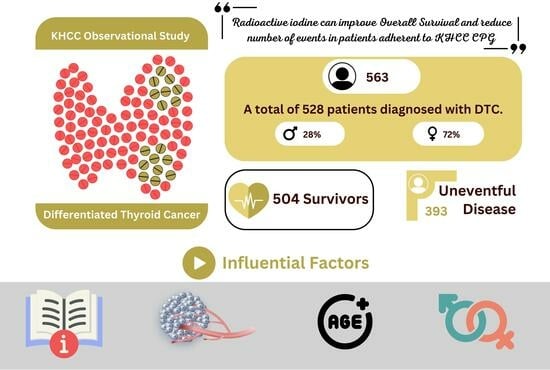
Long-Term Survival Analysis and Prognostic Factors of Arabic Patients with Differentiated Thyroid Carcinoma: A 20-Year Observational Study at the King Hussein Cancer Center (KHCC) Involving 528 Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Procedures for Therapeutic Radioiodine Administration
2.3. Procedure for Radioiodine WBS
2.4. Late Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Modalities
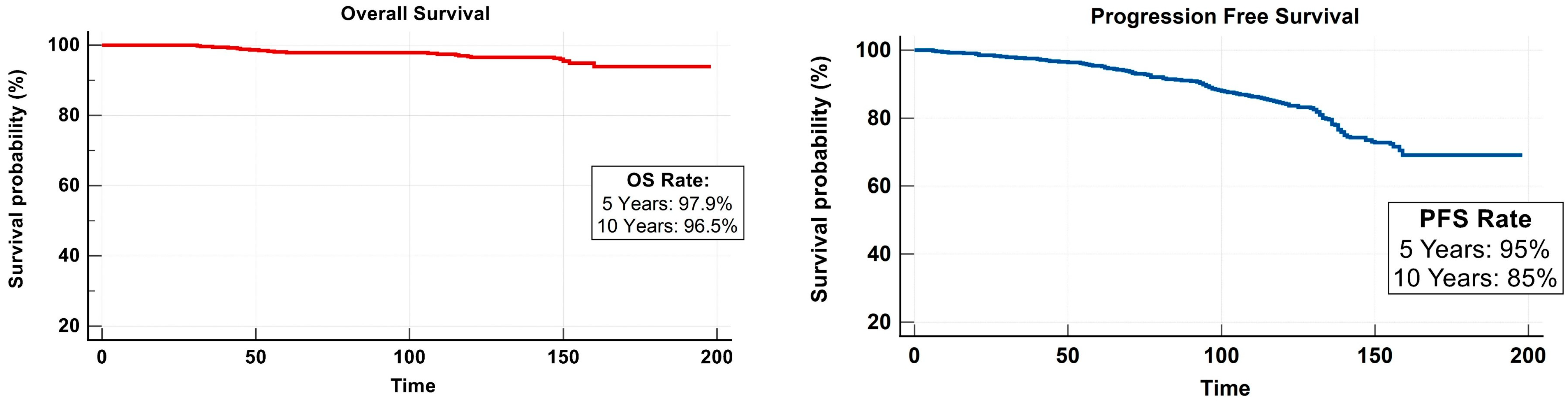
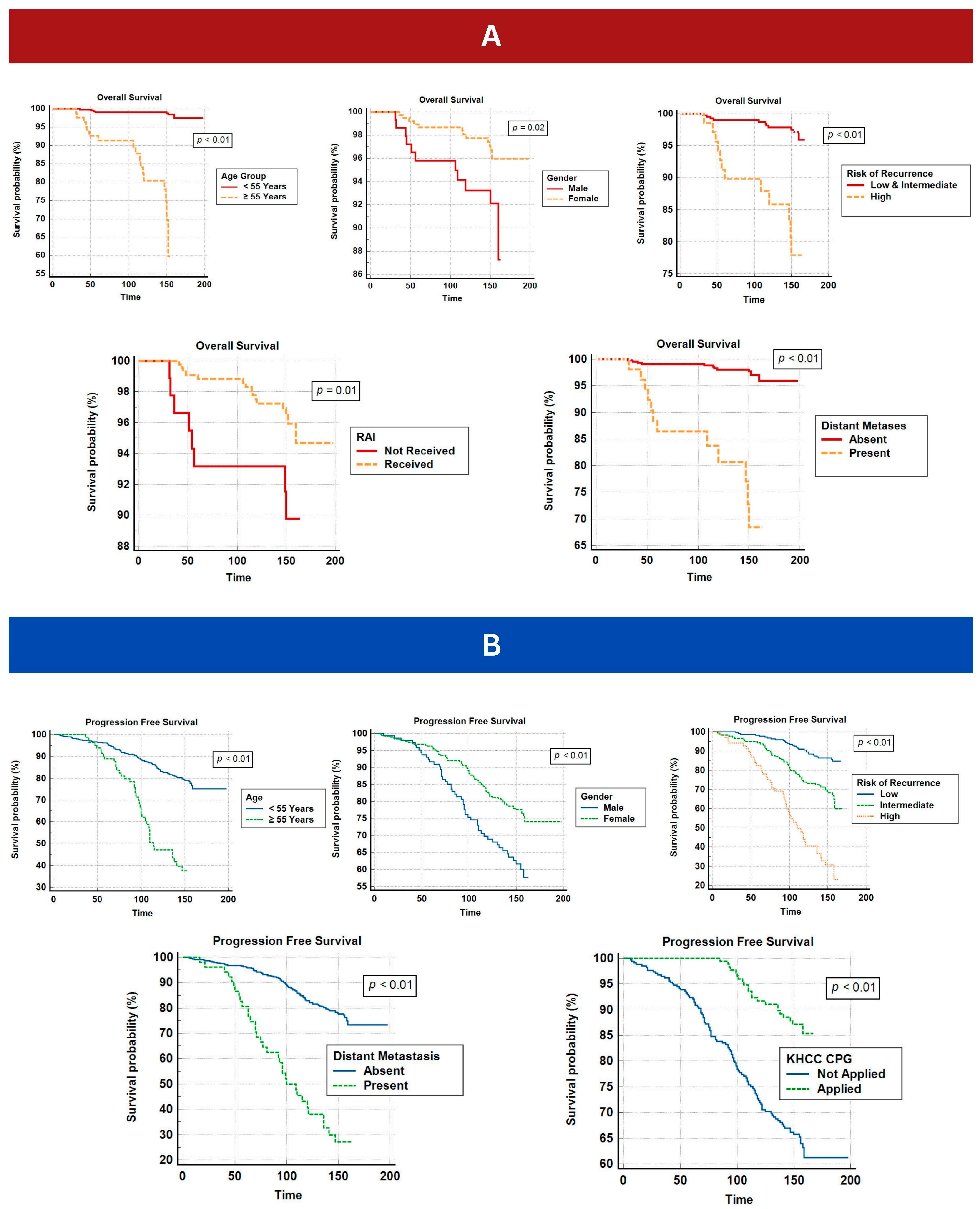
3.3. Survival Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brierley, J.D.; Panzarella, T.; Tsang, R.W.; Gospodarowicz, M.K.; O’Sullivan, B. A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer 1997, 79, 2414–2423. [Google Scholar] [CrossRef]
- Cady, B.; Rossi, R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 1988, 104, 947–953. [Google Scholar] [PubMed]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simoes, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.L.; Palme, C.E.; Audet, N.; Eski, S.; Walfish, P.G.; Freeman, J.L. Prognostic factors in well-differentiated thyroid carcinoma. Laryngoscope 2004, 114, 2110–2115. [Google Scholar] [CrossRef]
- Lamartina, L.; Godbert, Y.; Nascimento, C.; Do Cao, C.; Hescot, S.; Borget, I.; Al Ghuzlan, A.; Hartl, D.; Hadoux, J.; Pottier, E.; et al. Locally unresectable differentiated thyroid cancer: Outcomes and perspectives. Endocrine 2020, 69, 133–141. [Google Scholar] [CrossRef]
- Sit, D.; Koh, W.X.; Shokoohi, A.; Raycraft, T.; Vu, M.; Hamm, J.; Tran, E.; Berthelet, E.; Wu, J.; Olson, R.; et al. External Beam Radiation Therapy in pT4 Well-Differentiated Thyroid Cancer: A Population-Based Study of 405 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 468–478. [Google Scholar] [CrossRef]
- Jayarangaiah, A.; Sidhu, G.; Brown, J.; Barrett-Campbell, O.; Bahtiyar, G.; Youssef, I.; Arora, S.; Skwiersky, S.; McFarlane, S.I. Therapeutic options for advanced thyroid cancer. Int. J. Clin. Endocrinol. Metab. 2019, 5, 26. [Google Scholar] [CrossRef]
- Tang, J.; Kong, D.; Cui, Q.; Wang, K.; Zhang, D.; Liao, X.; Gong, Y.; Wu, G. The role of radioactive iodine therapy in papillary thyroid cancer: An observational study based on SEER. OncoTargets Ther. 2018, 11, 3551–3560. [Google Scholar] [CrossRef]
- Ibrahim, D.R.; Kelany, M.R.; Michael, M.M.; Elsayed, Z.M. Low-dose versus high-dose radioactive iodine ablation of differentiated thyroid carcinoma: A prospective randomized study. J. Clin. Oncol. 2016, 34, 6087. [Google Scholar] [CrossRef]
