Tumor and Nodal Disease Growth Rates in Patients with Oropharyngeal Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Imaging, Measurements, and Calculations
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Tumor Volumes, Growth Rates, and Doubling Time
3.3. Slow-Growing Versus Fast-Growing Tumors
3.4. Factors Influencing SGR
3.5. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dejaco, D.; Steinbichler, T.; Schartinger, V.H.; Fischer, N.; Anegg, M.; Dudas, J.; Posch, A.; Widmann, G.; Riechelmann, H. Specific growth rates calculated from CTs in patients with head and neck squamous cell carcinoma: A retrospective study performed in Austria. BMJ Open 2019, 9, e025359. [Google Scholar]
- Schutte, H.W.; Heutink, F.; Wellenstein, D.J.; van den Broek, G.B.; van den Hoogen, F.J.; Marres, H.A.; van Herpen, C.M.; Kaanders, J.H.; Merkx, T.M.; Takes, R.P. Impact of time to diagnosis and treatment in head and neck cancer: A systematic review. Otolaryngol. –Head Neck Surg. 2020, 162, 446–457. [Google Scholar] [CrossRef]
- Davis, K.S.; Lim, C.M.; Clump, D.A.; Heron, D.E.; Ohr, J.P.; Kim, S.; Duvvuri, U.; Johnson, J.T.; Ferris, R.L. Tumor volume as a predictor of survival in human papillomavirus–positive oropharyngeal cancer. Head Neck 2016, 38 (Suppl. 1), E1613–E1617. [Google Scholar] [CrossRef]
- Carpén, T.; Saarilahti, K.; Haglund, C.; Markkola, A.; Tarkkanen, J.; Hagström, J.; Mattila, P.; Mäkitie, A. Tumor volume as a prognostic marker in p16-positive and p16-negative oropharyngeal cancer patients treated with definitive intensity-modulated radiotherapy. Strahlenther. Onkol. 2018, 194, 759–770. [Google Scholar] [CrossRef]
- Murphy, C.T.; Galloway, T.J.; Handorf, E.A.; Wang, L.; Mehra, R.; Flieder, D.B.; Ridge, J.A. Increasing time to treatment initiation for head and neck cancer: An analysis of the National Cancer Database. Cancer 2015, 121, 1204–1213. [Google Scholar] [CrossRef]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.; Tong, Z.-Y.