Significance of Furin Expression in Thyroid Neoplastic Transformation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Samples
2.2. Patients’ Characteristics and Clinicopathological Data
2.3. Reverse Transcription of the RNA from Frozen Tissues
2.4. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.5. Immunohistochemistry
2.6. Immune Reactive Score (IRS)
2.7. Statistical Analysis
3. Results
3.1. Clinical Series Characterization
3.2. mRNA and Protein Analysis in the Thyroid Series
- First, data were analysed by comparing ATs with benign lesions, which included goitres and FTAs, and with carcinomas, which included all malignant thyroid neoplasms;
- Data were then stratified and separated according to the main histological groups: goitres, FTAs, PTCs, FTCs, OCAs, PDTCs, and MTCs;
- Finally, all the sub-histological groups were compared: goitres, FTAs, cPTCs, FVPTCs, DSVPTCs, OVPTCs, FTCs, OCAs, and PDTCs.
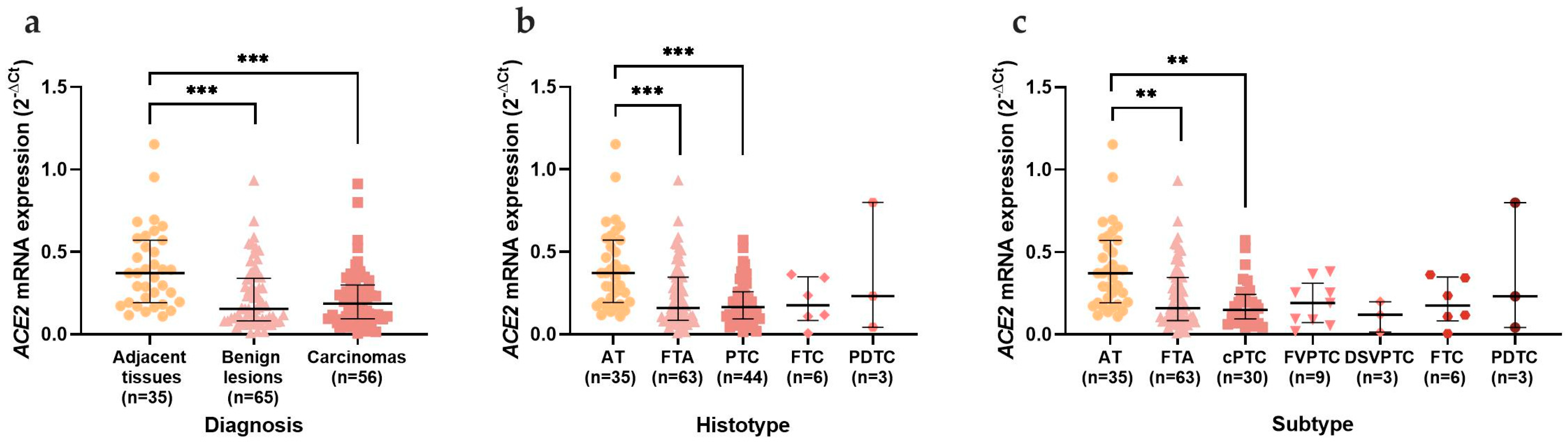
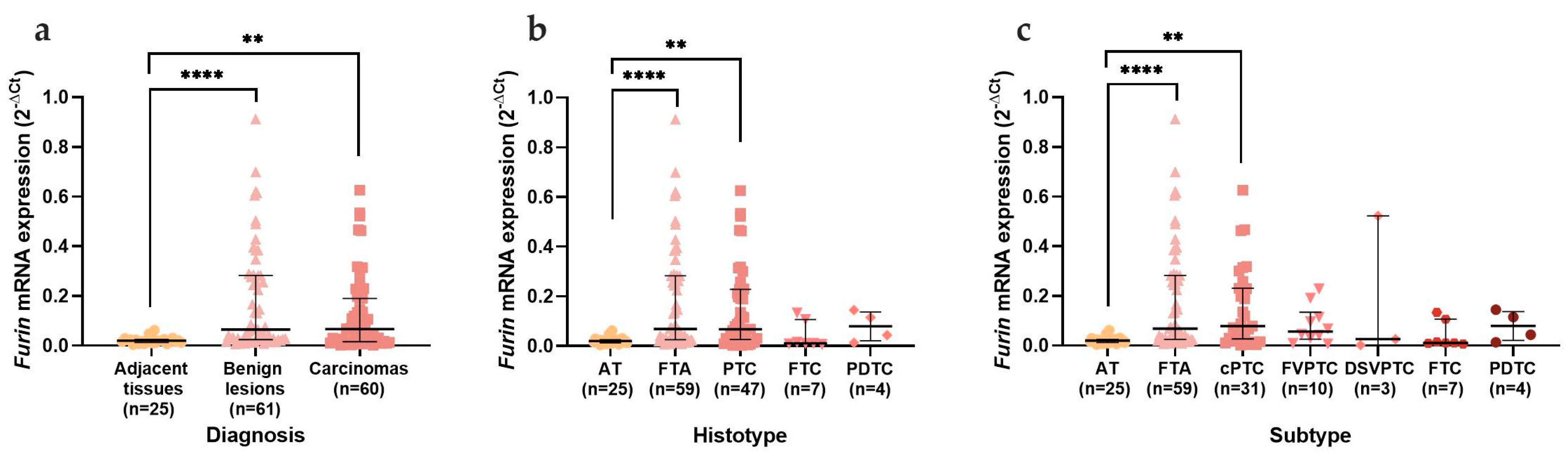
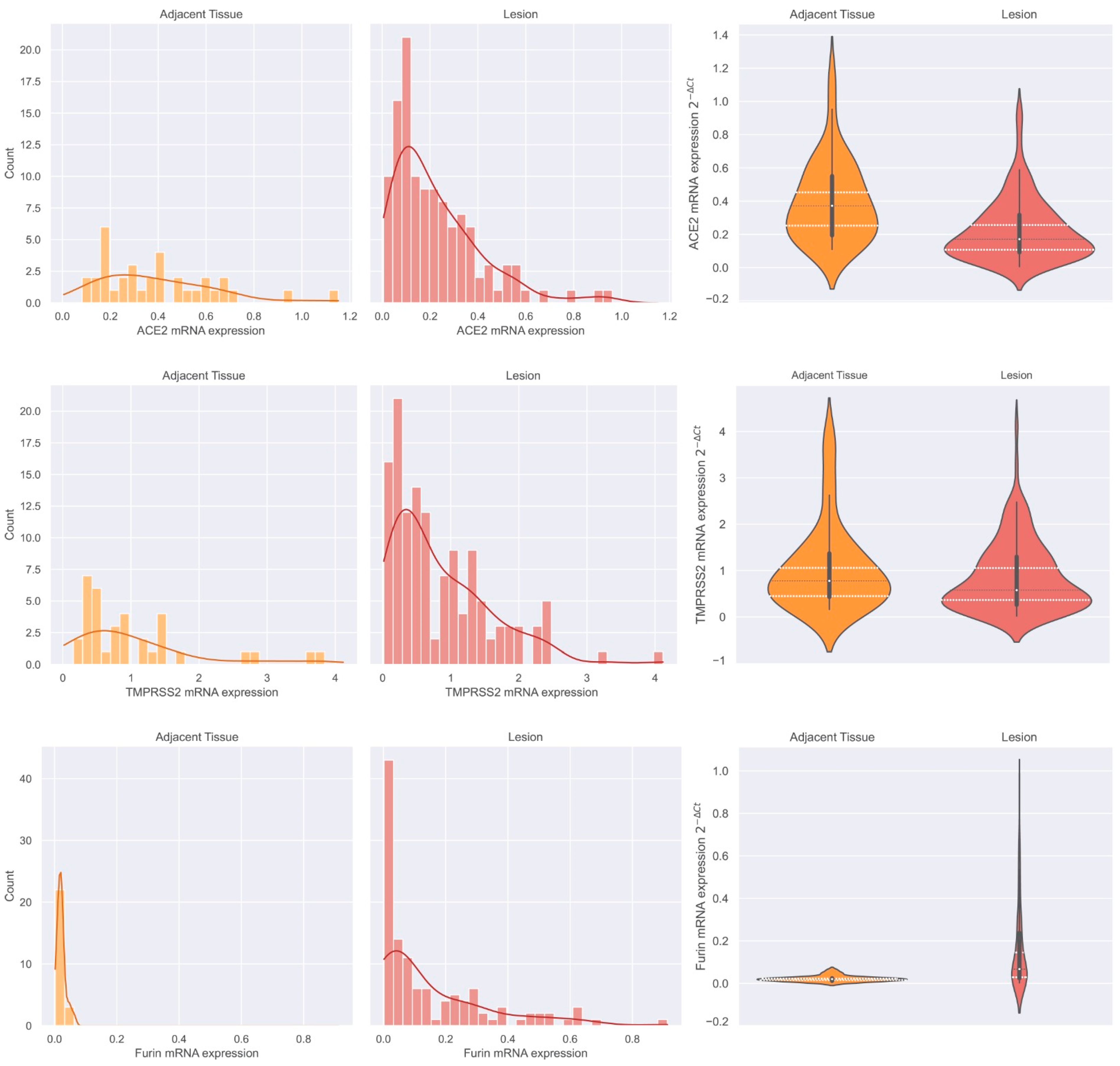
3.2.1. mRNA Expression in Thyroid Tissues
