM (2) | Stage II | Stage II | Stage III | Stage IV | |
| Severe IM (3) | Stage III | Stage III | Stage IV | Stage IV | |
| ESGE (2019) | BSG (2019) | AGA (2020) | |
|---|---|---|---|
| Extensive IM | Thrice yearly | Thrice yearly | Routine surveillance not recommended. Patients with GIM at higher risk for gastric cancer who place high value on potential but uncertain reductions in gastric cancer mortality, and who place a low value on the potential risks of surveillance endoscopies, may reasonably elect for surveillance. Consider surveillance 3–5 times a year. |
| Limited IM, no risk factors | No surveillance | No surveillance | |
| Limited IM, family history OR incomplete or persistent H pylori | Thrice yearly | Thrice yearly | |
| Limited IM, severe atrophy | Thrice yearly | Thrice yearly | |
| Limited IM, severe atrophy, family history | Considered 1–2 times a year | Thrice yearly | |
| Autoimmune gastritis | Considered 3–5 times a year |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tjandra, D.; Busuttil, R.A.; Boussioutas, A. Gastric Intestinal Metaplasia: Challenges and the Opportunity for Precision Prevention. Cancers 2023, 15, 3913. https://doi.org/10.3390/cancers15153913
Tjandra D, Busuttil RA, Boussioutas A. Gastric Intestinal Metaplasia: Challenges and the Opportunity for Precision Prevention. Cancers. 2023; 15(15):3913. https://doi.org/10.3390/cancers15153913
Chicago/Turabian StyleTjandra, Douglas, Rita A. Busuttil, and Alex Boussioutas. 2023. "Gastric Intestinal Metaplasia: Challenges and the Opportunity for Precision Prevention" Cancers 15, no. 15: 3913. https://doi.org/10.3390/cancers15153913
APA StyleTjandra, D., Busuttil, R. A., & Boussioutas, A. (2023). Gastric Intestinal Metaplasia: Challenges and the Opportunity for Precision Prevention. Cancers, 15(15), 3913. https://doi.org/10.3390/cancers15153913






