The Neurodevelopmental and Molecular Landscape of Medulloblastoma Subgroups: Current Targets and the Potential for Combined Therapies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Neurodevelopmental and Molecular Underpinnings of Medulloblastoma Subgroups
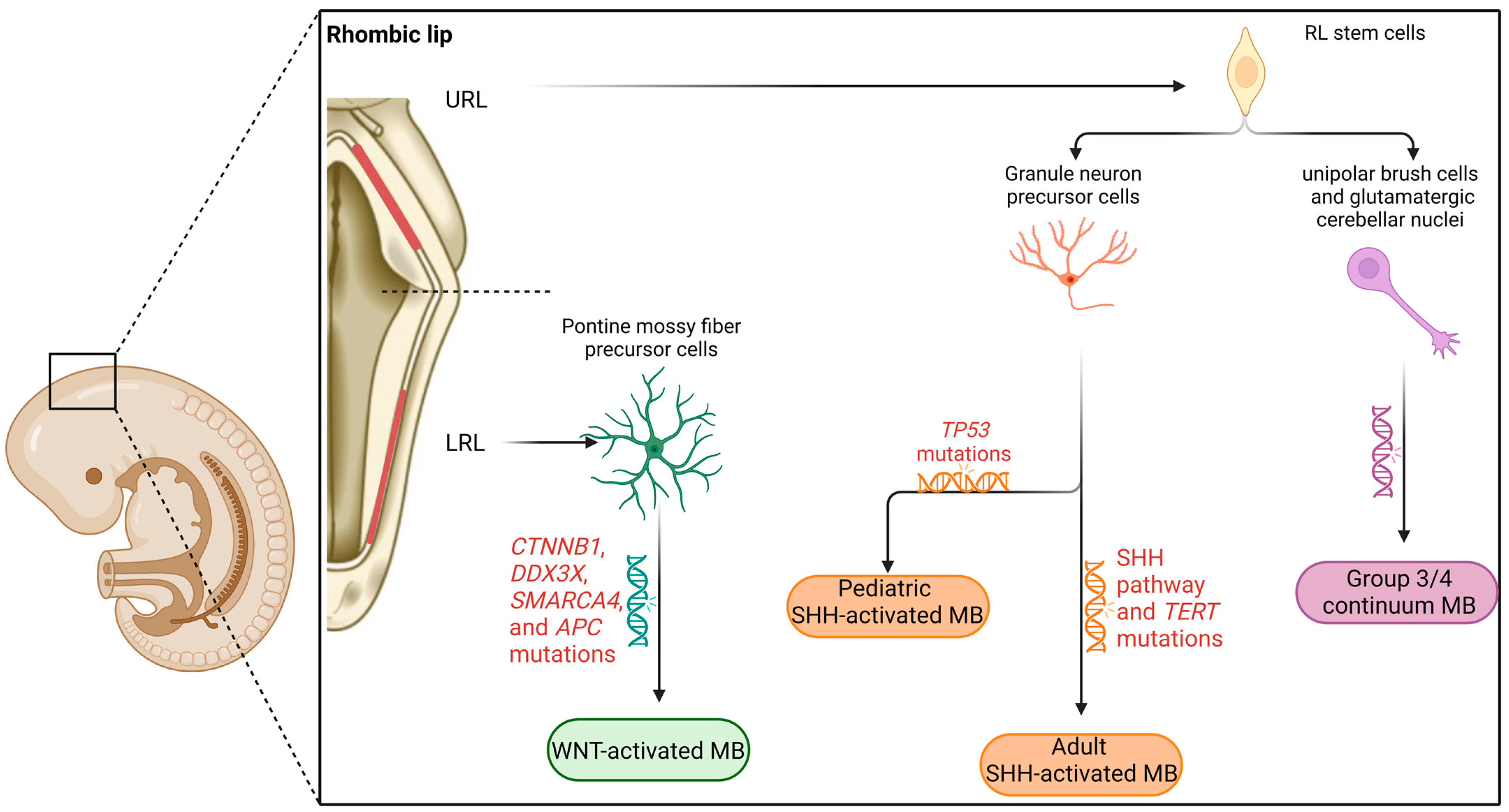
2.1. Cerebellar Embryonal Development
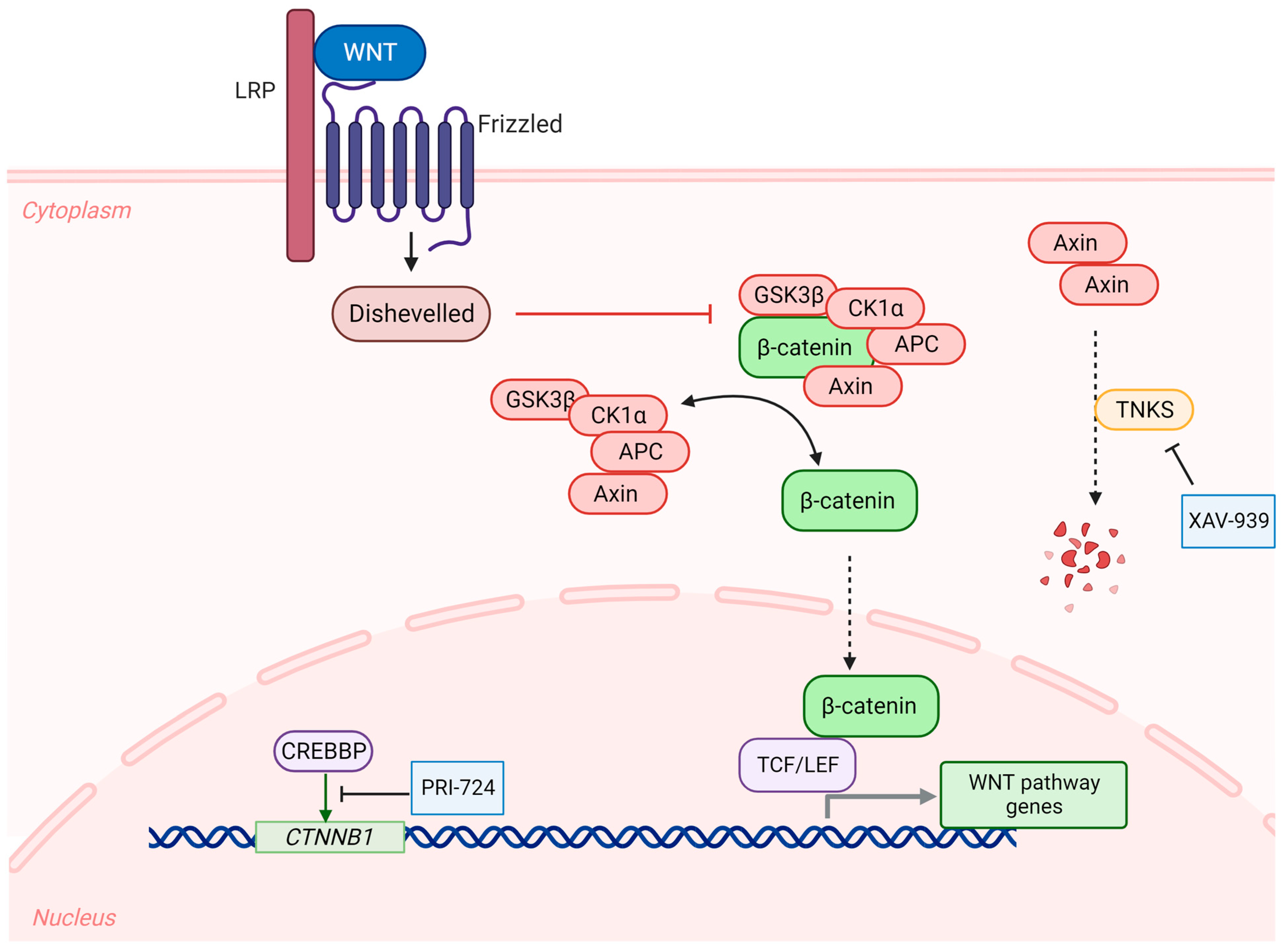
2.2. WNT-Activated Medulloblastoma
2.3. SHH-Activated Medulloblastoma
2.4. Group 3 Medulloblastoma
2.5. Group 4 Medulloblastoma
2.6. Intermediate Group 3/Group 4 Medulloblastoma
3. Subgroup-Specific Targeted Therapies in Medulloblastoma
3.1. WNT-Activated Medulloblastoma
3.2. SHH-Activated Medulloblastoma
3.3. Group 3 Medulloblastoma
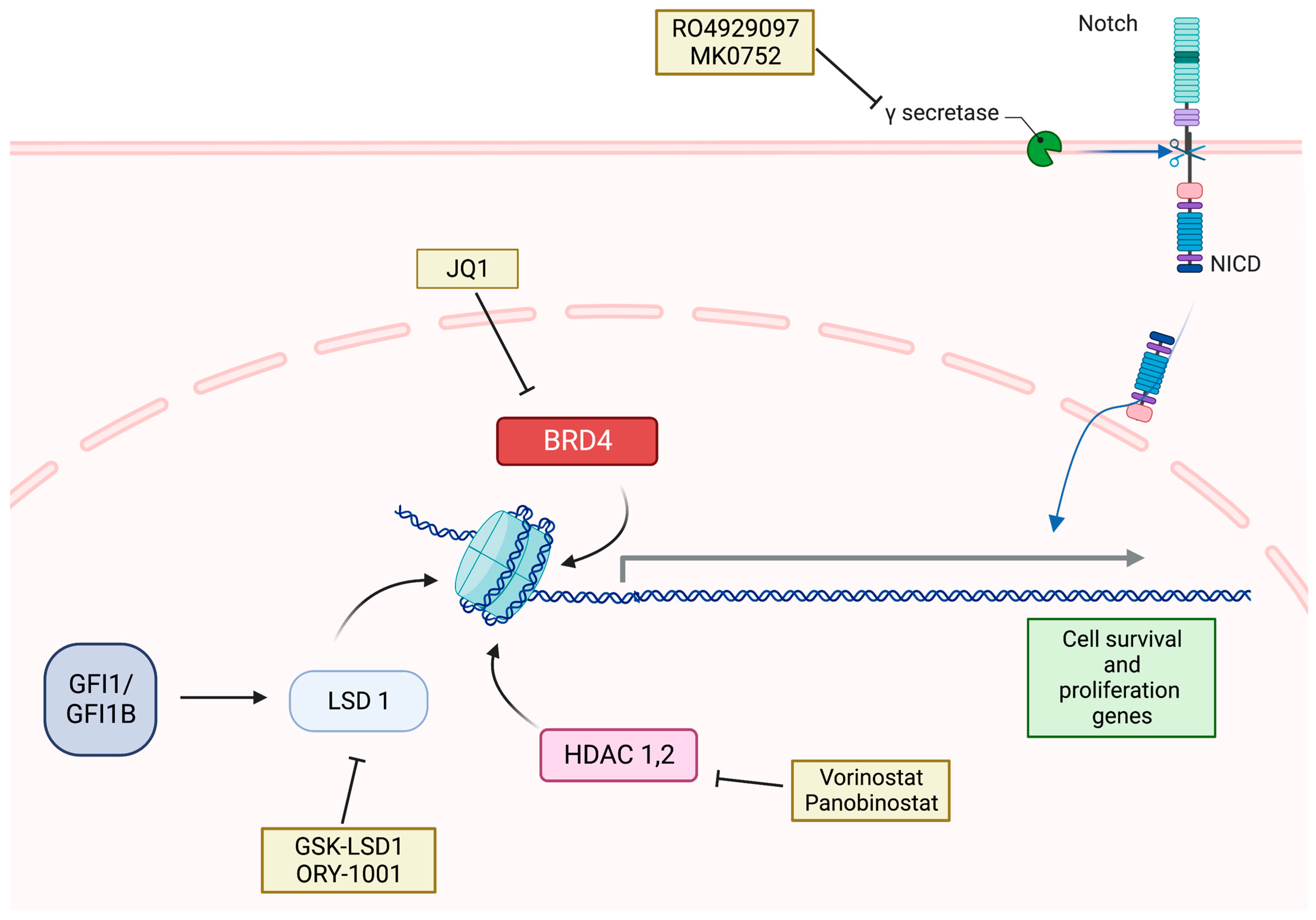
3.4. Group 4 Medulloblastoma
4. Other Non-Specific Combination Therapies in Medulloblastoma
4.1. Targeting the PI3K/mTOR Pathway
4.2. Targeting Tyrosine Kinases
4.3. Targeting Vascular Endothelial Growth Factor
4.4. Immunotherapy
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro. Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.R. Brain Tumors in Children. N. Engl. J. Med. 2022, 386, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Primers 2019, 5, 11. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Garancher, A.; Ramaswamy, V.; Wechsler-Reya, R.J. Medulloblastoma: From Molecular Subgroups to Molecular Targeted Therapies. Annu. Rev. Neurosci. 2018, 41, 207–232. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Semeria Mantelli, S.; Mosconi, M.; Foiadelli, T.; Savasta, S. Targeting the medulloblastoma: A molecular-based approach. Acta Biomed. 2020, 91, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.F.; Stripay, J.L. Epigenetic Drivers in Pediatric Medulloblastoma. Cerebellum 2018, 17, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Hovestadt, V.; Smith, K.S.; Bihannic, L.; Filbin, M.G.; Shaw, M.L.; Baumgartner, A.; De Witt, J.C.; Groves, A.; Mayr, L.; Weisman, H.R.; et al. Resolving medulloblastoma cellular architecture by single-cell genomics. Nature 2019, 572, 74–79. [Google Scholar] [CrossRef]
- Hendrikse, L.D.; Haldipur, P.; Saulnier, O.; Millman, J.; Sjoboen, A.H.; Erickson, A.W.; Ong, W.; Gordon, V.; Coudiere-Morrison, L.; Mercier, A.L.; et al. Failure of human rhombic lip differentiation underlies medulloblastoma formation. Nature 2022, 609, 1021–1028. [Google Scholar] [CrossRef]
- Luo, Z.; Xia, M.; Shi, W.; Zhao, C.; Wang, J.; Xin, D.; Dong, X.; Xiong, Y.; Zhang, F.; Berry, K.; et al. Human fetal cerebellar cell atlas informs medulloblastoma origin and oncogenesis. Nature 2022, 612, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Vladoiu, M.C.; El-Hamamy, I.; Donovan, L.K.; Farooq, H.; Holgado, B.L.; Sundaravadanam, Y.; Ramaswamy, V.; Hendrikse, L.D.; Kumar, S.; Mack, S.C.; et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature 2019, 572, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Leto, K.; Arancillo, M.; Becker, E.B.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef] [PubMed]
- Wingate, R.J. The rhombic lip and early cerebellar development. Curr. Opin. Neurobiol. 2001, 11, 82–88. [Google Scholar] [CrossRef]
- Wang, V.Y.; Rose, M.F.; Zoghbi, H.Y. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 2005, 48, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Belzunce, I.; Belmonte-Mateos, C.; Pujades, C. The interplay of atoh1 genes in the lower rhombic lip during hindbrain morphogenesis. PLoS ONE 2020, 15, e0228225. [Google Scholar] [CrossRef] [Green Version]
- Wullimann, M.F.; Mueller, T.; Distel, M.; Babaryka, A.; Grothe, B.; Koster, R.W. The long adventurous journey of rhombic lip cells in jawed vertebrates: A comparative developmental analysis. Front. Neuroanat. 2011, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.; Tong, Y.; Robinson, G.; Thompson, M.C.; Currle, D.S.; Eden, C.; Kranenburg, T.A.; Hogg, T.; Poppleton, H.; Martin, J.; et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 2010, 468, 1095–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, J.; Ha, T.J.; Swanson, D.J.; Choi, K.; Tong, Y.; Goldowitz, D. Wls provides a new compartmental view of the rhombic lip in mouse cerebellar development. J. Neurosci. 2014, 34, 12527–12537. [Google Scholar] [CrossRef] [Green Version]
- Consalez, G.G.; Goldowitz, D.; Casoni, F.; Hawkes, R. Origins, Development, and Compartmentation of the Granule Cells of the Cerebellum. Front. Neural Circuits 2020, 14, 611841. [Google Scholar] [CrossRef]
- Haldipur, P.; Aldinger, K.A.; Bernardo, S.; Deng, M.; Timms, A.E.; Overman, L.M.; Winter, C.; Lisgo, S.N.; Razavi, F.; Silvestri, E.; et al. Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science 2019, 366, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Hovestadt, V.; Ayrault, O.; Swartling, F.J.; Robinson, G.W.; Pfister, S.M.; Northcott, P.A. Medulloblastomics revisited: Biological and clinical insights from thousands of patients. Nat. Rev. Cancer 2020, 20, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Jessa, S.; Blanchet-Cohen, A.; Krug, B.; Vladoiu, M.; Coutelier, M.; Faury, D.; Poreau, B.; De Jay, N.; Hebert, S.; Monlong, J.; et al. Stalled developmental programs at the root of pediatric brain tumors. Nat. Genet. 2019, 51, 1702–1713. [Google Scholar] [CrossRef]
- Waszak, S.M.; Northcott, P.A.; Buchhalter, I.; Robinson, G.W.; Sutter, C.; Groebner, S.; Grund, K.B.; Brugieres, L.; Jones, D.T.W.; Pajtler, K.W.; et al. Spectrum and prevalence of genetic predisposition in medulloblastoma: A retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol. 2018, 19, 785–798. [Google Scholar] [CrossRef]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Grobner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754.e736. [Google Scholar] [CrossRef] [Green Version]
- Robinson, G.; Parker, M.; Kranenburg, T.A.; Lu, C.; Chen, X.; Ding, L.; Phoenix, T.N.; Hedlund, E.; Wei, L.; Zhu, X.; et al. Novel mutations target distinct subgroups of medulloblastoma. Nature 2012, 488, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Parsons, D.W.; Li, M.; Zhang, X.; Jones, S.; Leary, R.J.; Lin, J.C.; Boca, S.M.; Carter, H.; Samayoa, J.; Bettegowda, C.; et al. The genetic landscape of the childhood cancer medulloblastoma. Science 2011, 331, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Sexton-Oates, A.; MacGregor, D.; Dodgshun, A.; Saffery, R. The potential for epigenetic analysis of paediatric CNS tumours to improve diagnosis, treatment and prognosis. Ann. Oncol. 2015, 26, 1314–1324. [Google Scholar] [CrossRef]
- Shih, D.J.; Northcott, P.A.; Remke, M.; Korshunov, A.; Ramaswamy, V.; Kool, M.; Luu, B.; Yao, Y.; Wang, X.; Dubuc, A.M.; et al. Cytogenetic prognostication within medulloblastoma subgroups. J. Clin. Oncol. 2014, 32, 886–896. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez Castro, L.N.; Liu, I.; Filbin, M. Characterizing the biology of primary brain tumors and their microenvironment via single-cell profiling methods. Neuro. Oncol. 2023, 25, 234–247. [Google Scholar] [CrossRef]
- Kaderali, Z.; Lamberti-Pasculli, M.; Rutka, J.T. The changing epidemiology of paediatric brain tumours: A review from the Hospital for Sick Children. Childs Nerv. Syst. 2009, 25, 787–793. [Google Scholar] [CrossRef]
- Paugh, B.S.; Qu, C.; Jones, C.; Liu, Z.; Adamowicz-Brice, M.; Zhang, J.; Bax, D.A.; Coyle, B.; Barrow, J.; Hargrave, D.; et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J. Clin. Oncol. 2010, 28, 3061–3068. [Google Scholar] [CrossRef] [Green Version]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Zagozewski, J.; Shahriary, G.M.; Morrison, L.C.; Saulnier, O.; Stromecki, M.; Fresnoza, A.; Palidwor, G.; Porter, C.J.; Forget, A.; Ayrault, O.; et al. An OTX2-PAX3 signaling axis regulates Group 3 medulloblastoma cell fate. Nat. Commun. 2020, 11, 3627. [Google Scholar] [CrossRef]
- Lund, L.W.; Schmiegelow, K.; Rechnitzer, C.; Johansen, C. A systematic review of studies on psychosocial late effects of childhood cancer: StructuRes. of society and methodological pitfalls may challenge the conclusions. Pediatr. Blood Cancer 2011, 56, 532–543. [Google Scholar] [CrossRef]