3.2.2. ACE2, TMPRSS2, and Furin mRNA Expression Distribution in Adjacent Thyroid Tissue and Thyroid Neoplasms and Furin Discriminative Power
3.2.3. Protein Expression in Thyroid Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Macedo, S.; Pestana, L.A.; Santos, C.; Neves, S.; Guimarães, A.; Duarte-Neto, M.; Dolhnikoff, P.; Saldiva, G.; Alves, R.; Oliveira, D.; et al. Detection of SARS-CoV-2 Infection in Thyroid Follicular Cells from a COVID-19 Autopsy Series. Eur. Thyroid. J. 2022, 11, e220074. [Google Scholar] [CrossRef]
- Donoghue, M.; Hsieh, E.F.; Baronas, K.; Godbout, M.; Gosselin, N.; Stagliano, M.; Donovan, B.; Woolf, K.; Robison, R.; Jeyaseelan, R.; et al. A Novel Angiotensin-Converting Enzyme-Related Carboxypeptidase (Ace2) Converts Angiotensin I to Angiotensin 1-9. Circ. Res. 2000, 87, e1–e9. [Google Scholar] [CrossRef]
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A Human Homolog of Angiotensin-Converting Enzyme. Cloning and Functional Expression as a Captopril-Insensitive Carboxypeptidase. J. Biol. Chem. 2000, 275, 33238–33243. [Google Scholar] [CrossRef] [Green Version]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (Ace2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Bank, S.; De, S.K.; Bankura, B.; Maiti, S.; Das, M.; Khan, G.A. Ace/Ace2 Balance Might Be Instrumental to Explain the Certain Comorbidities Leading to Severe COVID-19 Cases. Biosci. Rep. 2021, 41, BSR20202014. [Google Scholar] [CrossRef]
- Chai, P.; Yu, J.; Ge, S.; Jia, R.; Fan, X. Genetic Alteration, Rna Expression, and DNA Methylation Profiling of Coronavirus Disease 2019 (COVID-19) Receptor Ace2 in Malignancies: A Pan-Cancer Analysis. J. Hematol. Oncol. 2020, 13, 43. [Google Scholar] [CrossRef]
- Dai, Y.J.; Hu, F.; Li, H.; Huang, H.Y.; Wang, D.W.; Liang, Y. A Profiling Analysis on the Receptor Ace2 Expression Reveals the Potential Risk of Different Type of Cancers Vulnerable to SARS-CoV-2 Infection. Ann. Transl. Med. 2020, 8, 481. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Gong, C.; Ma, S. Evaluation of COVID-19 Based on Ace2 Expression in Normal and Cancer Patients. Open Med. 2020, 15, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 20 May 2023).