- Ratki, S.K.R.; Fallahi, B.; Namiranian, N.; Emami-Ardekani, A.; Saghari, M.; Mirabzadeh, A.; Fard-Esfahani, A.; Beiki, D.; Eftekhari, M.; Pooyafard, F. Factors affecting the quality of life of well-differentiated thyroid carcinoma patients: A cross-sectional study on 435 Iranian patients. Iran J. Nucl. Med. 2016, 24, 92–98. [Google Scholar]
- Al-Qahtani, K.H.; Al Asiri, M.; Tunio, M.A.; Aljohani, N.J.; Bayoumi, Y.; Fatani, H.; AlHadab, A. Adjuvant Radioactive iodine 131 ablation in papillary microcarcinoma of thyroid: Saudi Arabian experience. J. Otolaryngol.-Head Neck Surg. 2015, 44, 51. [Google Scholar] [CrossRef] [PubMed]
- Juweid, M.E.; Rabadi, N.J.; Tulchinsky, M.; Aloqaily, M.; Al-Momani, A.; Arabiat, M.; Abu Ain, G.; Al Hawari, H.; Al-Momani, M.; Mismar, A.; et al. Assessing potential impact of 2015 American Thyroid Association guidelines on community standard practice for I-131 treatment of low-risk differentiated thyroid cancer: Case study of Jordan. Endocrine 2021, 73, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kilfoy, B.A.; Zheng, T.; Holford, T.R.; Han, X.; Ward, M.H.; Sjodin, A.; Zhang, Y.; Bai, Y.; Zhu, C.; Guo, G.L.; et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 2009, 20, 525–531. [Google Scholar] [CrossRef]
- Li, M.; Brito, J.P.; Vaccarella, S. Long-Term Declines of Thyroid Cancer Mortality: An International Age-Period-Cohort Analysis. Thyroid 2020, 30, 838–846. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, J.D.; Koifman, S.; Koifman, R.J. Differentiated thyroid carcinoma: A 5-years survival study at a referral hospital in Brazil. Rev. Saude Publica 2019, 53, 106. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Fraumeni, J.F., Jr. (Eds.) Cancer Epidemiology and Prevention; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1024. [Google Scholar]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Higashi, T.; Nishii, R.; Yamada, S.; Nakamoto, Y.; Ishizu, K.; Kawase, S.; Togashi, K.; Itasaka, S.; Hiraoka, M.; Misaki, T. Delayed initial radioactive iodine therapy resulted in poor survival in patients with metastatic differentiated thyroid carcinoma: A retrospective statistical analysis of 198 cases. J. Nucl. Med. 2011, 52, 683–689. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Kheetan, R.; Hamdan, B.; Ibrahimi, A.; Mohamad, I.; Ghatasheh, H.; Barakat, F.; Maraqa, B.; Abo-Sheikha, A. Clinical Practice Guidelines for Thyroid Cancer. Available KHCC Libr. 2023, 7, 38. [Google Scholar]