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012, 307, 693–703. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Masterson, L.; Moualed, D.; Liu, Z.W.; Howard, J.E.; Dwivedi, R.C.; Tysome, J.R.; Benson, R.; Sterling, J.C.; Sudhoff, H.; Jani, P. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis of current clinical trials. Eur. J. Cancer 2014, 50, 2636–2648. [Google Scholar]
- Mirghani, H.; Amen, F.; Blanchard, P.; Moreau, F.; Guigay, J.; Hartl, D.; Lacau St Guily, J. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: Ongoing trials, critical issues and perspectives. Int. J. Cancer 2015, 136, 1494–1503. [Google Scholar]
- Durkova, J.; Boldis, M.; Kovacova, S. Has the time come for de-escalation in the management of oropharyngeal carcinoma? Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2019, 163, 293–301. [Google Scholar] [CrossRef]
- Rogers, S.N.; Vedpathak, S.V.; Lowe, D. Reasons for delayed presentation in oral and oropharyngeal cancer: The patients perspective. Br. J. Oral Maxillofac. Surg. 2011, 49, 349–353. [Google Scholar] [CrossRef]
- Baxi, S.S.; Shuman, A.G.; Corner, G.W.; Shuk, E.; Sherman, E.J.; Elkin, E.B.; Hay, J.L.; Pfister, D.G. Sharing a diagnosis of HPV-related head and neck cancer: The emotions, the confusion, and what patients want to know. Head Neck 2013, 35, 1534–1541. [Google Scholar]
- Prouty, C.D.; Mazor, K.M.; Greene, S.M.; Roblin, D.W.; Firneno, C.L.; Lemay, C.A.; Robinson, B.E.; Gallagher, T.H. Providers’ perceptions of communication breakdowns in cancer care. J. Gen. Intern. Med. 2014, 29, 1122–1130. [Google Scholar] [CrossRef]
- Pfister, D. National comprehensive cancer network clinical practice guidelines in oncology. Head and neck cancers. J. Natl. Compr. Canc. Netw. 2011, 9, 596–650. [Google Scholar] [CrossRef]
- Van den Brekel, M.; Stel, H.; Castelijns, J.; Nauta, J.; Van der Waal, I.; Valk, J.; Meyer, C.; Snow, G. Cervical lymph node metastasis: Assessment of radiologic criteria. Radiology 1990, 177, 379–384. [Google Scholar] [CrossRef]
