The Bivalent Bromodomain Inhibitor MT-1 Inhibits Prostate Cancer Growth
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Reagents and Chemicals
2.2. Cell Viability
2.3. Cell Cycle Analysis
2.4. Protein Western Blot Analysis
2.5. PDX 3D Spheroid Culture
2.6. Mice PDX and Xenograft In Vivo Studies to Test the Efficacy of MT-1, 3jc48-3 or MT-1 and 3jc48-3 Combination
3. Results
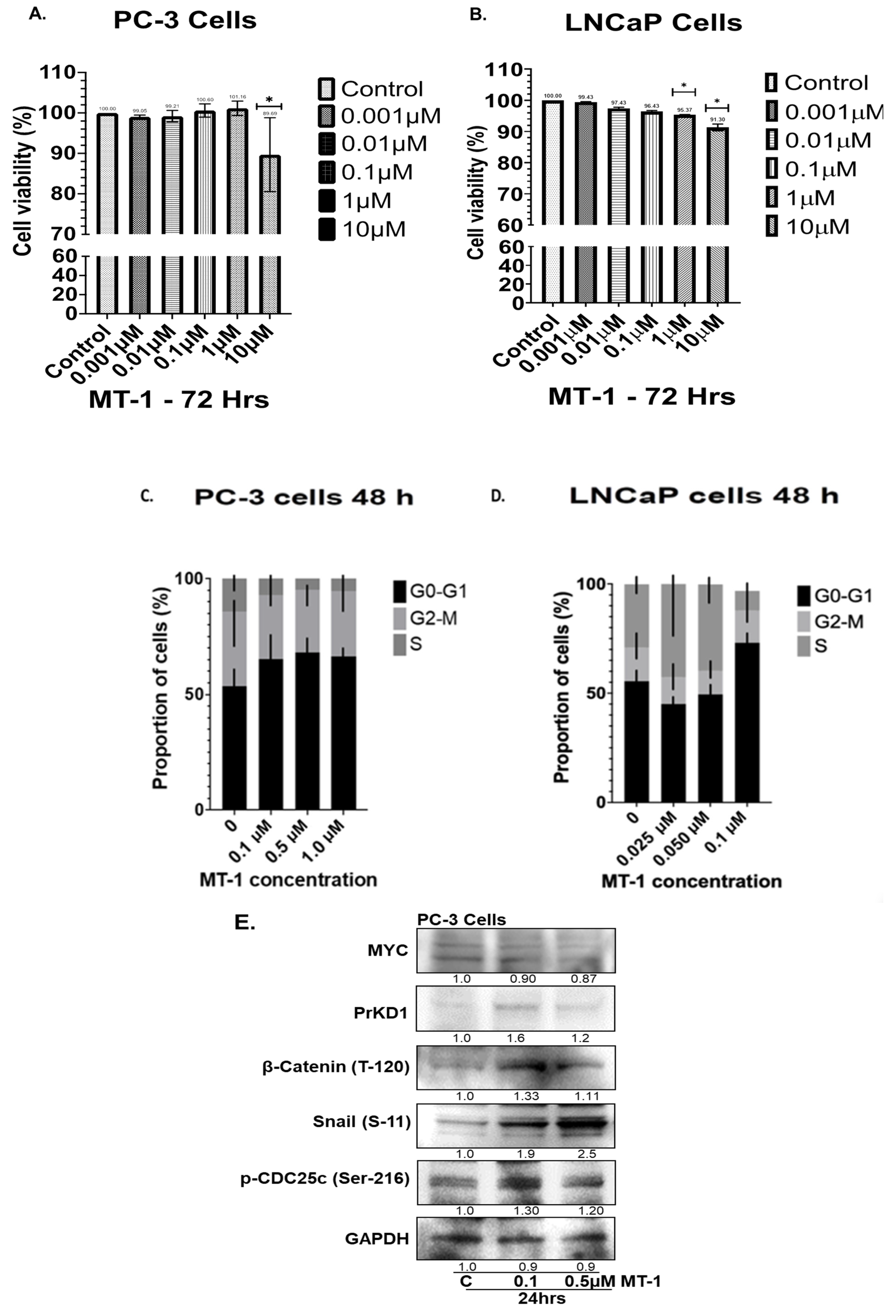
3.1. MT-1 Inhibits Growth of PC Cells and G0-G1 Cell Cycle Arrest In Vitro
3.2. MT-1 Treatment of PC3 Cells Increased PrKD Protein Levels and Kinase Activity
3.3. Combination with MT-1 and 3jc48-3 Results in Synergistic Inhibition of PC Cells’ Viability Associated with Increased PrKD Substrate Phosphorylation
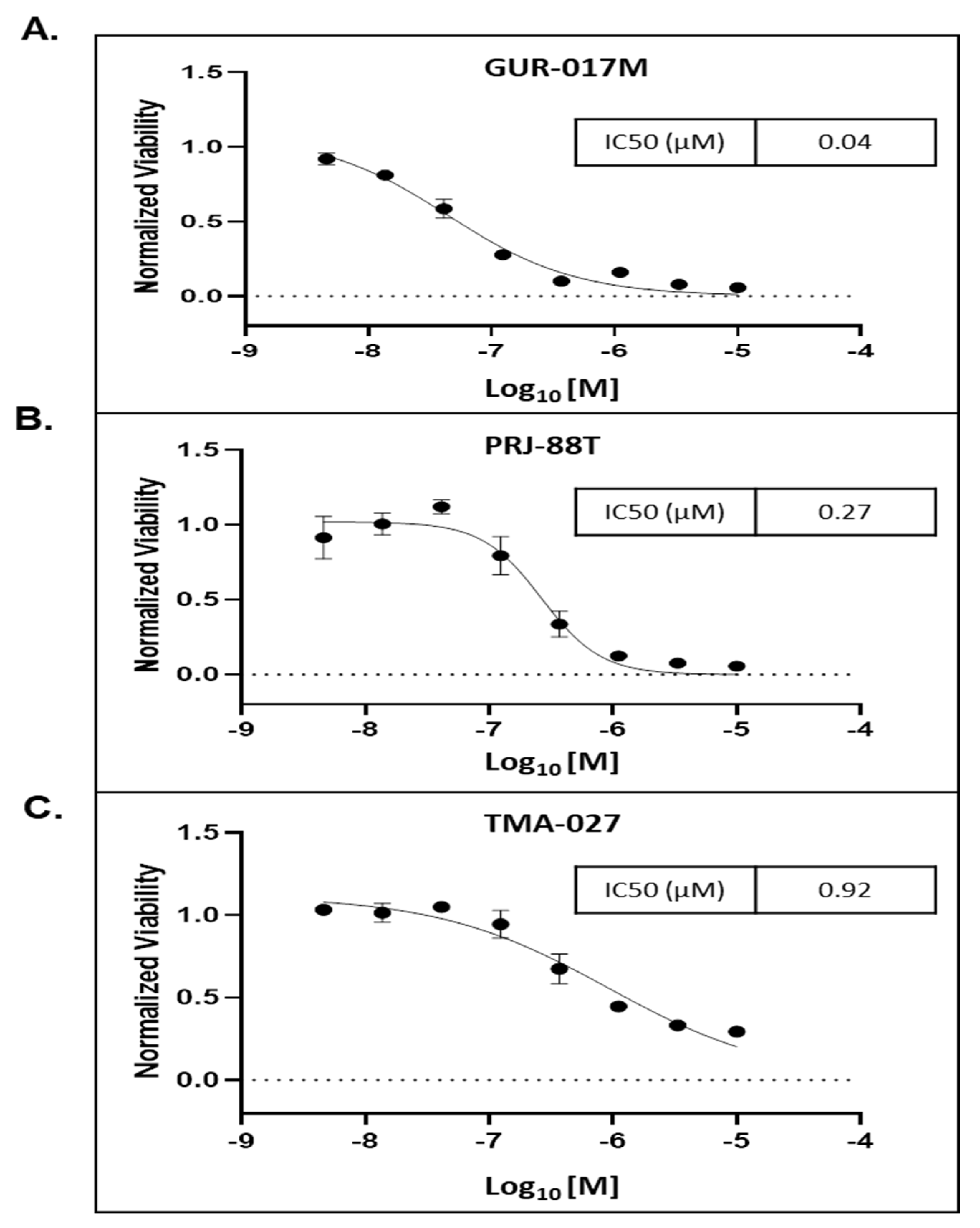
3.4. MT-1 Decreased the Growth of 3D Tumor Spheroids Derived from PDX Tumors Generated from PC Metastatic Tissues Samples from Heavily Pre-Treated Patients
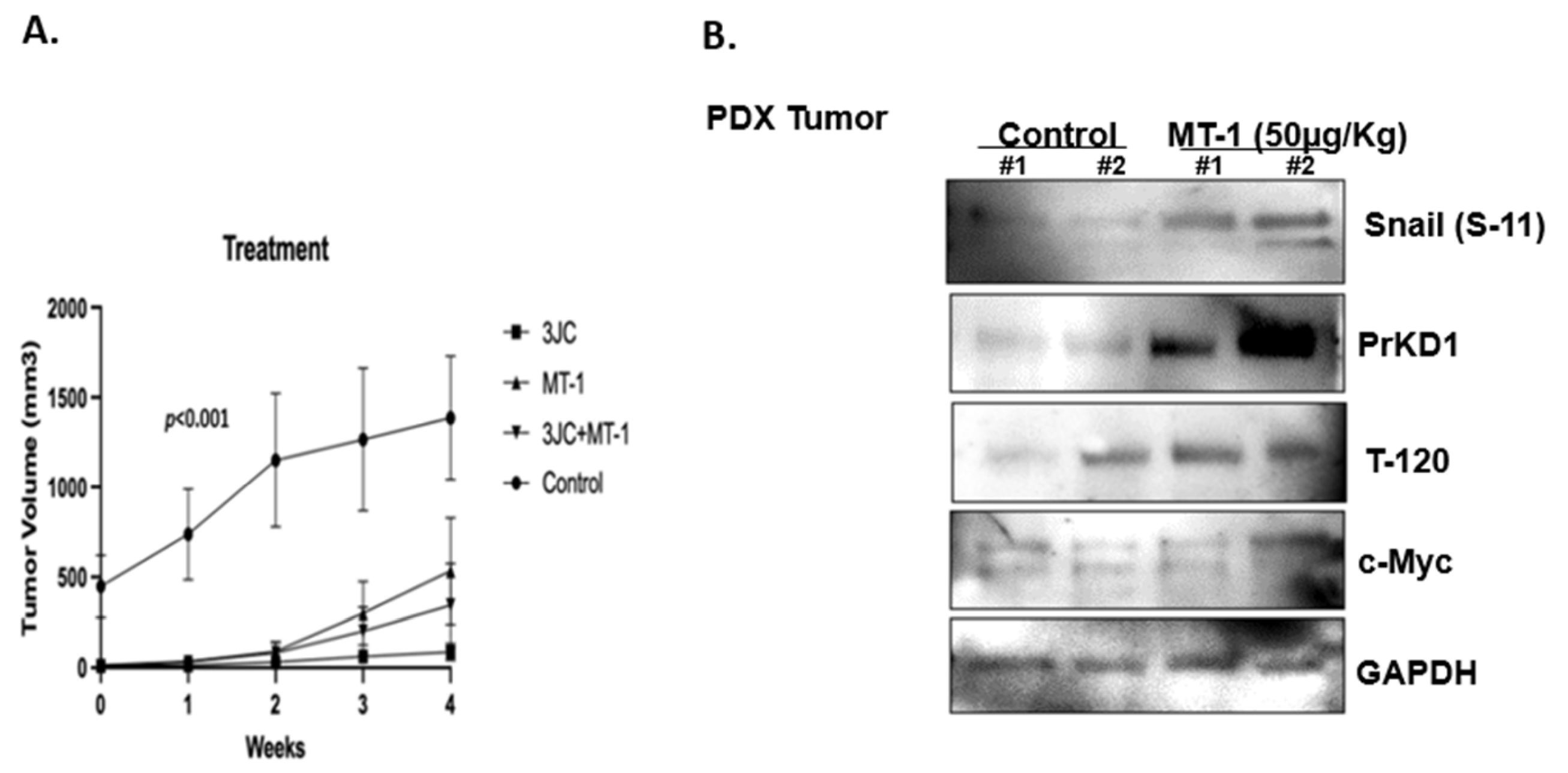
3.5. MT-1 and 3jc48-3 Synergistically Reduce Tumor Growth Rate in PDX PC Mice Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asangani, I.A.; Dommeti, V.L.; Wang, X.; Malik, R.; Cieslik, M.; Yang, R.; Escara-Wilke, J.; Wilder-Romans, K.; Dhanireddy, S.; Engelke, C. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 2014, 510, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Niu, X.; Jin, F.; Liu, Z.; Jin, C.; Lai, L. Structure-based inhibitor design for the intrinsically disordered protein c-Myc. Sci. Rep. 2016, 6, 22298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, N.; Giri, R. Therapeutic interventions of cancers using intrinsically disordered proteins as drug targets: C-Myc as model system. Cancer Inform. 2017, 16, 1176935117699408. [Google Scholar] [CrossRef] [PubMed]