- Rousseau, A.; Idbaih, A.; Ducray, F.; Criniere, E.; Fevre-Montange, M.; Jouvet, A.; Delattre, J.Y. Specific chromosomal imbalances as detected by array CGH in ependymomas in association with tumor location, histological subtype and grade. J. Neurooncol. 2010, 97, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Witt, H.; Hielscher, T.; Benner, A.; Remke, M.; Ryzhova, M.; Milde, T.; Bender, S.; Wittmann, A.; Schottler, A.; et al. Molecular staging of intracranial ependymoma in children and adults. J. Clin. Oncol. 2010, 28, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Rickert, C.H.; Strater, R.; Kaatsch, P.; Wassmann, H.; Jurgens, H.; Dockhorn-Dworniczak, B.; Paulus, W. Pediatric high-grade astrocytomas show chromosomal imbalances distinct from adult cases. Am. J. Pathol. 2001, 158, 1525–1532. [Google Scholar] [CrossRef] [Green Version]
- Dubuc, A.M.; Remke, M.; Korshunov, A.; Northcott, P.A.; Zhan, S.H.; Mendez-Lago, M.; Kool, M.; Jones, D.T.; Unterberger, A.; Morrissy, A.S.; et al. Aberrant patterns of H3K4 and H3K27 histone lysine methylation occur across subgroups in medulloblastoma. Acta Neuropathol. 2013, 125, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.S.; Bihannic, L.; Gudenas, B.L.; Haldipur, P.; Tao, R.; Gao, Q.; Li, Y.; Aldinger, K.A.; Iskusnykh, I.Y.; Chizhikov, V.V.; et al. Unified rhombic lip origins of group 3 and group 4 medulloblastoma. Nature 2022, 609, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Erkek, S.; Tong, Y.; Yin, L.; Federation, A.J.; Zapatka, M.; Haldipur, P.; Kawauchi, D.; Risch, T.; Warnatz, H.J.; et al. Active medulloblastoma enhancers reveal subgroup-specific cellular origins. Nature 2016, 530, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northcott, P.A.; Lee, C.; Zichner, T.; Stutz, A.M.; Erkek, S.; Kawauchi, D.; Shih, D.J.; Hovestadt, V.; Zapatka, M.; Sturm, D.; et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature 2014, 511, 428–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northcott, P.A.; Shih, D.J.; Peacock, J.; Garzia, L.; Morrissy, A.S.; Zichner, T.; Stutz, A.M.; Korshunov, A.; Reimand, J.; Schumacher, S.E.; et al. Subgroup-specific structural variation across 1000 medulloblastoma genomes. Nature 2012, 488, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, D.; Schwalbe, E.C.; Hicks, D.; Aldinger, K.A.; Lindsey, J.C.; Crosier, S.; Richardson, S.; Goddard, J.; Hill, R.M.; Castle, J.; et al. Medulloblastoma group 3 and 4 tumors comprise a clinically and biologically significant expression continuum reflecting human cerebellar development. Cell Rep. 2022, 40, 111162. [Google Scholar] [CrossRef]
- Ellison, D.W.; Onilude, O.E.; Lindsey, J.C.; Lusher, M.E.; Weston, C.L.; Taylor, R.E.; Pearson, A.D.; Clifford, S.C.; United Kingdom Children’s Cancer Study Group Brain Tumour Committee. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: The United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J. Clin. Oncol. 2005, 23, 7951–7957. [Google Scholar] [CrossRef]
- Clifford, S.C.; Lannering, B.; Schwalbe, E.C.; Hicks, D.; O’Toole, K.; Nicholson, S.L.; Goschzik, T.; Zur Muhlen, A.; Figarella-Branger, D.; Doz, F.; et al. Biomarker-driven stratification of disease-risk in non-metastatic medulloblastoma: Results from the multi-center HIT-SIOP-PNET4 clinical trial. Oncotarget 2015, 6, 38827–38839. [Google Scholar] [CrossRef] [Green Version]
- Phoenix, T.N.; Patmore, D.M.; Boop, S.; Boulos, N.; Jacus, M.O.; Patel, Y.T.; Roussel, M.F.; Finkelstein, D.; Goumnerova, L.; Perreault, S.; et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016, 29, 508–522. [Google Scholar] [CrossRef] [Green Version]
- Ris, M.D.; Packer, R.; Goldwein, J.; Jones-Wallace, D.; Boyett, J.M. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children’s Cancer Group study. J. Clin. Oncol. 2001, 19, 3470–3476. [Google Scholar] [CrossRef]
- Moxon-Emre, I.; Taylor, M.D.; Bouffet, E.; Hardy, K.; Campen, C.J.; Malkin, D.; Hawkins, C.; Laperriere, N.; Ramaswamy, V.; Bartels, U.; et al. Intellectual Outcome in Molecular Subgroups of Medulloblastoma. J. Clin. Oncol. 2016, 34, 4161–4170. [Google Scholar] [CrossRef]
- Michalski, J.M.; Janss, A.J.; Vezina, L.G.; Smith, K.S.; Billups, C.A.; Burger, P.C.; Embry, L.M.; Cullen, P.L.; Hardy, K.K.; Pomeroy, S.L.; et al. Children’s Oncology Group Phase III Trial of Reduced-Dose and Reduced-Volume Radiotherapy with Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J. Clin. Oncol. 2021, 39, 2685–2697. [Google Scholar] [CrossRef] [PubMed]
- Cooney, T.; Lindsay, H.; Leary, S.; Wechsler-Reya, R. Current studies and future directions for medulloblastoma: A review from the pacific pediatric neuro-oncology consortium (PNOC) disease working group. Neoplasia 2023, 35, 100861. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014, 13, 513–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, T.J.; Weeraratne, S.D.; Archer, T.C.; Pomeranz Krummel, D.A.; Auclair, D.; Bochicchio, J.; Carneiro, M.O.; Carter, S.L.; Cibulskis, K.; Erlich, R.L.; et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature 2012, 488, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tantravedi, S.; Vesuna, F.; Winnard, P.T., Jr.; Martin, A.; Lim, M.; Eberhart, C.G.; Berlinicke, C.; Raabe, E.; van Diest, P.J.; Raman, V. Targeting DDX3 in Medulloblastoma Using the Small Molecule Inhibitor RK-33. Transl. Oncol. 2019, 12, 96–105. [Google Scholar] [CrossRef]
- Huang, S.M.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Renna, C.; Salaroli, R.; Cocchi, C.; Cenacchi, G. XAV939-mediated ARTD activity inhibition in human MB cell lines. PLoS ONE 2015, 10, e0124149. [Google Scholar] [CrossRef]
- Ferri, M.; Liscio, P.; Carotti, A.; Asciutti, S.; Sardella, R.; Macchiarulo, A.; Camaioni, E. Targeting Wnt-driven cancers: Discovery of novel tankyrase inhibitors. Eur. J. Med. Chem. 2017, 142, 506–522. [Google Scholar] [CrossRef]
- Bassani, B.; Bartolini, D.; Pagani, A.; Principi, E.; Zollo, M.; Noonan, D.M.; Albini, A.; Bruno, A. Fenretinide (4-HPR) Targets Caspase-9, ERK 1/2 and the Wnt3a/beta-Catenin Pathway in Medulloblastoma Cells and Medulloblastoma Cell Spheroids. PLoS ONE 2016, 11, e0154111. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Hadden, M.K. Medulloblastoma drugs in development: Current leads, trials and drawbacks. Eur. J. Med. Chem. 2021, 215, 113268. [Google Scholar] [CrossRef]
- Ko, A.H.; Chiorean, E.G.; Kwak, E.L.; Lenz, H.-J.; Nadler, P.I.; Wood, D.L.; Fujimori, M.; Inada, T.; Kouji, H.; McWilliams, R.R. Final results of a phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J. Clin. Oncol. 2016, 34, e15721. [Google Scholar] [CrossRef]
- Lastowska, M.; Trubicka, J.; Niemira, M.; Paczkowska-Abdulsalam, M.; Karkucinska-Wieckowska, A.; Kaleta, M.; Drogosiewicz, M.; Tarasinska, M.; Perek-Polnik, M.; Kretowski, A.; et al. ALK Expression Is a Novel Marker for the WNT-activated Type of Pediatric Medulloblastoma and an Indicator of Good Prognosis for Patients. Am. J. Surg. Pathol. 2017, 41, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Lastowska, M.; Trubicka, J.; Karkucinska-Wieckowska, A.; Kaleta, M.; Tarasinska, M.; Perek-Polnik, M.; Sobocinska, A.A.; Dembowska-Baginska, B.; Grajkowska, W.; Matyja, E. Immunohistochemical detection of ALK protein identifies APC mutated medulloblastoma and differentiates the WNT-activated medulloblastoma from other types of posterior fossa childhood tumors. Brain Tumor Pathol. 2019, 36, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Vibhakar, R.; Foltz, G.; Yoon, J.G.; Field, L.; Lee, H.; Ryu, G.Y.; Pierson, J.; Davidson, B.; Madan, A. Dickkopf-1 is an epigenetically silenced candidate tumor suppressor gene in medulloblastoma. Neuro. Oncol. 2007, 9, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Lospinoso Severini, L.; Ghirga, F.; Bufalieri, F.; Quaglio, D.; Infante, P.; Di Marcotullio, L. The SHH/GLI signaling pathway: A therapeutic target for medulloblastoma. Expert Opin. Ther. Targets 2020, 24, 1159–1181. [Google Scholar] [CrossRef]
- Robarge, K.D.; Brunton, S.A.; Castanedo, G.M.; Cui, Y.; Dina, M.S.; Goldsmith, R.; Gould, S.E.; Guichert, O.; Gunzner, J.L.; Halladay, J.; et al. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg. Med. Chem. Lett. 2009, 19, 5576–5581. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Wu, X.; Jiang, J.; Gao, W.; Wan, Y.; Cheng, D.; Han, D.; Liu, J.; Englund, N.P.; Wang, Y.; et al. Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist. ACS Med. Chem. Lett. 2010, 1, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef] [Green Version]