- Mehrara, E.; Forssell-Aronsson, E.; Ahlman, H.; Bernhardt, P. Specific growth rate versus doubling time for quantitative characterization of tumor growth rate. Cancer Res. 2007, 67, 3970–3975. [Google Scholar] [CrossRef]
- Schwartz, M. A biomathematical approach to clinical tumor growth. Cancer 1961, 14, 1272–1294. [Google Scholar] [CrossRef]
- Murphy, C.T.; Devarajan, K.; Wang, L.S.; Mehra, R.; Ridge, J.A.; Fundakowski, C.; Galloway, T.J. Pre-treatment tumor-specific growth rate as a temporal biomarker that predicts treatment failure and improves risk stratification for oropharyngeal cancer. Oral Oncol. 2015, 51, 1034–1040. [Google Scholar] [CrossRef]
- Chao, K.C.; Ozyigit, G.; Blanco, A.I.; Thorstad, W.L.; Deasy, J.O.; Haughey, B.H.; Spector, G.J.; Sessions, D.G. Intensity-modulated radiation therapy for oropharyngeal carcinoma: Impact of tumor volume. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 43–50. [Google Scholar]
- Huang, S.H.; Xu, W.; Waldron, J.; Siu, L.; Shen, X.; Tong, L.; Ringash, J.; Bayley, A.; Kim, J.; Hope, A. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J. Clin. Oncol. 2015, 33, 836–845. [Google Scholar] [CrossRef]
- Jensen, A.R.; Nellemann, H.M.; Overgaard, J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother. Oncol. 2007, 84, 5–10. [Google Scholar] [CrossRef]
- Steel, G.; Lamerton, L. The growth rate of human tumours. Br. J. Cancer 1966, 20, 74. [Google Scholar] [CrossRef]
- Mehrara, E.; Forssell-Aronsson, E.; Ahlman, H.; Bernhardt, P. Quantitative analysis of tumor growth rate and changes in tumor marker level: Specific growth rate versus doubling time. Acta Oncol. 2009, 48, 591–597. [Google Scholar] [CrossRef]
- Kay, K.; Dolcy, K.; Bies, R.; Shah, D.K. Estimation of solid tumor doubling times from progression-free survival plots using a novel statistical approach. AAPS J. 2019, 21, 27. [Google Scholar] [CrossRef]