- Fahey III, T.; Reeve, T.; Delbridge, L. Increasing incidence and changing presentation of thyroid cancer over a 30-year period. Br. J. Surg. 1995, 82, 518–520. [Google Scholar] [CrossRef]
- Abdulmughni, Y.A.; Al-Hureibi, M.A.; Al-Hureibi, K.A.; Ghafoor, M.A.; Al-Wadan, A.H.; Al-Hureibi, Y.A. Thyroid cancer in Yemen. Saudi Med. J. 2004, 25, 55–59. [Google Scholar]
- Aboelnaga, E.M.; Ahmed, R.A. Difference between papillary and follicular thyroid carcinoma outcomes: An experience from Egyptian institution. Cancer Biol. Med. 2015, 12, 53. [Google Scholar] [PubMed]
- Boutros, R.H.; Arabi, A.; Shoucair, M.; Abbas, J.; Salti, I. Disease Free Survival of Well Differentiated Thyroid Cancer: 20 years Experience at a tertiary care center in Lebanon. Int. Arch. Med. 2018, 11. [Google Scholar] [CrossRef]
- Al-Zaher, N.; Al-Salam, S.; El Teraifi, H. Thyroid carcinoma in the United Arab Emirates: Perspectives and experience of a tertiary care hospital. Hematol. Oncol. Stem Cell Ther. 2008, 1, 14–21. [Google Scholar] [CrossRef]
- LiVolsi, V.A. Papillary thyroid carcinoma: An update. Mod. Pathol. 2011, 24 (Suppl. 2), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Grogan, R.H.; Kaplan, S.P.; Cao, H.; Weiss, R.E.; Degroot, L.J.; Simon, C.A.; Embia, O.M.; Angelos, P.; Kaplan, E.L.; Schechter, R.B. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 2013, 154, 1436–1446; discussion 1446–1437. [Google Scholar] [CrossRef]
- Lamartina, L.; Durante, C.; Filetti, S.; Cooper, D.S. Low-risk differentiated thyroid cancer and radioiodine remnant ablation: A systematic review of the literature. J. Clin. Endocrinol. Metab. 2015, 100, 1748–1761. [Google Scholar] [CrossRef]
- Sacks, W.; Fung, C.H.; Chang, J.T.; Waxman, A.; Braunstein, G.D. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: A systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid 2010, 20, 1235–1245. [Google Scholar] [CrossRef]
- Sawka, A.M.; Brierley, J.D.; Tsang, R.W.; Thabane, L.; Rotstein, L.; Gafni, A.; Straus, S.; Goldstein, D.P. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 457–480. [Google Scholar] [CrossRef]
- Nixon, I.J.; Shah, J.P.; Zafereo, M.; Simo, R.S.; Hay, I.D.; Suarez, C.; Zbaren, P.; Rinaldo, A.; Sanabria, A.; Silver, C.; et al. The role of radioactive iodine in the management of patients with differentiated thyroid cancer—An oncologic surgical perspective. Eur. J. Surg. Oncol. 2020, 46, 754–762. [Google Scholar] [CrossRef]