- Kalkat, M.; Resetca, D.; Lourenco, C.; Chan, P.-K.; Wei, Y.; Shiah, Y.-J.; Vitkin, N.; Tong, Y.; Sunnerhagen, M.; Done, S.J.; et al. MYC Protein Interactome Profiling Reveals Functionally Distinct Regions that Cooperate to Drive Tumorigenesis. Mol. Cell 2018, 72, 836–848.e7. [Google Scholar] [CrossRef]
- Whitfield, A.; Beaulieu, M.-E.; Soucek, L. Strategies to Inhibit Myc and Their Clinical Applicability. Front. Cell Dev. Biol. 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Azad, N.; Zahnow, C.A.; Rudin, C.M.; Baylin, S.B. The future of epigenetic therapy in solid tumours—Lessons from the past. Nat. Rev. Clin. Oncol. 2013, 10, 256–266. [Google Scholar] [CrossRef]
- Fitzel, R.; Secker-Grob, K.A.; Keppeler, H.; Korkmaz, F.; Schairer, R.; Erkner, E.; Schneidawind, D.; Lengerke, C.; Hentrich, T.; Schulze-Hentrich, J.M.; et al. Targeting MYC in combination with epigenetic regulators induces synergistic anti-leukemic effects in MLLr leukemia and simultaneously improves immunity. Neoplasia 2023, 41, 100902. [Google Scholar] [CrossRef]
- Davalos, V.; Esteller, M. Cancer epigenetics in clinical practice. CA Cancer J. Clin. 2022, 73, 376–424. [Google Scholar] [CrossRef]
- Morel, D.; Jeffery, D.; Aspeslagh, S.; Almouzni, G.; Postel-Vinay, S. Combining epigenetic drugs with other therapies for solid tumours—Past lessons and future promise. Nat. Rev. Clin. Oncol. 2020, 17, 91–107. [Google Scholar] [CrossRef]