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Lizbeth, H. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar]
- Larzabal, L.; Nguewa, P.A.; Pio, R.; Blanco, D.; Sanchez, B.; Rodríguez, M.J.; Pajares, M.J.; Catena, R.; Montuenga, L.M.; Calvo, A. Overexpression of Tmprss4 in Non-Small Cell Lung Cancer Is Associated with Poor Prognosis in Patients with Squamous Histology. Br. J. Cancer 2011, 105, 1608–1614. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.J.; Huang, C.C.; Lin, H.Y.; Juan, C.P.; Lan, S.W.; Shyu, H.Y.; Wu, S.R.; Hsiao, P.W.; Huang, H.P.; Shun, C.T.; et al. Androgen-Induced Tmprss2 Activates Matriptase and Promotes Extracellular Matrix Degradation, Prostate Cancer Cell Invasion, Tumor Growth, and Metastasis. Cancer Res. 2015, 75, 2949–2960. [Google Scholar] [CrossRef] [Green Version]
- Fraser, B.J.; Beldar, S.; Seitova, A.; Hutchinson, A.; Mannar, D.; Li, Y.; Kwon, D.; Tan, R.; Wilson, R.P.; Leopold, K.; et al. Structure and Activity of Human Tmprss2 Protease Implicated in SARS-CoV-2 Activation. Nat. Chem. Biol. 2022, 18, 963–971. [Google Scholar] [CrossRef]
- Choi, S.Y.; Bertram, S.; Glowacka, I.; Park, Y.W.; Pöhlmann, S. Type Ii Transmembrane Serine Proteases in Cancer and Viral Infections. Trends Mol. Med. 2009, 15, 303–312. [Google Scholar] [CrossRef]
- Lucas, J.M.; Heinlein, C.; Kim, T.; Hernandez, S.A.; Malik, M.S.; True, L.D.; Morrissey, C.; Corey, E.; Montgomery, B.; Mostaghel, E.; et al. The Androgen-Regulated Protease Tmprss2 Activates a Proteolytic Cascade Involving Components of the Tumor Microenvironment and Promotes Prostate Cancer Metastasis. Cancer Discov. 2014, 4, 1310–1325. [Google Scholar] [CrossRef] [Green Version]
- Lucas, J.M.; True, L.; Hawley, S.; Matsumura, M.; Morrissey, C.; Vessella, R.; Nelson, P.S. The Androgen-Regulated Type Ii Serine Protease Tmprss2 Is Differentially Expressed and Mislocalized in Prostate Adenocarcinoma. J. Pathol. 2008, 215, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Lee, M.-S.; Lucht, A.; Chou, F.-P.; Huang, W.; Havighurst, T.C.; Kim, K.; Wang, J.-K.; Antalis, T.M.; Johnson, M.D.; et al. Tmprss2, a Serine Protease Expressed in the Prostate on the Apical Surface of Luminal Epithelial Cells and Released into Semen in Prostasomes, Is Misregulated in Prostate Cancer Cells. Am. J. Pathol. 2010, 176, 2986–2996. [Google Scholar] [CrossRef]
- Baughn, L.B.; Sharma, N.; Elhaik, E.; Sekulic, A.; Bryce, A.H.; Fonseca, R. Targeting Tmprss2 in SARS-CoV-2 Infection. Mayo. Clin. Proc. 2020, 95, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Gkogkou, E.; Barnasas, G.; Vougas, K.; Trougakos, I.P. Expression Profiling Meta-Analysis of Ace2 and Tmprss2, the Putative Anti-Inflammatory Receptor and Priming Protease of SARS-CoV-2 in Human Cells, and Identification of Putative Modulators. Redox Biol. 2020, 36, 101615. [Google Scholar] [CrossRef] [PubMed]
- Piva, F.; Sabanovic, B.; Cecati, M.; Giulietti, M. Expression and Co-Expression Analyses of Tmprss2, a Key Element in COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 451–455. [Google Scholar] [CrossRef]
- Zhou, L.; Niu, Z.; Jiang, X.; Zhang, Z.; Zheng, Y.; Wang, Z.; Sun, Q. SARS-CoV-2 Targets by the Pscrna Profiling of Ace2, Tmprss2 and Furin Proteases. iScience 2020, 23, 101744. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, S. Pan-Cancer Analysis of Furin as a Potential Prognostic and Immunological Biomarker. Front. Mol. Biosci. 2021, 8, 648402. [Google Scholar] [CrossRef]