- Goldenberg, D.; Begum, S.; Westra, W.H.; Khan, Z.; Sciubba, J.; Pai, S.I.; Califano, J.A.; Tufano, R.P.; Koch, W.M. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck J. Sci. Spec. Head Neck 2008, 30, 898–903. [Google Scholar]
- Taberna, M.; Mena, M.; Pavón, M.; Alemany, L.; Gillison, M.; Mesía, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef]
- Slootweg, P.J.; Bishop, J.A. Oral and oropharyngeal cancer: Pathology and genetics. In Encyclopedia of Cancer; Elsevier: Amsterdam, The Netherlands, 2018; pp. 124–130. [Google Scholar]
- Stefanuto, P.; Doucet, J.-C.; Robertson, C. Delays in treatment of oral cancer: A review of the current literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 424–429. [Google Scholar] [CrossRef]
- Murphy, C.T.; Galloway, T.J.; Handorf, E.A.; Egleston, B.L.; Wang, L.S.; Mehra, R.; Flieder, D.B.; Ridge, J.A. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J. Clin. Oncol. 2016, 34, 169. [Google Scholar] [CrossRef]
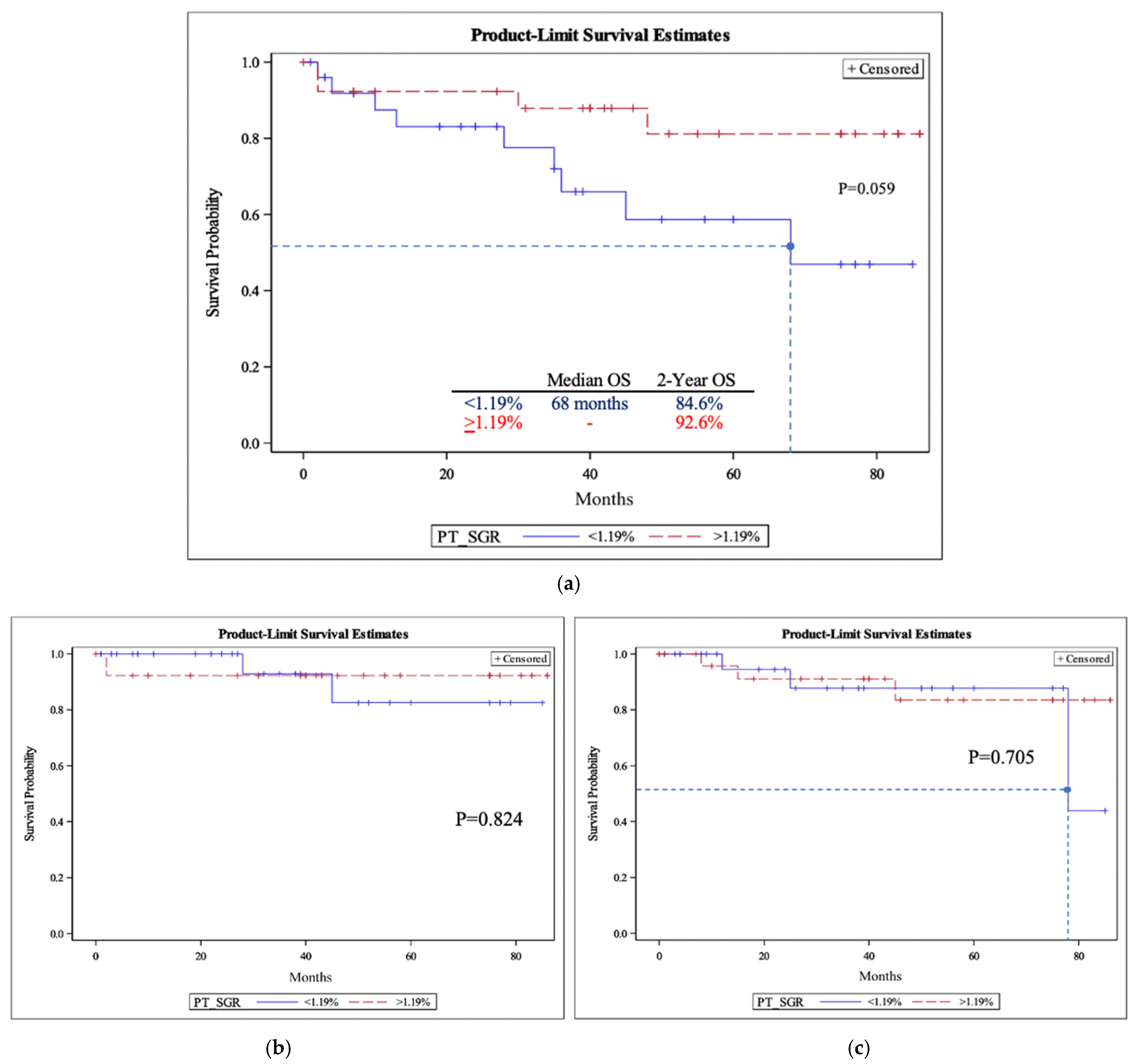
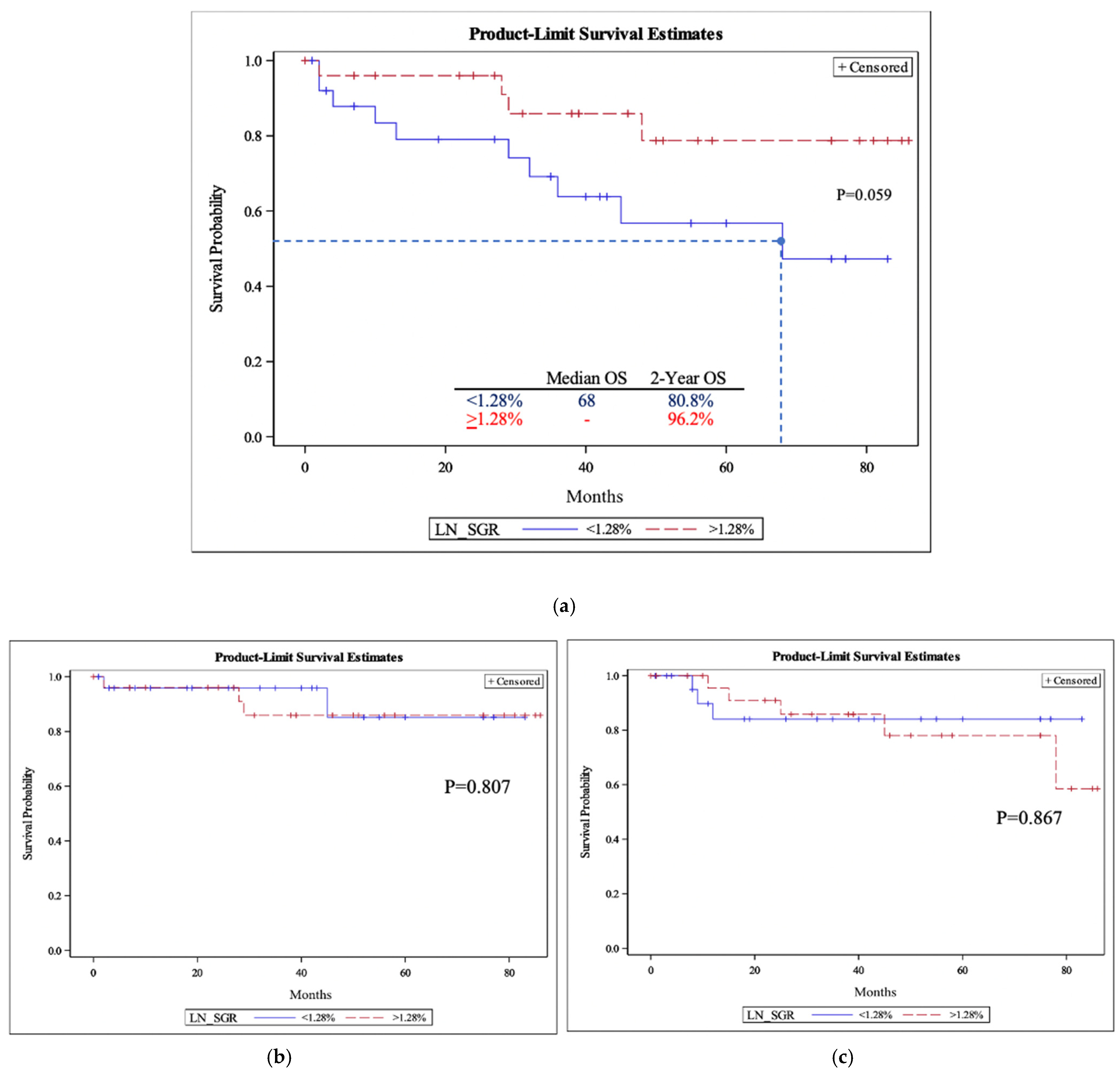
| All Patients (n = 54) | PT-SGR < 1.19% (n = 26) | PT-SGR ≥ 1.19% (n = 27) | p-Value | LN-SGR < 1.28% (n = 26) | LN-SGR ≥ 1.28% (n = 28) | p-Value | |
|---|---|---|---|---|---|---|---|
| Age (Years) | |||||||
| Mean ± SD | 62.3 ± 9.4 | 60.2 ± 9.8 | 64.7 ± 8.1 | 0.0813 | 62 ± 9.4 | 63 ± 9.5 | 0.6993 |
| Gender (n, %) | |||||||
| Male | 45 (83.3) | 19 (73) | 25 (92.6) | 21 (80.7) | 24 (85.7) | ||
| Female | 9 (16.6) | 7 (26.9) | 2 (7.4) | 0.0764 | 5 (19.2) | 4 (14.3) | 0.7237 |
| Smoking (n, %) | |||||||
| Never | 18 (33.3) | 7 (26.9) | 11 (40.7) | 9 (34.6) | 9 (32.1) | ||
| Former | 26 (48.1) | 14 (53.8) | 12 (41.7) | 14 (53.8) | 12 (42.8) | ||
| Current | 10 (18.5) | 5 (19.2) | 4 (14.8) | 0.8395 | 3 (11.5) | 7 (25) | 0.4317 |
| Subsite (n, %) | |||||||
| Tonsil | 26 (48.1) | 10 (38.5) | 15 (55.6) | 10 (38.5) | 16 (57.1) | ||
| Base of tongue | 27 (50) | 15 (57.7) | 12 (44.4) | 15 (57.7) | 12 (42.9) | ||
| Soft palate | 1 (1.9) | 1 (3.8) | 0 (0) | 0.3135 | 1 (3.8) | 0 (0) | 0.2658 |
| Stage (n, %) | |||||||
