Grey Matter Reshaping of Language-Related Regions Depends on Tumor Lateralization
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Brief Overview of Data Acquisition and Analysis
2.3. MRI Analyses
2.4. Statistical Approach
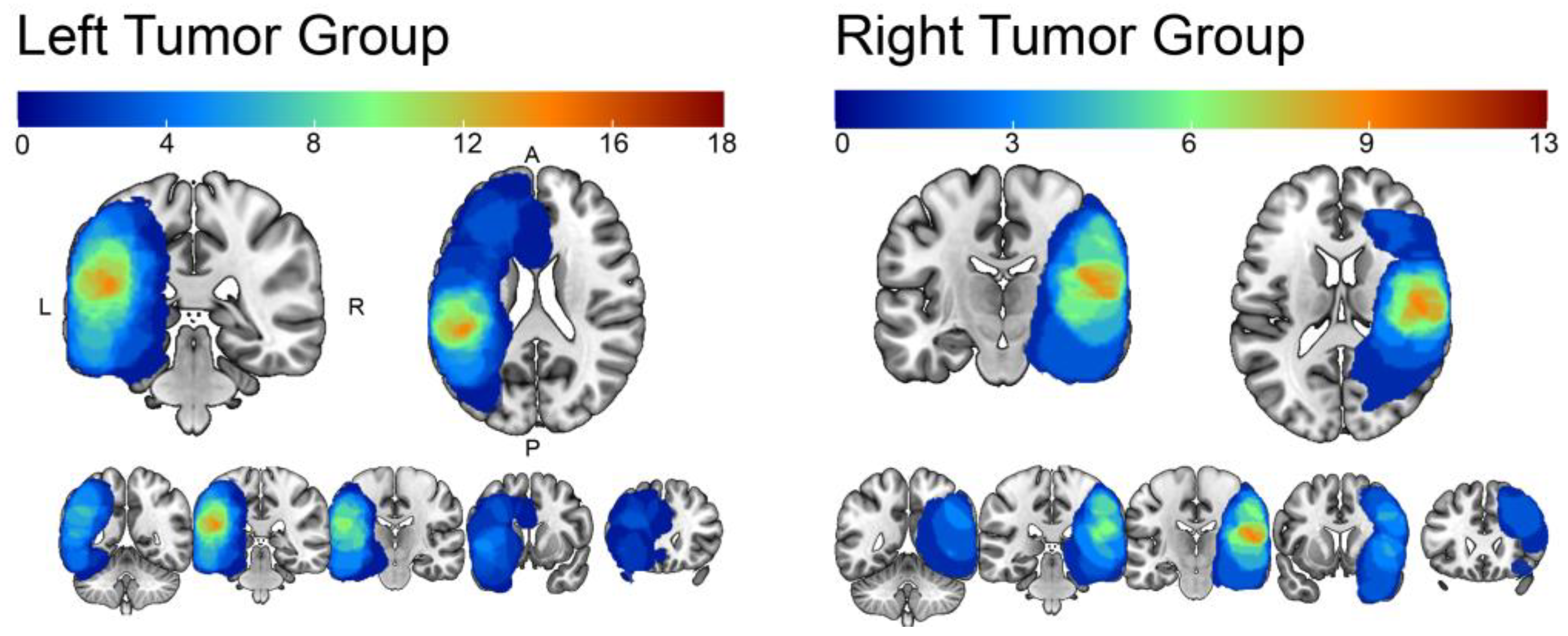
3. Results
Structural Reshaping in Patients with Brain Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herbet, G.; Maheu, M.; Costi, E.; Lafargue, G.; Duffau, H. Mapping Neuroplastic Potential in Brain-Damaged Patients. Brain 2016, 139, 829–844. [Google Scholar] [CrossRef]
- Ille, S.; Engel, L.; Albers, L.; Schroeder, A.; Kelm, A.; Meyer, B.; Krieg, S.M. Functional Reorganization of Cortical Language Function in Glioma Patients—A Preliminary Study. Front. Oncol. 2019, 9, 446. [Google Scholar] [CrossRef]
- Krieg, S.M.; Sollmann, N.; Hauck, T.; Ille, S.; Foerschler, A.; Meyer, B.; Ringel, F. Functional Language Shift to the Right Hemisphere in Patients with Language-Eloquent Brain Tumors. PLoS ONE 2013, 8, e75403. [Google Scholar] [CrossRef]
- Li, W.; An, D.; Tong, X.; Liu, W.; Xiao, F.; Ren, J.; Niu, R.; Tang, Y.; Zhou, B.; Lei, D.; et al. Different Patterns of White Matter Changes after Successful Surgery of Mesial Temporal Lobe Epilepsy. NeuroImage Clin. 2019, 21, 101631. [Google Scholar] [CrossRef]
- Quiñones, I.; Amoruso, L.; Pomposo Gastelu, I.C.; Gil-Robles, S.; Carreiras, M. What Can Glioma Patients Teach Us about Language (Re)Organization in the Bilingual Brain: Evidence from FMRI and MEG. Cancers 2021, 13, 2593. [Google Scholar] [CrossRef]
- Połczyńska, M.M.; Beck, L.; Kuhn, T.; Benjamin, C.F.; Ly, T.K.; Japardi, K.; Cavanagh, L.; Bookheimer, S.Y. Tumor Location and Reduction in Functional MRI Estimates of Language Laterality. J. Neurosurg. 2021, 135, 1674–1684. [Google Scholar] [CrossRef]
- Almairac, F.; Duffau, H.; Herbet, G. Contralesional Macrostructural Plasticity of the Insular Cortex in Patients with Glioma: A VBM Study. Neurology 2018, 91, e1902–e1908. [Google Scholar] [CrossRef]
- Hu, G.; Hu, X.; Yang, K.; Liu, D.; Xue, C.; Liu, Y.; Xiao, C.; Zou, Y.; Liu, H.; Chen, J. Restructuring of Contralateral Gray Matter Volume Associated with Cognition in Patients with Unilateral Temporal Lobe Glioma before and after Surgery. Hum. Brain Mapp. 2020, 41, 1786–1796. [Google Scholar] [CrossRef]
- Yuan, T.; Zuo, Z.; Ying, J.; Jin, L.; Kang, J.; Gui, S.; Wang, R.; Li, C. Structural and Functional Alterations in the Contralesional Medial Temporal Lobe in Glioma Patients. Front. Neurosci. 2020, 14, 10. [Google Scholar] [CrossRef]