- Nakayama, K. Furin: A Mammalian Subtilisin/Kex2p-Like Endoprotease Involved in Processing of a Wide Variety of Precursor Proteins. Biochem. J. 1997, 327, 625–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidah, N.G.; Prat, A. The Biology and Therapeutic Targeting of the Proprotein Convertases. Nat. Rev. Drug. Discov. 2012, 11, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, M.; Nagahama, M.; Kim, W.S.; Watanabe, T.; Hatsuzawa, K.; Ikemizu, J.; Murakami, K.; Nakayama, K. Arg-X-Lys/Arg-Arg Motif as a Signal for Precursor Cleavage Catalyzed by Furin within the Constitutive Secretory Pathway. J. Biol. Chem. 1991, 266, 12127–12130. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.S.; Bresnahan, P.A.; Leppla, S.H.; Klimpel, K.R.; Thomas, G. Human Furin Is a Calcium-Dependent Serine Endoprotease That Recognizes the Sequence Arg-X-X-Arg and Efficiently Cleaves Anthrax Toxin Protective Antigen. J. Biol. Chem. 1992, 267, 16396–16402. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Zhou, L.; Zhou, J.; Wan, H.; Li, Q.; Feng, Y. Ace2 Overexpression Inhibits Acquired Platinum Resistance-Induced Tumor Angiogenesis in Nsclc. Oncol. Rep. 2016, 36, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Bassi, D.E.; Mahloogi, H.; Al-Saleem, L.; De Cicco, R.L.; Ridge, J.A.; Klein-Szanto, A.J.P. Elevated Furin Expression in Aggressive Human Head and Neck Tumors and Tumor Cell Lines. Mol. Carcinog. 2001, 31, 224–232. [Google Scholar] [CrossRef]
- Schalken, J.A.; Roebroek, A.J.; Oomen, P.P.; Wagenaar, S.S.; Debruyne, F.M.; Bloemers, H.P.; Van de Ven, W.J. Fur Gene Expression as a Discriminating Marker for Small Cell and Nonsmall Cell Lung Carcinomas. J. Clin. Invest. 1987, 80, 1545–1549. [Google Scholar] [CrossRef]
- Page, R.E.; Klein-Szanto, A.J.; Litwin, S.; Nicolas, E.; Al-Jumaily, R.; Alexander, P.; Godwin, A.K.; Ross, E.A.; Schilder, R.J.; Bassi, D.E. Increased Expression of the Pro-Protein Convertase Furin Predicts Decreased Survival in Ovarian Cancer. Cell Oncol. 2007, 29, 289–299. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.X.; Zhang, J.; He, X.; Chen, S.; Liu, J.; Fu, S.; Fu, H.; Chen, J.; Fu, J.; et al. COVID-19 Disease and Malignant Cancers: The Impact for the Furin Gene Expression in Susceptibility to SARS-CoV-2. Int. J. Biol. Sci. 2021, 17, 3954–3967. [Google Scholar] [CrossRef] [PubMed]
- Poyil, P.K.; Siraj, A.K.; Padmaja, D.; Parvathareddy, S.K.; Diaz, R.; Thangavel, S.; Begum, R.; Haqawi, W.; Al-Mohanna, F.H.; Al-Sobhi, S.S.; et al. Overexpression of the Pro-Protein Convertase Furin Predicts Prognosis and Promotes Papillary Thyroid Carcinoma Progression and Metastasis through Raf/Mek Signaling. Mol. Oncol. 2023, 17, 13396. [Google Scholar] [CrossRef] [PubMed]