- Jabin, Z.; Kwon, S.Y.; Bom, H.-S.; Lin, Y.; Yang, K.; Inaki, A.; Dewi, A.R.; Al-Ibraheem, A.N.; Balooshi, B.A.; San Luis, T.O. Clinico-social factors to choose radioactive iodine dose in differentiated thyroid cancer patients: An Asian survey. Nucl. Med. Commun. 2018, 39, 283–289. [Google Scholar] [CrossRef]
| Demographics | |
| Age At Diagnosis (in Years) | |
| Mean | 39 years |
| Range | 4–88 years |
| Gender (Number, Percentage) | |
| Male | 148 (28%) |
| Female | 380 (72%) |
| Nationality | |
| Jordan | 354 (67%) |
| Palestine | 40 (7.6%) |
| Iraq | 33 (6.2%) |
| Libya | 31 (5.9%) |
| Egypt | 26 (4.9%) |
| Yemen | 22 (4.2%) |
| Syria | 17 (3.2%) |
| Sudan | 5 (1%) |
| Histopathological Characteristics | |
| Histologic Subtypes (Number, Percentage) | |
| Papillary | 468 (88.6%) |
| Follicular | 40 (7.6%) |
| Hürthle | 20 (3.8%) |
| Tumor Size (cm mean and range) | 2.7 cm (0.1–15 cm) |
| T-Category (Number, Percentage) | |
| T1a | 57 (10.8%) |
| T1b | 120 (22.7%) |
| T2 | 149 (28.2%) |
| T3a | 91 (17.3%) |
| T3b | 66 (12.5%) |
| T4a | 24 (4.5%) |
| T4b | 21 (4%) |
| N-Category (Number, Percentage) | |
| N0 | 262 (49.6%) |
| N1a | 111 (21%) |
| N1b | 155 (29.4%) |
| M-Category (Number, Percentage) | |
| M0 | 470 (89%) |
| M1 | 58 (11%) |
| Overall Staging (Number, Percentage), according to AJCC 1 8th edition | |
| Stage I | 349 (66.1%) |
| Stage II | 73 (13.8%) |
| Stage III | 46 (8.7%) |
| Stage IVa | 38 (7.2%) |
| Stage IVb | 14 (2.7%) |
| Stage IVc | 8 (1.5%) |
| Treatment Modality (Number, Percentage) | |
| Total Thyroidectomy | 438 (83%) |
| Partial Thyroidectomy | 32 (6%) |
| Completion thyroidectomy | 32 (6%) |
| RAI 2 | 431 (81.6%) |
| Tyrosine Kinase inhibitors | 10 (2.6%) |
| Palliative Therapy | 29 (5.5%) |
| RAI Treatment Received (Number, Percentage) | |
| Single Dose | 344 (65.2%) |
| Multiple Doses | 94 (17.8%) |
| Not Received | 90 (17%) |
| Response after RAI Therapy (Number, Percentage) | |
| Complete Response | 316 (59.8%) |
| Partial Response | 117 (21.2%) |
| No Response | 5 (1%) |
| Cumulative RAI dose (GBq mean and range) | 5.5 GBq (1.1–37 GBq) |
| Risk of Recurrence (Number, Percentage), according to 2015 ATA 3 guidelines | |
| Low Risk | 257 (48.7%) |
| Intermediate Risk | 190 (36%) |
| High Risk | 81 (15.3%) |
| Variable | PFT 1 | ST 2 | p Value | |
|---|---|---|---|---|
| Age at diagnosis | <55 Years | 173.7 | 196 | <0.01 (For PFS) |