- Gajjar, A.; Stewart, C.F.; Ellison, D.W.; Kaste, S.; Kun, L.E.; Packer, R.J.; Goldman, S.; Chintagumpala, M.; Wallace, D.; Takebe, N.; et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: A pediatric brain tumor consortium study. Clin. Cancer Res. 2013, 19, 6305–6312. [Google Scholar] [CrossRef] [Green Version]
- Robinson, G.W.; Orr, B.A.; Wu, G.; Gururangan, S.; Lin, T.; Qaddoumi, I.; Packer, R.J.; Goldman, S.; Prados, M.D.; Desjardins, A.; et al. Vismodegib Exerts Targeted Efficacy Against Recurrent Sonic Hedgehog-Subgroup Medulloblastoma: Results From Phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J. Clin. Oncol. 2015, 33, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Kieran, M.W.; Chisholm, J.; Casanova, M.; Brandes, A.A.; Aerts, I.; Bouffet, E.; Bailey, S.; Leary, S.; MacDonald, T.J.; Mechinaud, F.; et al. Phase I study of oral sonidegib (LDE225) in pediatric brain and solid tumors and a phase II study in children and adults with relapsed medulloblastoma. Neuro. Oncol. 2017, 19, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Ji, X.; De La Cruz, L.K.; Thareja, S.; Wang, B. Strategies to target the Hedgehog signaling pathway for cancer therapy. Med. Res. Rev. 2018, 38, 870–913. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Zhang, W.; Xu, Y.; Zhang, J.J. Design, synthesis and biological evaluation of anthranilamide derivatives as potent SMO inhibitors. Bioorg. Med. Chem. 2020, 28, 115354. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Tang, J.Y.; Gong, R.; Kim, J.; Lee, J.J.; Clemons, K.V.; Chong, C.R.; Chang, K.S.; Fereshteh, M.; Gardner, D.; et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell 2010, 17, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Aftab, B.T.; Tang, J.Y.; Kim, D.; Lee, A.H.; Rezaee, M.; Kim, J.; Chen, B.; King, E.M.; Borodovsky, A.; et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell 2013, 23, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Meco, D.; Attina, G.; Mastrangelo, S.; Navarra, P.; Ruggiero, A. Emerging Perspectives on the Antiparasitic Mebendazole as a Repurposed Drug for the Treatment of Brain Cancers. Int. J. Mol. Sci. 2023, 24, 1334. [Google Scholar] [CrossRef]
- Larsen, A.R.; Bai, R.Y.; Chung, J.H.; Borodovsky, A.; Rudin, C.M.; Riggins, G.J.; Bunz, F. Repurposing the antihelmintic mebendazole as a hedgehog inhibitor. Mol. Cancer Ther. 2015, 14, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Bodhinayake, I.; Symons, M.; Boockvar, J.A. Repurposing mebendazole for the treatment of medulloblastoma. Neurosurgery 2015, 76, N15–N16. [Google Scholar] [CrossRef] [Green Version]
- Lou, E.; Nelson, A.C.; Kool, M. Differential response of SHH-expressing adult medulloblastomas to the sonic hedgehog inhibitor vismodegib: Whole-genome analysis. Cancer Biol. Ther. 2019, 20, 1398–1402. [Google Scholar] [CrossRef]
- Kool, M.; Jones, D.T.; Jager, N.; Northcott, P.A.; Pugh, T.J.; Hovestadt, V.; Piro, R.M.; Esparza, L.A.; Markant, S.L.; Remke, M.; et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell 2014, 25, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Gholamin, S.; Schubert, S.; Willardson, M.I.; Lee, A.; Bandopadhayay, P.; Bergthold, G.; Masoud, S.; Nguyen, B.; Vue, N.; et al. Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nat. Med. 2014, 20, 732–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Jiang, W.; Sui, Y.; Meng, W.; Hou, L.; Li, T.; Li, M.; Zhang, L.; Mo, J.; Wang, J.; et al. CDK7 inhibition suppresses aberrant hedgehog pathway and overcomes resistance to smoothened antagonists. Proc. Natl. Acad. Sci. USA 2019, 116, 12986–12995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Li, S.; Sheng, H.; Cai, M.; Ma, L.Y.; Hu, L.; Xu, S.; Yu, L.S.; Zhang, N. Suppression of GLI sensitizes medulloblastoma cells to mitochondria-mediated apoptosis. J. Cancer Res. Clin. Oncol. 2016, 142, 2469–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konings, K.; Vandevoorde, C.; Belmans, N.; Vermeesen, R.; Baselet, B.; Walleghem, M.V.; Janssen, A.; Isebaert, S.; Baatout, S.; Haustermans, K.; et al. The Combination of Particle Irradiation With the Hedgehog Inhibitor GANT61 Differently Modulates the Radiosensitivity and Migration of Cancer Cells Compared to X-ray Irradiation. Front. Oncol. 2019, 9, 391. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.J.; Kim, J.; Gardner, D.; Beachy, P.A. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc. Natl. Acad. Sci. USA 2010, 107, 13432–13437. [Google Scholar] [CrossRef]
- Dos Santos Klinger, P.H.; Delsin, L.E.A.; Cruzeiro, G.A.V.; Andrade, A.F.; Lira, R.C.P.; de Andrade, P.V.; das Chagas, P.F.; de Paula Queiroz, R.G.; Trevisan, F.A.; de Oliveira, R.S.; et al. Arsenic Trioxide exerts cytotoxic and radiosensitizing effects in pediatric Medulloblastoma cell lines of SHH Subgroup. Sci. Rep. 2020, 10, 6836. [Google Scholar] [CrossRef] [Green Version]
- Cohen, K.J.; Gibbs, I.C.; Fisher, P.G.; Hayashi, R.J.; Macy, M.E.; Gore, L. A phase I trial of arsenic trioxide chemoradiotherapy for infiltrating astrocytomas of childhood. Neuro. Oncol. 2013, 15, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Peng, X.; Feng, C.; Xiong, X.; Li, J.; Liao, N.; Yang, Z.; Liu, A.; Wu, P.; Liang, X.; et al. Excellent Early Outcomes of Combined Chemotherapy with Arsenic Trioxide for Stage 4/M Neuroblastoma in Children: A Multicenter Nonrandomized Controlled Trial. Oncol. Res. 2021, 28, 791–800. [Google Scholar] [CrossRef]
- Faria, C.C.; Golbourn, B.J.; Dubuc, A.M.; Remke, M.; Diaz, R.J.; Agnihotri, S.; Luck, A.; Sabha, N.; Olsen, S.; Wu, X.; et al. Foretinib is effective therapy for metastatic sonic hedgehog medulloblastoma. Cancer Res. 2015, 75, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Luo, J.; Mosley, Y.Y.; Hedrick, V.E.; Paul, L.N.; Chang, J.; Zhang, G.; Wang, Y.K.; Banko, M.R.; Brunet, A.; et al. AMP-Activated Protein Kinase Directly Phosphorylates and Destabilizes Hedgehog Pathway Transcription Factor GLI1 in Medulloblastoma. Cell Rep. 2015, 12, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonnissen, A.; Isebaert, S.; McKee, C.M.; Muschel, R.J.; Haustermans, K. The Effect of Metformin and GANT61 Combinations on the Radiosensitivity of Prostate Cancer Cells. Int. J. Mol. Sci. 2017, 18, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Wei, B.; Lu, C.; Huang, X.; Li, P.; Chen, L. Metformin suppresses the expression of Sonic hedgehog in gastric cancer cells. Mol. Med. Rep. 2017, 15, 1909–1915. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.; Hu, Z.; Ye, J.; Liu, Y.; Lai, Z.; Zhang, M.; Ji, W.; Huang, L.; Zou, H.; Chen, B.; et al. Metformin exerts anti-tumor effects via Sonic hedgehog signaling pathway by targeting AMPK in HepG2 cells. Biochem. Cell Biol. 2022, 100, 142–151. [Google Scholar] [CrossRef]
- Northcott, P.A.; Fernandez, L.A.; Hagan, J.P.; Ellison, D.W.; Grajkowska, W.; Gillespie, Y.; Grundy, R.; Van Meter, T.; Rutka, J.T.; Croce, C.M.; et al. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009, 69, 3249–3255. [Google Scholar] [CrossRef] [Green Version]
- Murphy, B.L.; Obad, S.; Bihannic, L.; Ayrault, O.; Zindy, F.; Kauppinen, S.; Roussel, M.F. Silencing of the miR-17~92 cluster family inhibits medulloblastoma progression. Cancer Res. 2013, 73, 7068–7078. [Google Scholar] [CrossRef] [Green Version]
- Tan, I.L.; Arifa, R.D.N.; Rallapalli, H.; Kana, V.; Lao, Z.; Sanghrajka, R.M.; Sumru Bayin, N.; Tanne, A.; Wojcinski, A.; Korshunov, A.; et al. CSF1R inhibition depletes tumor-associated macrophages and attenuates tumor progression in a mouse sonic Hedgehog-Medulloblastoma model. Oncogene 2021, 40, 396–407. [Google Scholar] [CrossRef]
- Read, T.A.; Fogarty, M.P.; Markant, S.L.; McLendon, R.E.; Wei, Z.; Ellison, D.W.; Febbo, P.G.; Wechsler-Reya, R.J. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell 2009, 15, 135–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markant, S.L.; Esparza, L.A.; Sun, J.; Barton, K.L.; McCoig, L.M.; Grant, G.A.; Crawford, J.R.; Levy, M.L.; Northcott, P.A.; Shih, D.; et al. Targeting sonic hedgehog-associated medulloblastoma through inhibition of Aurora and Polo-like kinases. Cancer Res. 2013, 73, 6310–6322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; He, X.; Liu, X.; Zhang, F.; Huang, L.F.; Potter, A.S.; Xu, L.; Zhou, W.; Zheng, T.; Luo, Z.; et al. Single-Cell Transcriptomics in Medulloblastoma Reveals Tumor-Initiating Progenitors and Oncogenic Cascades during Tumorigenesis and Relapse. Cancer Cell 2019, 36, 302–318.e307. [Google Scholar] [CrossRef]