- Arrowsmith, C.H.; Bountra, C.; Fish, P.V.; Lee, K.; Schapira, M. Epigenetic protein families: A new frontier for drug discovery. Nat. Rev. Drug Discov. 2012, 11, 384–400. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, Y.; Wang, X.; Xie, X.; Liu, M.; Zhang, K.; Wang, L.; Bai, D.; Foster, L.J.; Shu, R.; et al. Targeting bromodomain-containing proteins: Research advances of drug discovery. Mol. Biomed. 2023, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, A.; Meshorer, E. Chromatin remodeling and bivalent histone modifications in embryonic stem cells. EMBO Rep. 2015, 16, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y. The bromodomain and extra-terminal domain (BET) family: Functional anatomy of BET paralogous proteins. Int. J. Mol. Sci. 2016, 17, 1849. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Pearson, R.B.; Hannan, R.D.; Furic, L. Therapeutic approaches targeting MYC-driven prostate cancer. Genes 2017, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- French, C.A. Small-Molecule Targeting of BET Proteins in Cancer. Adv. Cancer Res. 2016, 131, 21–58. [Google Scholar] [CrossRef]
- Tanaka, M.; Roberts, J.M.; Seo, H.S.; Souza, A.; Paulk, J.; Scott, T.G.; DeAngelo, S.L.; Dhe-Paganon, S.; Bradner, J.E. Design and characterization of bivalent BET inhibitors. Nat. Chem. Biol. 2016, 12, 1089–1096. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, P.; Chen, H.; Wold, E.A.; Tian, B.; Brasier, A.R.; Zhou, J. Drug Discovery Targeting Bromodomain-Containing Protein 4. J. Med. Chem. 2017, 60, 4533–4558. [Google Scholar] [CrossRef]