| ≥55 Years | 116.7 | 139.3 | <0.01 (For OS) | |
| Gender | Female | 172.9 | 194.4 | <0.01 (For PFS) |
| Male | 134.4 | 156.4 | 0.02 (For OS) | |
| Risk of recurrence | Low | 157.4 | <0.01 (For PFS) <0.01 (For OS) | |
| IM 3 | 142.3 | |||
| Low and IM | 166 | |||
| High | 110.2 | 149 | ||
| RAI 4 | Received | 193 | 0.01 (For OS) | |
| Not Received | 155.3 | |||
| Distant Metastasis | Absent | 173 | 194.7 | <0.01 (For PFS) |
| Present | 106.5 | 143 | <0.01 (For OS) | |
| CPG 5 | Applied | 161.3 | <0.01 (For PFS) | |
| Not Applied | 157 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ibraheem, A.; Al-Rasheed, U.; Mashhadani, N.; Abdlkadir, A.S.; Al-Adhami, D.A.; Ruzzeh, S.; Istatieh, F.; Mansour, A.; Hamdan, B.; Kheetan, R.; et al. Long-Term Survival Analysis and Prognostic Factors of Arabic Patients with Differentiated Thyroid Carcinoma: A 20-Year Observational Study at the King Hussein Cancer Center (KHCC) Involving 528 Patients. Cancers 2023, 15, 4102. https://doi.org/10.3390/cancers15164102
Al-Ibraheem A, Al-Rasheed U, Mashhadani N, Abdlkadir AS, Al-Adhami DA, Ruzzeh S, Istatieh F, Mansour A, Hamdan B, Kheetan R, et al. Long-Term Survival Analysis and Prognostic Factors of Arabic Patients with Differentiated Thyroid Carcinoma: A 20-Year Observational Study at the King Hussein Cancer Center (KHCC) Involving 528 Patients. Cancers. 2023; 15(16):4102. https://doi.org/10.3390/cancers15164102
Chicago/Turabian StyleAl-Ibraheem, Akram, Ula Al-Rasheed, Noor Mashhadani, Ahmed Saad Abdlkadir, Dhuha Ali Al-Adhami, Saad Ruzzeh, Feras Istatieh, Areen Mansour, Basem Hamdan, Reem Kheetan, and et al. 2023. "Long-Term Survival Analysis and Prognostic Factors of Arabic Patients with Differentiated Thyroid Carcinoma: A 20-Year Observational Study at the King Hussein Cancer Center (KHCC) Involving 528 Patients" Cancers 15, no. 16: 4102. https://doi.org/10.3390/cancers15164102
APA StyleAl-Ibraheem, A., Al-Rasheed, U., Mashhadani, N., Abdlkadir, A. S., Al-Adhami, D. A., Ruzzeh, S., Istatieh, F., Mansour, A., Hamdan, B., Kheetan, R., Al-Shatti, M., Mohamad, I., Juweid, M. E., Abu Sheikha, A., Al-Rabi, K., Sykiotis, G. P., Kreissl, M. C., Ismael, T., Sultan, I., & Abdel-Razeq, H. (2023). Long-Term Survival Analysis and Prognostic Factors of Arabic Patients with Differentiated Thyroid Carcinoma: A 20-Year Observational Study at the King Hussein Cancer Center (KHCC) Involving 528 Patients. Cancers, 15(16), 4102. https://doi.org/10.3390/cancers15164102