- Buonamici, S.; Williams, J.; Morrissey, M.; Wang, A.; Guo, R.; Vattay, A.; Hsiao, K.; Yuan, J.; Green, J.; Ospina, B.; et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2010, 2, 51ra70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, S.A.; Warrington, N.M.; Taylor, S.; Kfoury, N.; Luo, J.; Rubin, J.B. Reprogramming Medulloblastoma-Propagating Cells by a Combined Antagonism of Sonic Hedgehog and CXCR4. Cancer Res. 2017, 77, 1416–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandopadhayay, P.; Bergthold, G.; Nguyen, B.; Schubert, S.; Gholamin, S.; Tang, Y.; Bolin, S.; Schumacher, S.E.; Zeid, R.; Masoud, S.; et al. BET bromodomain inhibition of MYC-amplified medulloblastoma. Clin. Cancer Res. 2014, 20, 912–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataraman, S.; Alimova, I.; Balakrishnan, I.; Harris, P.; Birks, D.K.; Griesinger, A.; Amani, V.; Cristiano, B.; Remke, M.; Taylor, M.D.; et al. Inhibition of BRD4 attenuates tumor cell self-renewal and suppresses stem cell signaling in MYC driven medulloblastoma. Oncotarget 2014, 5, 2355–2371. [Google Scholar] [CrossRef] [Green Version]
- Hanaford, A.R.; Archer, T.C.; Price, A.; Kahlert, U.D.; Maciaczyk, J.; Nikkhah, G.; Kim, J.W.; Ehrenberger, T.; Clemons, P.A.; Dancik, V.; et al. DiSCoVERing Innovative Therapies for Rare Tumors: Combining Genetically Accurate Disease Models with In Silico Analysis to Identify Novel Therapeutic Targets. Clin. Cancer Res. 2016, 22, 3903–3914. [Google Scholar] [CrossRef] [Green Version]
- Cook Sangar, M.L.; Genovesi, L.A.; Nakamoto, M.W.; Davis, M.J.; Knobluagh, S.E.; Ji, P.; Millar, A.; Wainwright, B.J.; Olson, J.M. Inhibition of CDK4/6 by Palbociclib Significantly Extends Survival in Medulloblastoma Patient-Derived Xenograft Mouse Models. Clin. Cancer Res. 2017, 23, 5802–5813. [Google Scholar] [CrossRef]
- Faria, C.C.; Agnihotri, S.; Mack, S.C.; Golbourn, B.J.; Diaz, R.J.; Olsen, S.; Bryant, M.; Bebenek, M.; Wang, X.; Bertrand, K.C.; et al. Identification of alsterpaullone as a novel small molecule inhibitor to target group 3 medulloblastoma. Oncotarget 2015, 6, 21718–21729. [Google Scholar] [CrossRef] [Green Version]
- Van Mater, D.; Gururangan, S.; Becher, O.; Campagne, O.; Leary, S.; Phillips, J.J.; Huang, J.; Lin, T.; Poussaint, T.Y.; Goldman, S.; et al. A phase I trial of the CDK 4/6 inhibitor palbociclib in pediatric patients with progressive brain tumors: A Pediatric Brain Tumor Consortium study (PBTC-042). Pediatr. Blood Cancer 2021, 68, e28879. [Google Scholar] [CrossRef]
- Archer, T.C.; Ehrenberger, T.; Mundt, F.; Gold, M.P.; Krug, K.; Mah, C.K.; Mahoney, E.L.; Daniel, C.J.; LeNail, A.; Ramamoorthy, D.; et al. Proteomics, Post-translational Modifications, and Integrative Analyses Reveal Molecular Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2018, 34, 396–410.e398. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, S.; Weeraratne, S.D.; Sun, H.; Phallen, J.; Rallapalli, S.K.; Teider, N.; Kosaras, B.; Amani, V.; Pierre-Francois, J.; Tang, Y.; et al. alpha5-GABAA receptors negatively regulate MYC-amplified medulloblastoma growth. Acta Neuropathol. 2014, 127, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Jonas, O.; Calligaris, D.; Methuku, K.R.; Poe, M.M.; Francois, J.P.; Tranghese, F.; Changelian, A.; Sieghart, W.; Ernst, M.; Krummel, D.A.; et al. First In Vivo Testing of Compounds Targeting Group 3 Medulloblastomas Using an Implantable Microdevice as a New Paradigm for Drug Development. J. Biomed. Nanotechnol. 2016, 12, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Veo, B.; Pierce, A.; Fosmire, S.; Madhavan, K.; Balakrishnan, I.; Donson, A.; Alimova, I.; Sullivan, K.D.; Joshi, M.; et al. A novel PLK1 inhibitor onvansertib effectively sensitizes MYC-driven medulloblastoma to radiotherapy. Neuro. Oncol. 2022, 24, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Veo, B.; Danis, E.; Pierce, A.; Sola, I.; Wang, D.; Foreman, N.K.; Jin, J.; Ma, A.; Serkova, N.; Venkataraman, S.; et al. Combined functional genomic and chemical screens identify SETD8 as a therapeutic target in MYC-driven medulloblastoma. JCI Insight 2019, 4, e122933. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sui, Y.; Li, Q.; Zhao, Y.; Dong, X.; Yang, J.; Liang, Z.; Han, Y.; Tang, Y.; Ma, J. Effective inhibition of MYC-amplified group 3 medulloblastoma by FACT-targeted curaxin drug CBL0137. Cell Death Dis. 2020, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Casaos, J.; Gorelick, N.L.; Huq, S.; Choi, J.; Xia, Y.; Serra, R.; Felder, R.; Lott, T.; Kast, R.E.; Suk, I.; et al. The Use of Ribavirin as an Anticancer Therapeutic: Will It Go Viral? Mol. Cancer Ther. 2019, 18, 1185–1194. [Google Scholar] [CrossRef]
- Huq, S.; Kannapadi, N.V.; Casaos, J.; Lott, T.; Felder, R.; Serra, R.; Gorelick, N.L.; Ruiz-Cardozo, M.A.; Ding, A.S.; Cecia, A.; et al. Preclinical efficacy of ribavirin in SHH and group 3 medulloblastoma. J. Neurosurg. Pediatr. 2021, 27, 482–488. [Google Scholar] [CrossRef]
- Serra, R.; Zhao, T.; Huq, S.; Gorelick, N.L.; Casaos, J.; Cecia, A.; Mangraviti, A.; Eberhart, C.; Bai, R.; Olivi, A.; et al. DisulfirAm. and copper combination therapy targets NPL4, cancer stem cells and extends survival in a medulloblastoma model. PLoS ONE 2021, 16, e0251957. [Google Scholar] [CrossRef]
- Lee, C.; Rudneva, V.A.; Erkek, S.; Zapatka, M.; Chau, L.Q.; Tacheva-Grigorova, S.K.; Garancher, A.; Rusert, J.M.; Aksoy, O.; Lea, R.; et al. Lsd1 as a therapeutic target in Gfi1-activated medulloblastoma. Nat. Commun. 2019, 10, 332. [Google Scholar] [CrossRef] [Green Version]
- Gholamin, S.; Mitra, S.S.; Feroze, A.H.; Liu, J.; Kahn, S.A.; Zhang, M.; Esparza, R.; Richard, C.; Ramaswamy, V.; Remke, M.; et al. Disrupting the CD47-SIRPalpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 2017, 9, eaaf2968. [Google Scholar] [CrossRef] [Green Version]
- Kahn, S.A.; Wang, X.; Nitta, R.T.; Gholamin, S.; Theruvath, J.; Hutter, G.; Azad, T.D.; Wadi, L.; Bolin, S.; Ramaswamy, V.; et al. Notch1 regulates the initiation of metastasis and self-renewal of Group 3 medulloblastoma. Nat. Commun. 2018, 9, 4121. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasan, L.; Wang, H.; Yap, S.Q.; Leclair, P.; Tam, A.; Lim, C.J. Autocrine IL-6/STAT3 signaling aids development of acquired drug resistance in Group 3 medulloblastoma. Cell Death Dis. 2020, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, L.; Li, L.V.; Leclair, P.; Lim, C.J. Targeting the gp130/STAT3 Axis Attenuates Tumor Microenvironment Mediated Chemoresistance in Group 3 Medulloblastoma Cells. Cells 2022, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, V.; Chaudhary, A.K.; Coulter, D.W.; McGuire, T.; Mahato, R.I. Impact of miRNA-mRNA Profiling and Their Correlation on Medulloblastoma Tumorigenesis. Mol. Ther. Nucleic Acids 2018, 12, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Weeraratne, S.D.; Amani, V.; Teider, N.; Pierre-Francois, J.; Winter, D.; Kye, M.J.; Sengupta, S.; Archer, T.; Remke, M.; Bai, A.H.; et al. Pleiotropic effects of miR-183~96~182 converge to regulate cell survival, proliferation and migration in medulloblastoma. Acta Neuropathol. 2012, 123, 539–552. [Google Scholar] [CrossRef]
- Bharambe, H.S.; Joshi, A.; Yogi, K.; Kazi, S.; Shirsat, N.V. Restoration of miR-193a expression is tumor-suppressive in MYC amplified Group 3 medulloblastoma. Acta Neuropathol. Commun. 2020, 8, 70. [Google Scholar] [CrossRef]
- Yogi, K.; Sridhar, E.; Goel, N.; Jalali, R.; Goel, A.; Moiyadi, A.; Thorat, R.; Panwalkar, P.; Khire, A.; Dasgupta, A.; et al. MiR-148a, a microRNA upregulated in the WNT subgroup tumors, inhibits invasion and tumorigenic potential of medulloblastoma cells by targeting Neuropilin 1. Oncoscience 2015, 2, 334–348. [Google Scholar] [CrossRef] [Green Version]
- Katsushima, K.; Lee, B.; Yuan, M.; Kunhiraman, H.; Stapleton, S.; Jallo, G.; Raabe, E.; Eberhart, C.; Perera, R. CSIG-32. microRNA 211, A POTENTIAL THERAPEUTIC AGENT FOR GROUP 3 MEDULLOBLASTOMA IN CHILDREN. Neuro-Oncology 2021, 23, vi40. [Google Scholar] [CrossRef]