- Jaaks, P.; Bernasconi, M. The Proprotein Convertase Furin in Tumour Progression. Int. J. Cancer 2017, 141, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Pestana, A.; Batista, R.; Celestino, R.; Canberk, S.; Sobrinho-Simões, M.; Soares, P. Comprehensive Assessment of Tert Mrna Expression across a Large Cohort of Benign and Malignant Thyroid Tumours. Cancers 2020, 12, 1846. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different Approaches for Interpretation and Reporting of Immunohistochemistry Analysis Results in the Bone Tissue-a Review. Diagn. Pathol. 2014, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Waskom, M.L. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 Cell Receptor Gene Ace2 in a Wide Variety of Human Tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Luo, W.; Huang, L.; Xiao, J.; Li, F.; Qin, S.; Song, X.; Wu, Y.; Zeng, Q.; et al. A Comprehensive Investigation of the Mrna and Protein Level of Ace2, the Putative Receptor of SARS-CoV-2, in Human Tissues and Blood Cells. Int. J. Med. Sci. 2020, 17, 1522–1531. [Google Scholar] [CrossRef]
- Cao, W.; Feng, Q.; Wang, X. Computational Analysis of Tmprss2 Expression in Normal and SARS-CoV-2-Infected Human Tissues. Chem. Biol. Interact. 2021, 346, 109583. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The Protein Expression Profile of Ace2 in Human Tissues. Mol. Syst Biol. 2020, 16, e9610. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on Ace2 and Tmprss2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Park, G.-C.; Lee, H.-W.; Kim, J.-M.; Han, J.-M.; Kim, H.-I.; Shin, S.-C.; Cheon, Y.-I.; Sung, E.-S.; Lee, M.; Lee, J.-C.; et al. Ace2 and Tmprss2 Immunolocalization and COVID-19-Related Thyroid Disorder. Biology 2022, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Coperchini, F.; Ricci, G.; Denegri, M.; Croce, L.; Ngnitejeu, S.T.; Villani, L.; Magri, F.; Latrofa, F.; Chiovato, L. Detection of SARS-CoV-2 Receptor Ace-2 Mrna in Thyroid Cells: A Clue for COVID-19-Related Subacute Thyroiditis. J. Endocrinol. Invest. 2021, 44, 1085–1090. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Pitoia, F.; Esposito, K.; Piccardo, A.; Trimboli, P. Impact of COVID-19 on the Thyroid Gland: An Update. Rev. Endocr. Metab. Disord. 2021, 22, 803–815. [Google Scholar] [CrossRef]
- Bao, R.; Hernandez, K.; Huang, L.; Luke, J.J. Ace2 and Tmprss2 Expression by Clinical, Hla, Immune, and Microbial Correlates across 34 Human Cancers and Matched Normal Tissues: Implications for SARS-CoV-2 COVID-19. J. Immunother Cancer 2020, 8, 271–280. [Google Scholar] [CrossRef]
- Narayan, S.S.; Lorenz, K.; Ukkat, J.; Hoang-Vu, C.; Trojanowicz, B. Angiotensin Converting Enzymes Ace and Ace2 in Thyroid Cancer Progression. Neoplasma 2020, 67, 402–409. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, S.; Li, T.; Yu, L.; Zhang, Y.; Zeng, H.; Qian, X.; Bi, J.; Lin, Y. Ace2 Inhibits Breast Cancer Angiogenesis Via Suppressing the Vegfa/Vegfr2/Erk Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 173. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Zhu, Z.; Shao, E.; Bao, J. Ace2 Is a Prognostic Biomarker and Associated with Immune Infiltration in Kidney Renal Clear Cell Carcinoma: Implication for COVID-19. J. Oncol. 2021, 2021, 8847307. [Google Scholar] [CrossRef]