| I | 22 (41)/20 (38) | 9 (35) | 13 (48) | 0.328 | 12 (46) | 8 (31) | 0.446 |
| II | 17 (32)/17 (33) | 9 (35) | 8 (30) | 6 (23) | 11 (42) | ||
| III | 11 (21)/12 (23) | 5 (19) | 6 (22) | 7 (27) | 5 (19) | ||
| IV | 3 (6)/3 (6) | 3 (11) | 0 (0) | 1 (4) | 2 (8) | ||
| P16 Status (n, %) * | |||||||
| Positive | 49 (92.5) | 23 (88.5) | 26 (96.3) | 24 (92.3) | 25 (92.6) | ||
| Negative | 4 (7.5) | 3 (11.5) | 1 (3.7) | 0.3507 | 2 (7.7) | 2 (7.4) | >0.99 |
| Primary Therapy (n, %) * | |||||||
| RT | 5 (9.4) | 1 (3.8) | 4 (14.8) | 1 (3.8) | 4 (14.8) | ||
| CRT | 47 (88.7) | 24 (92.3) | 23 (85.2) | 0.3517 | 25 (96.2) | 22 (81.5) | 0.3497 |
| Follow-Up (Months) | |||||||
| Mean ± SD | 39 ± 28.6 | 33.9 ± 26.6 | 44.9 ± 28.9 | 35.8 ± 27.0 | 40.5 ± 28.9 | ||
| Median (range) | 37.5 (1–86) | 33.5 (1–85) | 39.5 (2–86) | 0.157 | 22.5 (1–77) | 37.5 (2–86) | 0.548 |
| Initial Volume (mL) | Final Volume (mL) | Specific Growth Rate (%/day) | Doubling Time (days) | |
|---|---|---|---|---|
| Primary tumor (mean ± SD) | 16.8 ± 12.6 | 26 ± 18.9 | 1.2 ± 2.2% | 71.7 ± 228.1 |
| (Median, 25th; 75th percentile) | 1.2 (0.6; 2.3) | 36.5 (17.4; 74.5) | ||
| Lymph node (mean ± SD) | 15.1 ± 15 | 23.9 ± 23.2 | 1.6 ± 1.9% | 50.2 ± 286.4 |
| (Median, 25th; 75th percentile) | 1.3 (0.6; 2.2) | 38.9 (21.6; 91.5) |
| N | PT-SGR (%/Day) | p-Value | LN-SGR (%/Day) | p-Value | |
|---|---|---|---|---|---|
| Tumor Subsite | |||||
| Tonsils | 26 | 2.2 ± 2.8 | 2.0 ± 2.6 | ||
| Base of tongue | 27 | 1.3 ± 1.2 | 1.3 ± 1.1 | ||
| Soft palate | 1 | 0.5 ± 0 | 0.2551 | 1.1 ± 0 | 0.4786 |
| P16 Status | |||||
| Positive | 50 | 1.7 ± 2.2 | 1.65 ± 2.0 | ||
| Negative | 4 | 1.3 ± 1.1 | 0.7021 | 1.32 ± 0.6 | 0.7456 |
| Time Between Scans (Days) | |||||
| 0–25 | 16 | 3.4 ± 3.2 | 2.93 ± 3.0 | ||
| 26–50 | 24 | 1.1 ± 1.0 | 1.14 ± 0.8 | ||
| >50 | 14 | 0.8 ± 0.5 | 0.0040 | 1.00 ± 0.9 | 0.0042 |
| Initial PT Volume | |||||
| <15 mL | 30 | 2.0 ± 2.8 | 1.70 ± 2.4 | ||
| ≥15 mL | 24 | 1.3 ± 0.9 | 0.2322 | 1.53 ± 1.2 | 0.7530 |