- Shukla, S.; Fletcher, S.; Chauhan, J.; Chalfant, V.; Riveros, C.; Mackeyev, Y.; Singh, P.K.; Krishnan, S.; Osumi, T.; Balaji, K.C. 3JC48-3 (methyl 4′-methyl-5-(7-nitrobenzo[c](1,2,5)oxadiazol-4-yl)-[1,1′-biphenyl]-3-carboxylate): A novel MYC/MAX dimerization inhibitor reduces prostate cancer growth. Cancer Gene Ther. 2022, 29, 1550–1557. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Xu, S.; Tandon, M.; LaValle, C.R.; Deng, F.; Wang, Q.J. Androgen suppresses protein kinase D1 expression through fibroblast growth factor receptor substrate 2 in prostate cancer cells. Oncotarget 2017, 8, 12800–12811. [Google Scholar] [CrossRef] [PubMed]
- Gabay, M.; Li, Y.; Felsher, D.W. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect. Med. 2014, 4, a014241. [Google Scholar] [CrossRef]
- Tsimberidou, A.M. Targeted therapy in cancer. Cancer Chemother. Pharmacol. 2015, 76, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, H.; Ali, S.; Essapen, S. Therapeutic application of monoclonal antibodies in cancer: Advances and challenges. Br. Med. Bull. 2012, 104, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Choucair, K.; Ashraf, M.; Hammouda, D.M.; Alloghbi, A.; Khan, T.; Senzer, N.; Nemunaitis, J. Bromodomain and extra-terminal motif inhibitors: A review of preclinical and clinical advances in cancer therapy. Future Sci. OA 2019, 5, FSO372. [Google Scholar] [CrossRef] [PubMed]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 904–917. [Google Scholar] [CrossRef]