- Saji, M.; Xydas, S.; Westra, W.H.; Liang, C.K.; Clark, D.P.; Udelsman, R.; Umbricht, C.B.; Sukumar, S.; Zeiger, M.A. Human Telomerase Reverse Transcriptase (Htert) Gene Expression in Thyroid Neoplasms. Clin. Cancer Res. 1999, 5, 1483–1489. [Google Scholar]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High Expression of Ace2 Receptor of 2019-Ncov on the Epithelial Cells of Oral Mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-Cell Rna Expression Profiling of Ace2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 756–759. [Google Scholar]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-Converting Enzyme 2 (Ace2) as a SARS-CoV-2 Receptor: Molecular Mechanisms and Potential Therapeutic Target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Coperchini, F.; Ricci, G.; Croce, L.; Denegri, M.; Ruggiero, R.; Villani, L.; Magri, F.; Chiovato, L.; Rotondi, M. Modulation of Ace-2 Mrna by Inflammatory Cytokines in Human Thyroid Cells: A Pilot Study. Endocrine 2021, 74, 638–645. [Google Scholar] [CrossRef]
- Lazartigues, E.; Qadir, M.M.F.; Mauvais-Jarvis, F. Endocrine Significance of SARS-CoV-2’s Reliance on Ace2. Endocrinology 2020, 161, bqaa108. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Shan, H.; Niu, Y.; Wang, P.; Li, D.; Zhang, Y.; Wang, J.; Wu, Y.; Jiang, H. Tmprss2 Serves as a Prognostic Biomarker and Correlated with Immune Infiltrates in Breast Invasive Cancer and Lung Adenocarcinoma. Front. Mol. Biosci. 2022, 9, 647826. [Google Scholar] [CrossRef]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered Tmprss2 Usage by SARS-CoV-2 Omicron Impacts Infectivity and Fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, S.; Tebaldi, M.; Fonzi, E.; Angeli, D.; Mazza, M.; Nicolini, F.; Lucchesi, A.; Fanini, F.; Pirini, F.; Tumedei, M.M.; et al. Ace2 and Tmprss2 Potential Involvement in Genetic Susceptibility to SARS-CoV-2 in Cancer Patients. Cell Transpl. 2020, 29, 963689720968749. [Google Scholar] [CrossRef] [PubMed]
- Nilubol, N.; Zhang, L.J.; Kebebew, E.; Yi, Y.S.; Kim, S.S.; Kim, W.J.; Bae, M.J.; Kang, J.H.; Choi, B.G.; Jeon, Y.K.; et al. Multivariate Analysis of the Relationship between Male Sex, Disease-Specific Survival, and Features of Tumor Aggressiveness in Thyroid Cancer of Follicular Cell Origin. Thyroid 2013, 23, 695–702. [Google Scholar] [CrossRef]
- Insilla, A.C.; Proietti, A.; Borrelli, N.; Macerola, E.; Niccoli, C.; Vitti, P.; Miccoli, P.; Basolo, F. Tert Promoter Mutations and Their Correlation with Braf and Ras Mutations in a Consecutive Cohort of 145 Thyroid Cancer Cases. Oncol. Lett. 2018, 15, 2763–2770. [Google Scholar] [PubMed] [Green Version]
- Xing, M. Clinical Utility of Ras Mutations in Thyroid Cancer: A Blurred Picture Now Emerging Clearer. BMC Med. 2016, 14, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricardo, S.; Canão, P.; Martins, D.; Magalhães, A.C.; Pereira, M.; Ribeiro-Junior, U.; de Mello, E.S.; Alves, V.A.; Pinto, R.; Leitão, D.; et al. Searching for SARS-CoV-2 in Cancer Tissues: Results of an Extensive Methodologic Approach Based on Ace2 and Furin Expression. Cancers 2022, 14, 2582. [Google Scholar] [CrossRef]
- Wang, D. Discrepancy between Mrna and Protein Abundance: Insight from Information Retrieval Process in Computers. Comput. Biol. Chem. 2008, 32, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegler, C.; Ölander, M.; Wiśniewski, J.R.; Lundquist, P.; Zettl, K.; Åsberg, A.; Hjelmesæth, J.; Andersson, T.B.; Artursson, P. Global Variability Analysis of Mrna and Protein Concentrations across and within Human Tissues. NAR Genom. Bioinform. 2020, 2, lqz010. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Patient Characteristics | Frozen Tissue | FFPE Tissue |