- Perumal, N.; Kanchan, R.K.; Doss, D.; Bastola, N.; Atri, P.; Chirravuri-Venkata, R.; Thapa, I.; Vengoji, R.; Maurya, S.K.; Klinkebiel, D.; et al. MiR-212-3p functions as a tumor suppressor gene in group 3 medulloblastoma via targeting nuclear factor I/B (NFIB). Acta Neuropathol. Commun. 2021, 9, 195. [Google Scholar] [CrossRef]
- Rea, J.; Carissimo, A.; Trisciuoglio, D.; Illi, B.; Picard, D.; Remke, M.; Laneve, P.; Caffarelli, E. Identification and Functional Characterization of Novel MYC-Regulated Long Noncoding RNAs in Group 3 Medulloblastoma. Cancers 2021, 13, 3853. [Google Scholar] [CrossRef]
- Katsushima, K.; Lee, B.; Kunhiraman, H.; Zhong, C.; Murad, R.; Yin, J.; Liu, B.; Garancher, A.; Gonzalez-Gomez, I.; Monforte, H.L.; et al. The long noncoding RNA lnc-HLX-2-7 is oncogenic in Group 3 medulloblastomas. Neuro. Oncol. 2021, 23, 572–585. [Google Scholar] [CrossRef]
- Bolin, S.; Borgenvik, A.; Persson, C.U.; Sundstrom, A.; Qi, J.; Bradner, J.E.; Weiss, W.A.; Cho, Y.J.; Weishaupt, H.; Swartling, F.J. Combined BET bromodomain and CDK2 inhibition in MYC-driven medulloblastoma. Oncogene 2018, 37, 2850–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, N.K.; Kling, M.J.; Griggs, C.N.; Kesherwani, V.; Shukla, M.; McIntyre, E.M.; Ray, S.; Liu, Y.; McGuire, T.R.; Sharp, J.G.; et al. A Novel Combination Approach Targeting an Enhanced Protein Synthesis Pathway in MYC-driven (Group 3) Medulloblastoma. Mol. Cancer Ther. 2020, 19, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Kling, M.J.; Kesherwani, V.; Mishra, N.K.; Alexander, G.; McIntyre, E.M.; Ray, S.; Challagundla, K.B.; Joshi, S.S.; Coulter, D.W.; Chaturvedi, N.K. A novel dual epigenetic approach targeting BET proteins and HDACs in Group 3 (MYC-driven) Medulloblastoma. J. Exp. Clin. Cancer Res. 2022, 41, 321. [Google Scholar] [CrossRef] [PubMed]
- Menyhart, O.; Giangaspero, F.; Gyorffy, B. Molecular markers and potential therapeutic targets in non-WNT/non-SHH (group 3 and group 4) medulloblastomas. J. Hematol. Oncol. 2019, 12, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroni, M.; Guardia, G.D.A.; Lei, X.; Kosti, A.; Qiao, M.; Landry, T.; Mau, K.; Galante, P.A.F.; Penalva, L.O.F. The RNA-Binding Protein Musashi1 Regulates a Network of Cell Cycle Genes in Group 4 Medulloblastoma. Cells 2021, 11, 56. [Google Scholar] [CrossRef]
- Alimova, I.; Venkataraman, S.; Harris, P.; Marquez, V.E.; Northcott, P.A.; Dubuc, A.; Taylor, M.D.; Foreman, N.K.; Vibhakar, R. Targeting the enhancer of zeste homologue 2 in medulloblastoma. Int. J. Cancer 2012, 131, 1800–1809. [Google Scholar] [CrossRef] [Green Version]
- Park, A.K.; Lee, J.Y.; Cheong, H.; Ramaswamy, V.; Park, S.H.; Kool, M.; Phi, J.H.; Choi, S.A.; Cavalli, F.; Taylor, M.D.; et al. Subgroup-specific prognostic signaling and metabolic pathways in pediatric medulloblastoma. BMC Cancer 2019, 19, 571. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhang, M.; Xuan, Q.; Wang, Z.; Lian, X.; Zhang, Q. Jagged1 promotes aromatase inhibitor resistance by modulating tumor-associated macrophage differentiation in breast cancer patients. Breast Cancer Res. Treat 2017, 166, 95–107. [Google Scholar] [CrossRef]
- Shen, Q.; Cohen, B.; Zheng, W.; Rahbar, R.; Martin, B.; Murakami, K.; Lamorte, S.; Thompson, P.; Berman, H.; Zuniga-Pflucker, J.C.; et al. Notch Shapes the Innate Immunophenotype in Breast Cancer. Cancer Discov. 2017, 7, 1320–1335. [Google Scholar] [CrossRef] [Green Version]
- Sierra, R.A.; Trillo-Tinoco, J.; Mohamed, E.; Yu, L.; Achyut, B.R.; Arbab, A.; Bradford, J.W.; Osborne, B.A.; Miele, L.; Rodriguez, P.C. Anti-Jagged Immunotherapy Inhibits MDSCs and Overcomes Tumor-Induced Tolerance. Cancer Res. 2017, 77, 5628–5638. [Google Scholar] [CrossRef] [Green Version]
- Meurette, O.; Mehlen, P. Notch Signaling in the Tumor Microenvironment. Cancer Cell 2018, 34, 536–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, L.M.; Fouladi, M.; Olson, J.; Daryani, V.M.; Stewart, C.F.; Wetmore, C.; Kocak, M.; Onar-Thomas, A.; Wagner, L.; Gururangan, S.; et al. Phase I trial of weekly MK-0752 in children with refractory central nervous system malignancies: A pediatric brain tumor consortium study. Childs Nerv. Syst. 2015, 31, 1283–1289. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Katsushima, K.; Pokhrel, R.; Yuan, M.; Stapleton, S.; Jallo, G.; Wechsler-Reya, R.J.; Eberhart, C.G.; Ray, A.; Perera, R.J. The long non-coding RNA SPRIGHTLY and its binding partner PTBP1 regulate exon 5 skipping of SMYD3 transcripts in group 4 medulloblastomas. Neurooncol. Adv. 2022, 4, vdac120. [Google Scholar] [CrossRef]
- Paul, R.; Bapat, P.; Deogharkar, A.; Kazi, S.; Singh, S.K.V.; Gupta, T.; Jalali, R.; Sridhar, E.; Moiyadi, A.; Shetty, P.; et al. MiR-592 activates the mTOR kinase, ERK1/ERK2 kinase signaling and imparts neuronal differentiation signature characteristic of Group 4 medulloblastoma. Hum. Mol. Genet. 2021, 30, 2416–2428. [Google Scholar] [CrossRef]
- Dimitrova, V.; Arcaro, A. Targeting the PI3K/AKT/mTOR signaling pathway in medulloblastoma. Curr. Mol. Med. 2015, 15, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, N.K.; Kling, M.J.; Coulter, D.W.; McGuire, T.R.; Ray, S.; Kesherwani, V.; Joshi, S.S.; Sharp, J.G. Improved therapy for medulloblastoma: Targeting hedgehog and PI3K-mTOR signaling pathways in combination with chemotherapy. Oncotarget 2018, 9, 16619–16633. [Google Scholar] [CrossRef] [Green Version]
- Jonchere, B.; Williams, J.; Zindy, F.; Liu, J.; Robinson, S.; Farmer, D.M.; Min, J.; Yang, L.; Stripay, J.L.; Wang, Y.; et al. Combination of Ribociclib with BET-Bromodomain and PI3K/mTOR Inhibitors for Medulloblastoma Treatment In Vitro and In Vivo. Mol. Cancer Ther. 2023, 22, 37–51. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, K.W.; Wang, J.; Garancher, A.; Tao, R.; Esparza, L.A.; Maier, D.L.; Udaka, Y.T.; Murad, N.; Morrissy, S.; et al. HDAC and PI3K Antagonists Cooperate to Inhibit Growth of MYC-Driven Medulloblastoma. Cancer Cell 2016, 29, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Calnan, D.R.; Brunet, A. The FoxO code. Oncogene 2008, 27, 2276–2288. [Google Scholar] [CrossRef] [Green Version]
- Pei, Y.; Moore, C.E.; Wang, J.; Tewari, A.K.; Eroshkin, A.; Cho, Y.J.; Witt, H.; Korshunov, A.; Read, T.A.; Sun, J.L.; et al. An animal model of MYC-driven medulloblastoma. Cancer Cell 2012, 21, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Marino, A.M.; Frijhoff, J.; Calero, R.; Baryawno, N.; Ostman, A.; Johnsen, J.I. Effects of epigenetic modificators in combination with small molecule inhibitors of receptor tyrosine kinases on medulloblastoma growth. Biochem. Biophys. Res. Commun. 2014, 450, 1600–1605. [Google Scholar] [CrossRef]
- Marino, A.M.; Sofiadis, A.; Baryawno, N.; Johnsen, J.I.; Larsson, C.; Vukojevic, V.; Ekstrom, T.J. Enhanced effects by 4-phenylbutyrate in combination with RTK inhibitors on proliferation in brain tumor cell models. Biochem. Biophys. Res. Commun. 2011, 411, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Guessous, F.; Yang, Y.; Johnson, E.; Marcinkiewicz, L.; Smith, M.; Zhang, Y.; Kofman, A.; Schiff, D.; Christensen, J.; Abounader, R. Cooperation between c-Met and focal adhesion kinase family members in medulloblastoma and implications for therapy. Mol. Cancer Ther. 2012, 11, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, W.; Wick, A.; Schulz, J.B.; Dichgans, J.; Rodemann, H.P.; Weller, M. Prevention of irradiation-induced glioma cell invasion by temozolomide involves caspase 3 activity and cleavage of focal adhesion kinase. Cancer Res. 2002, 62, 1915–1919. [Google Scholar]