- Waring, M.J.; Chen, H.; Rabow, A.A.; Walker, G.; Bobby, R.; Boiko, S.; Bradbury, R.H.; Callis, R.; Clark, E.; Dale, I.; et al. Potent and selective bivalent inhibitors of BET bromodomains. Nat. Chem. Biol. 2016, 12, 1097–1104. [Google Scholar] [CrossRef]
- Mandl, A.; Markowski, M.C.; Carducci, M.A.; Antonarakis, E.S. Role of bromodomain and extraterminal (BET) proteins in prostate cancer. Expert Opin. Investig. Drugs 2023, 32, 213–228. [Google Scholar] [CrossRef]
- Orme, J.J.; Pagliaro, L.C.; Quevedo, J.F.; Park, S.S.; Costello, B.A. Rational Second-Generation Antiandrogen Use in Prostate Cancer. Oncologist 2022, 27, 110–124. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Gao, F.; Ma, Y.; Wei, D.; Lu, Z.; Chen, S.; Wang, M.; Wang, Y.; Xu, K.; et al. c-Myc-Targeting PROTAC Based on a TNA-DNA Bivalent Binder for Combination Therapy of Triple-Negative Breast Cancer. J. Am. Chem. Soc. 2023, 145, 9334–9342. [Google Scholar] [CrossRef]
- Beaulieu, M.E.; Jauset, T.; Massó-Vallés, D.; Martínez-Martín, S.; Rahl, P.; Maltais, L.; Zacarias-Fluck, M.F.; Casacuberta-Serra, S.; Serrano Del Pozo, E.; Fiore, C.; et al. Intrinsic cell-penetrating activity propels Omomyc from proof of concept to viable anti-MYC therapy. Sci. Transl. Med. 2019, 11, eaar5012. [Google Scholar] [CrossRef]

| Clinical Exposure Prior to Biopsy (Outcome) | Treatment after Biopsy (Outcome) | Mutations | |
|---|---|---|---|
| GUR-017M | Abiraterone (resistant) Enzalutamide (resistant) Docetaxel (partial response) Cabazitaxel (resistant) | N/A | CCND1 amplification HRSA Q61R FGF 19, 4, 3 amplification LYN amplification MYC amplification TP53 splice site 672+2T>G |