| Initial LN Volume | |||||
| <15 mL | 32 | 2.1 ± 2.6 | 1.9 ± 2.3 | ||
| ≥15 mL | 22 | 1.1 ± 1.1 | 0.0954 | 1.2 ± 1.1 | 0.1912 |
| Tumor Stage | |||||
| I | 20/22 | 1.3 ± 0.9 | 1.8 ± 1.8 | ||
| II | 17 | 2.3 ± 3.0 | 0.300 | 1.9 ± 3.1 | 0.753 |
| III | 11/12 | 1.1 ± 1.0 | 1.3 ± 0.9 | ||
| IV | 3 | 1.6 ± 0.5 | 0.8 ± 2.1 |
| Time to Death | Time to Recurrence | |||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | p-Value | n | Mean (SD) | p-Value | |
| LN-SGR | ||||||
| <1.28% | 10 | 24.1 (21.8) | 0.836 | 3 | 9.7 (2) | 0.177 |
| >1.28% | 4 | 26.8 (18.9) | 5 | 34.8 (27.5) | ||
| PT-SGR | ||||||
| <1.19% | 9 | 26.8 (21.8) | 0.644 | 3 | 38.3 (34.9) | 0.536 |
| >1.19% | 4 | 20.5 (22.6) | 3 | 22.7 (19.7) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farber, N.I.; Li, Y.; Solis, R.N.; Chen, J.; Masheeb, Z.; Wilson, M.; Bewley, A.F.; Abouyared, M.; Rao, S.; Rong, Y.; et al. Tumor and Nodal Disease Growth Rates in Patients with Oropharyngeal Squamous Cell Carcinoma. Cancers 2023, 15, 3865. https://doi.org/10.3390/cancers15153865
Farber NI, Li Y, Solis RN, Chen J, Masheeb Z, Wilson M, Bewley AF, Abouyared M, Rao S, Rong Y, et al. Tumor and Nodal Disease Growth Rates in Patients with Oropharyngeal Squamous Cell Carcinoma. Cancers. 2023; 15(15):3865. https://doi.org/10.3390/cancers15153865
Chicago/Turabian StyleFarber, Nicole I., Yimin Li, Roberto N. Solis, Joy Chen, Zahrah Masheeb, Machelle Wilson, Arnaud F. Bewley, Marianne Abouyared, Shyam Rao, Yi Rong, and et al. 2023. "Tumor and Nodal Disease Growth Rates in Patients with Oropharyngeal Squamous Cell Carcinoma" Cancers 15, no. 15: 3865. https://doi.org/10.3390/cancers15153865
APA StyleFarber, N. I., Li, Y., Solis, R. N., Chen, J., Masheeb, Z., Wilson, M., Bewley, A. F., Abouyared, M., Rao, S., Rong, Y., & Birkeland, A. C. (2023). Tumor and Nodal Disease Growth Rates in Patients with Oropharyngeal Squamous Cell Carcinoma. Cancers, 15(15), 3865. https://doi.org/10.3390/cancers15153865