- Roberts, E.; Cossigny, D.A.; Quan, G.M. The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer 2013, 2013, 418340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quero, L.; Dubois, L.; Lieuwes, N.G.; Hennequin, C.; Lambin, P. miR-210 as a marker of chronic hypoxia, but not a therapeutic target in prostate cancer. Radiother Oncol. 2011, 101, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Cheng, X.; Gao, Y.; Zheng, J.; Xu, Q.; Sun, Y.; Guan, H.; Yu, H.; Sun, Z. Apigenin induces autophagic cell death in human papillary thyroid carcinoma BCPAP cells. Food Funct. 2015, 6, 3464–3472. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A.; Murray, F.; Yokoyama, U.; Romano, S.; Yun, H.; Brown, L.; Snead, A.; Lu, D.; Aroonsakool, N. cAMP and Epac in the regulation of tissue fibrosis. Br. J. Pharmacol. 2012, 166, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Valencia-Cervantes, J.; Huerta-Yepez, S.; Aquino-Jarquin, G.; Rodriguez-Enriquez, S.; Martinez-Fong, D.; Arias-Montano, J.A.; Davila-Borja, V.M. Hypoxia increases chemoresistance in human medulloblastoma DAOY cells via hypoxia-inducible factor 1alpha-mediated downregulation of the CYP2B6, CYP3A4 and CYP3A5 enzymes and inhibition of cell proliferation. Oncol. Rep. 2019, 41, 178–190. [Google Scholar] [CrossRef]
- Lasky, J.L., 3rd; Bradford, K.L.; Wang, Y.; Pak, Y.; Panosyan, E.H. Chemotherapy Can Synergize With Adoptive Immunotherapy to Inhibit Medulloblastoma Growth. Anticancer Res. 2022, 42, 1697–1706. [Google Scholar] [CrossRef]
- Donovan, L.K.; Delaidelli, A.; Joseph, S.K.; Bielamowicz, K.; Fousek, K.; Holgado, B.L.; Manno, A.; Srikanthan, D.; Gad, A.Z.; Van Ommeren, R.; et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020, 26, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Menyhart, O.; Gyorffy, B. Molecular stratifications, biomarker candidates and new therapeutic options in current medulloblastoma treatment approaches. Cancer Metastasis Rev. 2020, 39, 211–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, C.D.; Flores, C.; Yang, C.; Pinheiro, E.M.; Yearley, J.H.; Sayour, E.J.; Pei, Y.; Moore, C.; McLendon, R.E.; Huang, J.; et al. Differential Immune Microenvironments and Response to Immune Checkpoint Blockade among Molecular Subtypes of Murine Medulloblastoma. Clin. Cancer Res. 2016, 22, 582–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenthal, D.T.; Yalon, M.; Vainer, G.W.; Lossos, A.; Yust, S.; Tzach, L.; Cagnano, E.; Limon, D.; Bokstein, F. Pembrolizumab: First experience with recurrent primary central nervous system (CNS) tumors. J. Neurooncol. 2016, 129, 453–460. [Google Scholar] [CrossRef]
- Gorsi, H.S.; Malicki, D.M.; Barsan, V.; Tumblin, M.; Yeh-Nayre, L.; Milburn, M.; Elster, J.D.; Crawford, J.R. Nivolumab in the Treatment of Recurrent or Refractory Pediatric Brain Tumors: A Single Institutional Experience. J. Pediatr. Hematol. Oncol. 2019, 41, e235–e241. [Google Scholar] [CrossRef]
- Rodon, J.; Tawbi, H.A.; Thomas, A.L.; Stoller, R.G.; Turtschi, C.P.; Baselga, J.; Sarantopoulos, J.; Mahalingam, D.; Shou, Y.; Moles, M.A.; et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 1900–1909. [Google Scholar] [CrossRef] [Green Version]
- Peukert, S.; He, F.; Dai, M.; Zhang, R.; Sun, Y.; Miller-Moslin, K.; McEwan, M.; Lagu, B.; Wang, K.; Yusuff, N.; et al. Discovery of NVP-LEQ506, a second-generation inhibitor of smoothened. ChemMedChem 2013, 8, 1261–1265. [Google Scholar] [CrossRef]
- Hummel, T.R.; Wagner, L.; Ahern, C.; Fouladi, M.; Reid, J.M.; McGovern, R.M.; Ames, M.M.; Gilbertson, R.J.; Horton, T.; Ingle, A.M.; et al. A pediatric phase 1 trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: A Children’s Oncology Group phase 1 consortium study. Pediatr. Blood Cancer 2013, 60, 1452–1457. [Google Scholar] [CrossRef] [Green Version]
- Muscal, J.A.; Thompson, P.A.; Horton, T.M.; Ingle, A.M.; Ahern, C.H.; McGovern, R.M.; Reid, J.M.; Ames, M.M.; Espinoza-Delgado, I.; Weigel, B.J.; et al. A phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: A Children’s Oncology Group phase I consortium study (ADVL0916). Pediatr. Blood Cancer 2013, 60, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Leary, S.E.S.; Kilburn, L.; Geyer, J.R.; Kocak, M.; Huang, J.; Smith, K.S.; Hadley, J.; Ermoian, R.; MacDonald, T.J.; Goldman, S.; et al. Vorinostat and isotretinoin with chemotherapy in young children with embryonal brain tumors: A report from the Pediatric Brain Tumor Consortium (PBTC-026). Neuro. Oncol. 2022, 24, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, M.; Park, J.R.; Stewart, C.F.; Gilbertson, R.J.; Schaiquevich, P.; Sun, J.; Reid, J.M.; Ames, M.M.; Speights, R.; Ingle, A.M.; et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: A Children’s Oncology Group phase I consortium report. J. Clin. Oncol. 2010, 28, 3623–3629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeWire, M.D.; Fuller, C.; Campagne, O.; Lin, T.; Pan, H.; Young Poussaint, T.; Baxter, P.A.; Hwang, E.I.; Bukowinski, A.; Dorris, K.; et al. A Phase I and Surgical Study of Ribociclib and Everolimus in Children with Recurrent or Refractory Malignant Brain Tumors: A Pediatric Brain Tumor Consortium Study. Clin. Cancer Res. 2021, 27, 2442–2451. [Google Scholar] [CrossRef]
- Kieran, M.W.; Chi, S.; Goldman, S.; Onar-Thomas, A.; Poussaint, T.Y.; Vajapeyam, S.; Fahey, F.; Wu, S.; Turner, D.C.; Stewart, C.F.; et al. A phase I trial and PK study of cediranib (AZD2171), an orally bioavailable pan-VEGFR inhibitor, in children with recurrent or refractory primary CNS tumors. Childs Nerv. Syst. 2015, 31, 1433–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fangusaro, J.; Cefalo, M.G.; Garre, M.L.; Marshall, L.V.; Massimino, M.; Benettaib, B.; Biserna, N.; Poon, J.; Quan, J.; Conlin, E.; et al. Phase 2 Study of Pomalidomide (CC-4047) Monotherapy for Children and Young Adults With Recurrent or Progressive Primary Brain Tumors. Front. Oncol. 2021, 11, 660892. [Google Scholar] [CrossRef]
- Slavc, I.; Mayr, L.; Stepien, N.; Gojo, J.; Aliotti Lippolis, M.; Azizi, A.A.; Chocholous, M.; Baumgartner, A.; Hedrich, C.S.; Holm, S.; et al. Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a “MEMMAT-like” Metronomic Antiangiogenic Approach. Cancers 2022, 14, 5128. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Rood, B.R.; Turner, D.C.; Stewart, C.F.; Fisher, M.; Smith, C.; Young-Pouissant, T.; Goldman, S.; Lulla, R.; Banerjee, A.; et al. Phase I and pharmacokinetic trial of PTC299 in pediatric patients with refractory or recurrent central nervous system tumors: A PBTC study. J. Neurooncol. 2015, 121, 217–224. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, T.J.; Stewart, C.F.; Kocak, M.; Goldman, S.; Ellenbogen, R.G.; Phillips, P.; Lafond, D.; Poussaint, T.Y.; Kieran, M.W.; Boyett, J.M.; et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium Study PBTC-012. J. Clin. Oncol. 2008, 26, 919–924. [Google Scholar] [CrossRef] [Green Version]
- Saulnier-Sholler, G.; Duda, D.G.; Bergendahl, G.; Ebb, D.; Snuderl, M.; Laetsch, T.W.; Michlitsch, J.; Hanson, D.; Isakoff, M.S.; Bielamowicz, K.; et al. A Phase I Trial of TB-403 in Relapsed Medulloblastoma, Neuroblastoma, Ewing Sarcoma, and Alveolar Rhabdomyosarcoma. Clin. Cancer Res. 2022, 28, 3950–3957. [Google Scholar] [CrossRef]

| WNT-Activated | SHH-Activated | Group 3 | Group 4 | |
|---|---|---|---|---|
| Prevalence | 10% | 30% | 25% | 35% |
| 5-year survival | >90% | 70% | 50% | 75% |
| Neurodevelopmental origin | Pontine mossy fibers of the lower rhombic lip | Granule neuron precursor cells of the upper rhombic lip | Unipolar brush cells and glutamatergic cerebellar nuclei of the upper rhombic lip | |
| Commonly mutated genes | CTNNB1, DDX3X, CREBBP, SMARC4 | TP53, TERT, PTCH1, GLI2, SMO, SUFU | MYC, SOX11, PVT1, OTX2, GFI1/GFI1B | MYCN, SNCAIP, GFI1/GFI1B |
| Important epigenetic players | ARID1, ARID2, SMARC4, promoter methylation of CDH1 | MLL2/KMT2D, MLL3/KMT2C, NCOR2, LDB1 | LSD1, PRC2, EZH2, BRD | KDM6A/UTX, LSD1 |
| Group | Agent | Mechanism of Action | Trials | Type/Design | Population | Intervention | Status |
|---|---|---|---|---|---|---|---|
| SMO inhibitors | Sonidegib (LDE-225) | Binds to the transmembrane portion of the SMO protein and inhibits downstream signaling | NCT04402073 (PersoMed-I) | Phase II Comparative Randomized | Adult and post-pubertal patients with SHH-activated medulloblastoma | Sonidegib and reduced dose radiotherapy | Recruiting |
| NCT01708174 | Phase II Single arm | Pediatric and adult patients with relapsed SHH-activated medulloblastoma | Sonidegib | Completed. Results available on ClinicalTrials.gov (accessed on 24 July 2023) | |||
| NCT01208831 | Phase I Dose escalation | Adult patients with advanced solid tumors (including medulloblastoma) | Sonidegib | Completed. Results available on Novartis website | |||