| TMA-027 | Enzalutamide (resistant) Docetaxel (partial response) | Enzalutamide (resistant) Cabazitaxel (partial response) | AR v7 Detected TP53 C.81/C>T NTRK1 c.2311 C>T |
| PRJ-88T | Enzalutamide (resistant) | Docetaxel + Pembrolizumab (partial response) | BRCA2 E1035 PTEN K263 PIK3R1 I405del SETD2 Q7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, S.; Riveros, C.; Al-Toubat, M.; Chardon-Robles, J.; Osumi, T.; Serrano, S.; Kase, A.M.; Petit, J.L.; Meurice, N.; Gleba, J.; et al. The Bivalent Bromodomain Inhibitor MT-1 Inhibits Prostate Cancer Growth. Cancers 2023, 15, 3851. https://doi.org/10.3390/cancers15153851
Shukla S, Riveros C, Al-Toubat M, Chardon-Robles J, Osumi T, Serrano S, Kase AM, Petit JL, Meurice N, Gleba J, et al. The Bivalent Bromodomain Inhibitor MT-1 Inhibits Prostate Cancer Growth. Cancers. 2023; 15(15):3851. https://doi.org/10.3390/cancers15153851
Chicago/Turabian StyleShukla, Sanjeev, Carlos Riveros, Mohammed Al-Toubat, Jonathan Chardon-Robles, Teruko Osumi, Samuel Serrano, Adam M. Kase, Joachim L. Petit, Nathalie Meurice, Justyna Gleba, and et al. 2023. "The Bivalent Bromodomain Inhibitor MT-1 Inhibits Prostate Cancer Growth" Cancers 15, no. 15: 3851. https://doi.org/10.3390/cancers15153851
APA StyleShukla, S., Riveros, C., Al-Toubat, M., Chardon-Robles, J., Osumi, T., Serrano, S., Kase, A. M., Petit, J. L., Meurice, N., Gleba, J., Copland, J. A., III, Chauhan, J., Fletcher, S., & Balaji, K. C. (2023). The Bivalent Bromodomain Inhibitor MT-1 Inhibits Prostate Cancer Growth. Cancers, 15(15), 3851. https://doi.org/10.3390/cancers15153851