|---|---|---|
| n = 165 | n =75 | |
| Age Mean ± SD Median ± IQR | n= 155 | n= 71 |
| 43.4 ± 15.4 | 42.2 ± 15.4 | |
| 43.0 ± 21.0 | 44.0 ± 22.0 | |
| Min–Max | 11–82 | 11–76 |
| Age <55 years | n= 155 | n= 71 |
| 121 (78.1%) | 58 (81.7%) | |
| ≥55 years | 34 (21.9%) | 13 (18.3%) |
| Sex Female | n= 158 | n= 71 |
| 130 (82.3%) | 59 (83.1%) | |
| Male | 28 (17.7%) | 12 (16.9%) |
| Clinicopathological features | Frozen tissue | FFPE tissue |
| n= 168 | n= 75 | |
| Number oflesions | n= 137 | n= 35 |
| 1 | 82 (59.9%) | 24 (68.6%) |
| >1 | 55 (40.1%) | 11 (31.4%) |
| Tumour size (cm) | n= 130 | n= 35 |
| Mean ± SD | 3.3 ± 1.8 | 3.6 ± 1.7 |
| Median ± IQR | 3.0 ± 2.5 | 3.5 ± 2.8 |
| Min–Max (cm) | 0.5–10.0 | 0.5–7.0 |
| Tumour size | n= 130 | n= 35 |
| ≤3 cm | 77 (59.2%) | 16 (45.7%) |
| >3 cm | 53 (40.8%) | 19 (54.3%) |
| Tumour capsule | n= 142 | n= 33 |
| Presence | 95 (66.9%) | 23 (69.7%) |
| Absence | 47 (33.1%) | 10 (30.3%) |
| Capsule invasion/infiltration | n= 94 | n= 22 |
| Presence | 13 (13.8%) | 3 (13.6%) |
| Absence | 81 (86.2%) | 19 (86.4%) |
| Lymphovascular invasion | n= 139 | n= 34 |
| Presence | 28 (20.1%) | 9 (26.5%) |
| Absence | 111 (79.9%) | 25 (73.5%) |
| Lymph node metastases | n= 35 | n= 8 |
| Presence | 17 (48.6%) | 4 (50.0%) |
| Absence | 18 (51.4%) | 4 (50.0%) |
| Microscopical extrathyroidal extension | n= 102 | n= 22 |
| Presence | 23 (22.5%) | 4 (18.2%) |
| Absence | 79 (77.5%) | 18 (81.8%) |
| Lymphocytic infiltration | n= 140 | n= 36 |
| Presence | 50 (35.7%) | 16 (44.4%) |
| Absence | 90 (64.3%) | 20 (55.6%) |
| Chronic lymphocytic thyroiditis | n= 132 | n= 72 |
| Presence | 43 (32.6%) | 13 (18.1%) |
| Absence | 88 (66.7%) | 59 (81.9%) |
| TERTp | n= 151 | n= 38 |
| Wild-Type | 147 (97.4%) | 37 (97.4%) |
| Mutated | 4 (2.6%) | 1 (2.6%) |
| TERT expression | n= 159 | n= 38 |
| Positive | 107(67.3%) | 25 (65.8%) |
| Negative | 52 (32.7%) | 13 (34.2%) |
| NRAS mutation | n= 152 | n= 38 |
| Wild-Type | 136(89.5%) | 33 (86.8%) |
| Mutated | 16 (10.5%) | 5 (13.2%) |
| BRAF mutation | n= 89 | n= 27 |
| Wild-Type | 69 (77.5%) | 23 (85.2%) |
| Mutated | 20 (22.5%) | 4 (14.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, M.T.; Macedo, S.; Canberk, S.; Cardoso, L.; Gaspar, T.B.; Pestana, A.; Batista, R.; Sobrinho-Simões, M.; Soares, P. Significance of Furin Expression in Thyroid Neoplastic Transformation. Cancers 2023, 15, 3909. https://doi.org/10.3390/cancers15153909
Azevedo MT, Macedo S, Canberk S, Cardoso L, Gaspar TB, Pestana A, Batista R, Sobrinho-Simões M, Soares P. Significance of Furin Expression in Thyroid Neoplastic Transformation. Cancers. 2023; 15(15):3909. https://doi.org/10.3390/cancers15153909
Chicago/Turabian StyleAzevedo, Maria Teresa, Sofia Macedo, Sule Canberk, Luís Cardoso, Tiago Bordeira Gaspar, Ana Pestana, Rui Batista, Manuel Sobrinho-Simões, and Paula Soares. 2023. "Significance of Furin Expression in Thyroid Neoplastic Transformation" Cancers 15, no. 15: 3909. https://doi.org/10.3390/cancers15153909
APA StyleAzevedo, M. T., Macedo, S., Canberk, S., Cardoso, L., Gaspar, T. B., Pestana, A., Batista, R., Sobrinho-Simões, M., & Soares, P. (2023). Significance of Furin Expression in Thyroid Neoplastic Transformation. Cancers, 15(15), 3909. https://doi.org/10.3390/cancers15153909