| NCT01125800 | Phase I/II Dose escalation | Pediatric and adult patients with recurrent or refractory SHH-activated medulloblastoma | Sonidegib | Completed. Results published [72] | |||
| NCT00880308 | Phase I Dose escalation | Adult patients with advanced solid tumors (including medulloblastoma) | Sonidegib | Completed. Results published [167] | |||
| Vismodegib (GDC-0449) | Binds to the transmembrane portion of the SMO protein and inhibits downstream signaling | NCT01878617 | Phase II Parallel assignment Non-randomized | Skeletally mature patients with newly diagnosed standard and high-risk SHH-activated medulloblastoma | Standard chemoradiotherapy with vesmodegib added to maintenance therapy | Active, not recruiting | |
| NCT01601184 | Phase I/II Parallel assignment Randomized | Adult patients with recurrent or refractory SHH-activated medulloblastomas | Vismodegib plus temozolomide versus temozolomide alone | Terminated (number of successes not reached) | |||
| NCT01208831 PBTC-032 | Phase II Single group | Pediatric patients with recurrent or refractory medulloblastoma without (stratum A) or with (Stratum B) SHH activation | Vismodegib | Completed. Published results [71] | |||
| NCT00939484 PBTC-025B | Phase II Single group | Adult patients with recurrent or refractory medulloblastoma without (stratum A) or with (Stratum B) SHH activation | Vismodegib | Completed. Published results [71] | |||
| NCT00822458 PBTC-025 | Phase I Dose finding | Young patients with recurrent or refractory medulloblastoma | Vismodegib | Completed. Published results [70] | |||
| Taladegib (ENV-101) | Binds to the transmembrane portion of the SMO protein and inhibits downstream signaling | NCT05199584 | Phase II Parallel assignment Randomized | Adult patients with refractory advanced solid tumors (including medulloblastoma) with loss of function mutations in the PTCH1 gene | Taladegib | Recruiting | |
| NCT01697514 | Phase I Single group | Pediatric patients with recurrent or refractory medulloblastoma or rhabdomyosarcoma | Taladegib | Withdrawn (poor recruitment) | |||
| ZSP1602 | SMO antagonist (specific mechanism not known) | NCT03734913 | Phase I Parallel assignment Non-randomized | Adult patients with advanced solid tumors | ZSP1602 | Unknown (last update in July 2020 was recruiting) | |
| LEQ506 | Second generation SMO antagonist (specific mechanism not known) [168] | NCT01106508 | Phase I Dose finding | Adult patients with advanced solid tumors | LEQ506 | Completed. Results available on Novartis website | |
| GLI inhibitors | ATO | Direct inhibitor of GLI | NCT00024258 | Phase II Single group | Pediatric and adult patients with neuroblastoma and other pediatric solid tumors (nonmyeloid and nonlymphoid) | ATO | Completed. Results available on ClinicalTraial.gov (accessed on 24 July 2023) |
| Silmitasertib (CX-4945) | CK2 antagonist that reduces the transcription of GLI genes | NCT03904862 | Phase I/II Parallel assignment Non-randomized | Skeletally immature (phase I) and skeletally mature (phase II) patients with recurrent SHH-activated medulloblastomas | Silmitasertib with or without surgical resection | Recruiting | |
| HDAC inhibitors | Vorinostat | Inhibitor of class I and II HDACs | NCT01076530 | Phase I Single group | Young patients with relapsed or refractory primary CNS tumors | Vorinostat plus temozolomide | Completed. Published results [169] |
| NCT00994500 | Phase I Single group | Young patients with refractory or recurrent solid tumors (including medulloblastoma) | Vorinostat and Bortezomib (ubiquitin-proteosome pathway inhibitor) | Completed. Published results [170] | |||
| NCT00867178 | Phase I Single group | Younger patients with newly diagnosed CNS embryonal tumors | Adding vorinostat and isotretinoin to induction chemotherapy (cisplatin, etoposide, vincristine, cyclophosphamide) | Completed. Published results [171] | |||
| NCT00217412 | Phase I Parallel assignment Non-randomized | Young patients with recurrent or refractory solid tumors (including medulloblastoma), lymphoma, or leukemia | Vorinostat plus isotretinoin | Completed. Published results [172] | |||
| Panobinostat (MTX110) | Pan-HDAC inhibitor | NCT04315064 | Phase I Single group | Pediatric and adult patients with recurrent medulloblastoma | Infusions of Panobinostat into the fourth ventricle of the brain or tumor resection cavity | Recruiting | |
| Fimepinostat | Pan-HDAC and PI3K inhibitor | NCT03893487 PNOC016 | Phase I Single group | Pediatric and adult patients with newly diagnosed DIPG, recurrent medulloblastoma (any subtype), or recurrent high-grade glioma | Fimepinostat 2 days preoperatively followed by surgical resection, then maintenance with fimepinostat | Active, not recruiting | |
| Romidepsin (FR901228) | HDAC inhibitor | NCT00053963 | Phase I Single group | Pediatric patients with refractory or recurrent solid tumors | Romidepsin | Completed. Results not available | |
| Cell cycle-disrupting agents | Prexasertib (LY2606368) | Checkpoint kinases 1 and 2 (CHK1/2) inhibitor | NCT04023669 (St. Jude ELIOT) | Phase I Parallel assignment Non-randomized | Pediatric and adult (up to 24 years old) patients with refractory or recurrent SHH-activated, group 3, or group 4 medulloblastoma | Prexasertib in combination with cyclophosphamide (all three subtypes) or gemcitabine (only groups 3 and 4) | Active, not recruiting |
| Palbociclib | CDK4/6 inhibitor | NCT03709680 | Phase I-Dose escalation Phase II-Randomized | Pediatric patients with refractory or recurrent solid tumors (including medulloblastoma) | Palboociclib combined with chemotherapy (temozolomide plus irinotecan or topotecan plus cyclophosphamide) | Recruiting | |
| NCT03526250 (Subprotocol of the NCI-COG Pediatric MATCH trial) | Phase II Single group | Pediatric patients with relapsed or refractory Rb-positive solid tumors non-Hodgkin lymphoma, or histiocytic disorders with activating alterations in cell cycle genes | Palbociclib | Active, not recruiting | |||
| NCT02255461 (PBTC-042) | Phase I Single group | Pediatric patients with Rb-positive recurrent, progressive, or refractory primary CNS tumors. | Palbociclib | Completed. Published results [108] | |||
| Ribociclib (LEE011) | CDK4/6 inhibitor | NCT05429502 | Phase I/II Parallel assignment Randomized | Pediatric patients with relapsed or refractory solid tumors | Ribociclib combined with topotecan and temozolomide | Recruiting | |
| NCT03434262 (SJDAWN) | Phase I Parallel assignment Non-randomized | Pediatric and adult patients with refractory or recurrent brain tumors | Stratum A: ribociclib and gemcitabine for patients with recurrent/refractory group 3/4 medulloblastoma or ependymoma Stratum B: ribociclib and trametinib for recurrent/refractory WNT-activated or SHH-activated medulloblastoma and other CNS tumors Stratum C: ribociclib and sonidegib for skeletally mature patients with recurrent/refractory SHH-activated medulloblastoma | Active, not recruiting | |||
| NCT03387020 | Phase I Single group | Pediatric patients with recurrent, progressive, or refractory CNS tumors | Ribociclib and everolimus (mTOR inhibitor) | Completed. Published results [173] | |||
| Abemaciclib | CDK4/6 inhibitor | NCT04238819 | Phase I Dose escalation | Pediatric patients with recurrent or refractory solid tumors | Abemaciclib combined with temozolomide alone or with irinotecan and temozolomide | Recruiting | |
| Tyrosine kinase inhibitors (TKIs) | Apatinib | TKI that blocks the activity of vascular endothelial growth factor receptor 2 (VEGFR2) | NCT04501718 | Phase II Single group | Pediatric patients with recurrent medulloblastoma | Apatinib combined with temozolomide and etoposide | Recruiting |
| Volitinib | TKI that blocks cMET signaling | NCT03598244 | Phase I Single group | Pediatric patients with refractory, progressive, or recurrent primary CNS tumors | Volitinib | Recruiting | |
| Erdafitinib | TKI that blocks fibroblast growth factor receptor | NCT03210714 (Subprotocol of the NCI-COG Pediatric MATCH trial) | Phase II Single group | Pediatric patients with relapsed or refractory solid tumors non-Hodgkin lymphoma, or histiocytic disorders with FGFR mutations | Erdafitinib | Active, not recruiting | |
| Entrectinib (Rxdx-101) | TKI that blocks the activity of tropomyosin receptor kinases, ROS1, and ALK | NCT02650401 | Phase I/II Single group | Pediatric patients with locally advanced, metastatic, or refractory solid or primary CNS tumors | Entrectinib | Active, not recruiting | |
| Adavosertib (MK-1775) | TKI that block the activity of WEE1 | NCT02095132 | Phase I/II Single group | Pediatric patients with relapsed or refractory solid tumors | Adavosertib combined with irinotecan | Active, not recruiting | |
| Cediranib (AZD-2171) | TKI that blocks the activity of VEGF | NCT00326664 | Phase I Single group | Pediatric patients with recurrent, progressive, or refractory primary CNS tumors | Cediranib | Completed. Published results [174] | |
| Lapatinib | Dual TKI that blocks epidermal growth factor receptor and HER2 signaling | NCT00095940 | Phase I/II Single group | Pediatric patients with recurrent or refractory CNS tumors | Lapatinib | Completed. Results available on ClinicalTrials.gov (accessed on 24 July 2023) | |
| Antiangiogenic factors | Pomalidomide | Decreases the concentrations of VEGF and HIF1α. Increases the production of immune-stimulatory cytokines | NCT03257631 | Phase II Single group | Pediatric patients with recurrent or progressive primary brain tumors | Pomalidomide | Completed. Published results [175] |
| Bevacizumab and other drugs (multidrug) | Bevacizumab is a monoclonal antibody that binds VEGF. Thalidomide, celecoxib, and fenofibrate also have antiangiogenic effects [176] | NCT01356290 | Phase II Single group | Pediatric patients with recurrent or progressive medulloblastoma, ependymoma, or ATRT. | Bevacizumab in combination with 5 oral drugs (thalidomide, celecoxib, fenofibrate, etoposide, and cyclophosphamide) | Recruiting | |
| PTC-299 | Targets VEGF mRNA and inhibits their translation | NCT01158300 | Phase I Single group | Pediatric patients with recurrent or refractory primary CNS tumors | PTC-299 | Completed. Published results [177] | |
| Cilengitide | Integrin antagonist that disrupts endothelial interactions | NCT00063973 PBTC-012 | Phase I Single group | Pediatric patients with refractory primary brain tumors | Cilengitide | Completed. Published results [178] | |
| Immunomodulatory agents | Nivolumab | Monoclonal antibody against the immune checkpoint protein programmed death 1 (PD1) | NCT03585465 | Phase I/II Parallel assignment Randomized | Pediatric patients with relapsed or refractory solid tumors | Nivolumab combined with cyclophosphamide and vinblastine (Arm A), capecitabine (Arm B), or metronomic chemotherapy (Metronomic+ Nivolumab arm) | Recruiting |
| NCT03173950 | Phase II Parallel assignment Non-randomized | Adult patients with recurrent select rare CNS cancers (including medulloblastoma) | Nivolumab | Recruiting | |||
| Pembrolizamab | Monoclonal antibody against the immune checkpoint protein PD1 | NCT02359565 | Phase I Single group | Pediatric patients with recurrent, progressive, or refractory high-grade gliomas, DIPGs, hypermutated brain tumors, ependymoma, or medulloblastoma | Pembrolizumab | Recruiting | |
| Cemiplimab (REGN2810) | Monoclonal antibody against the immune checkpoint protein PD1 | NCT03690869 | Phase I | Pediatric patients with relapsed or refractory solid or CNS tumors | Cemiplimab | Recruiting | |
| Indoximod | Inhibitor of the immune-suppressive enzyme Indoleamine-2,3-dioxygenase (IDO) | NCT05106296 | Phase I Single group | Patients aged 12–25 years with pediatric brain tumors | Indoximod combined with ibrutinib (Bruton’s tyrosine kisase inhibitor, and chemoradiotherapy | Recruiting | |
| NCT04049669 | Phase II Crossover Non-randomized | Pediatric patients with relapsed brain tumors or newly diagnosed DIPG | Indoximod administered during chemotherapy and/or radiation therapy | Recruiting | |||
| NCT02502708 | Phase I Parallel assignment Non-randomized | Pediatric patients with progressive primary brain tumors | Indoximod in combination with temozolomide-based chemotherapy | Completed. No results available | |||
| Sotigalimab (APX005M) | CD40 agonist that activates antigen-presenting cells | NCT03389802 | Phase I Sequential Non-randomized | Pediatric patients with recurrent, progressive, or refractory primary malignant CNS tumor | Sotigalimab | Active, not recruiting | |
| EZH2 inhibitors | Tazemostat | EZH2 inhibitor | NCT03213665 (Subprotocol of the NCI-COG Pediatric MATCH trial) | Phase II Single group | Pediatric patients with relapsed or refractory solid tumors, non-Hodgkin lymphoma, or histiocytic disorders with gain of function mutations in EZH2 or loss of function mutations in SMARCB1 or SMARCA4 | Tazemostat | Active, not recruiting |
| PI3K/mTOR inhibitors | Samotolisib (LY3023414) | Dual PI3K and mTOR inhibitor | NCT03213678 (Subprotocol of the NCI-COG Pediatric MATCH trial) | Phase II Single group | Pediatric patients with relapsed or refractory solid tumors, non-Hodgkin lymphoma, or histiocytic disorders with TSC loss of function mutations, and/or other PI3K/mTOR activating mutations | Samotolisib | Recruiting |
| Sirolimus | mTOR inhibitor | NCT02574728 | Phase II Single group | Pediatric patients with relapsed or refractory solid or CNS tumors | Sirolimus in combination with metronomic chemotherapy | Recruiting | |
| BRD inhibitors | BMS-986158 and BMS-986378 | BRD inhibitors that prevent the interaction between BET proteins and histones | NCT03936465 | Phase I Parallel assignment Non-randomized | Pediatric patients with relapsed or progressive solid or CNS tumors | BMS-986158 or BMS-986378 as monotherapies | Recruiting |
| Gamma secretase inhibitors | RO492909 7 | Blocks the cleavage of Notch intracellular domain (NICD) and its translocation to the nucleus to induce the expression of Notch pathway effector genes | NCT01088763 | Phase I/II Single group | Pediatric patients with relapsed or refractory solid tumors, CNS tumors, lymphoma, or T-cell leukemia | RO4929097 | Terminated |
| MK0752 | Blocks the cleavage of Notch intracellular domain (NICD) and its translocation to the nucleus to induce the expression of Notch pathway effector genes | NCT00572182 | Phase I Single group | Pediatric patients with recurrent or refractory CNS tumors | MK0752 | Terminated due to discontinued financial support | |
| JAK/STAT inhibitors | WP1066 | JAK2/STAT3 pathway inhibitor | NCT04334863 | Phase I Single group | Pediatric patients with recurrent or progressive malignant brain tumors | WP1066 | Completed. No results available |
| Others | TB-403 | Monoclonal antibody against placental growth factor (PIGF) | NCT02748135 | Phase I Single group | Pediatric patients with relapsed or refractory medulloblastoma, neuroblastoma, Ewing sarcoma, and alveolar rhabdomyosarcoma | TB-403 | Completed. Published results [179] |
| Mebendazole | Antiparasitic drug that has been shown to have anti-proliferative and proapoptotic roles in several cancer types via its ability to modulate several oncogenic pathways (including SHH, MEK/ERK, and STAT1/2) | NCT02644291 | Phase I Single group | Pediatric patients with recurrent or progressive brain tumors | Mebendazole | Completed. No results available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slika, H.; Alimonti, P.; Raj, D.; Caraway, C.; Alomari, S.; Jackson, E.M.; Tyler, B. The Neurodevelopmental and Molecular Landscape of Medulloblastoma Subgroups: Current Targets and the Potential for Combined Therapies. Cancers 2023, 15, 3889. https://doi.org/10.3390/cancers15153889
Slika H, Alimonti P, Raj D, Caraway C, Alomari S, Jackson EM, Tyler B. The Neurodevelopmental and Molecular Landscape of Medulloblastoma Subgroups: Current Targets and the Potential for Combined Therapies. Cancers. 2023; 15(15):3889. https://doi.org/10.3390/cancers15153889
Chicago/Turabian StyleSlika, Hasan, Paolo Alimonti, Divyaansh Raj, Chad Caraway, Safwan Alomari, Eric M. Jackson, and Betty Tyler. 2023. "The Neurodevelopmental and Molecular Landscape of Medulloblastoma Subgroups: Current Targets and the Potential for Combined Therapies" Cancers 15, no. 15: 3889. https://doi.org/10.3390/cancers15153889
APA StyleSlika, H., Alimonti, P., Raj, D., Caraway, C., Alomari, S., Jackson, E. M., & Tyler, B. (2023). The Neurodevelopmental and Molecular Landscape of Medulloblastoma Subgroups: Current Targets and the Potential for Combined Therapies. Cancers, 15(15), 3889. https://doi.org/10.3390/cancers15153889