- Pasquini, L.; Jenabi, M.; Yildirim, O.; Silveira, P.; Peck, K.K.; Holodny, A.I. Brain Functional Connectivity in Low- and High-Grade Gliomas: Differences in Network Dynamics Associated with Tumor Grade and Location. Cancers 2022, 14, 3327. [Google Scholar] [CrossRef]
- Batouli, S.A.H.; Hasani, N.; Gheisari, S.; Behzad, E.; Oghabian, M.A. Evaluation of the Factors Influencing Brain Language Laterality in Presurgical Planning. Phys. Med. 2016, 32, 1201–1209. [Google Scholar] [CrossRef]
- Kristo, G.; Raemaekers, M.; Rutten, G.-J.; de Gelder, B.; Ramsey, N.F. Inter-Hemispheric Language Functional Reorganization in Low-Grade Glioma Patients after Tumour Surgery. Cortex J. Devoted Study Nerv. Syst. Behav. 2015, 64, 235–248. [Google Scholar] [CrossRef]
- Partovi, S.; Jacobi, B.; Rapps, N.; Zipp, L.; Karimi, S.; Rengier, F.; Lyo, J.K.; Stippich, C. Clinical Standardized FMRI Reveals Altered Language Lateralization in Patients with Brain Tumor. AJNR Am. J. Neuroradiol. 2012, 33, 2151–2157. [Google Scholar] [CrossRef]
- Petrovich, N.M.; Holodny, A.I.; Brennan, C.W.; Gutin, P.H. Isolated Translocation of Wernicke’s Area to the Right Hemisphere in a 62-Year-Man with a Temporo-Parietal Glioma. AJNR Am. J. Neuroradiol. 2004, 25, 130–133. [Google Scholar]
- Ulmer, J.L.; Hacein-Bey, L.; Mathews, V.P.; Mueller, W.M.; DeYoe, E.A.; Prost, R.W.; Meyer, G.A.; Krouwer, H.G.; Schmainda, K.M. Lesion-Induced Pseudo-Dominance at Functional Magnetic Resonance Imaging: Implications for Preoperative Assessments. Neurosurgery 2004, 55, 569–579; discussion 580–581. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Yang, X.; Olson, J.J.; Gopinath, K.; Fan, T.; Mao, H. Group Independent Component Analysis and Functional MRI Examination of Changes in Language Areas Associated with Brain Tumors at Different Locations. PLoS ONE 2013, 8, e59657. [Google Scholar] [CrossRef]
- Ius, T.; Angelini, E.; Thiebaut de Schotten, M.; Mandonnet, E.; Duffau, H. Evidence for Potentials and Limitations of Brain Plasticity Using an Atlas of Functional Resectability of WHO Grade II Gliomas: Towards a “Minimal Common Brain”. NeuroImage 2011, 56, 992–1000. [Google Scholar] [CrossRef]
- Sarubbo, S.; De Benedictis, A.; Merler, S.; Mandonnet, E.; Balbi, S.; Granieri, E.; Duffau, H. Towards a Functional Atlas of Human White Matter. Hum. Brain Mapp. 2015, 36, 3117–3136. [Google Scholar] [CrossRef]
- Sarubbo, S.; Tate, M.; De Benedictis, A.; Merler, S.; Moritz-Gasser, S.; Herbet, G.; Duffau, H. Mapping Critical Cortical Hubs and White Matter Pathways by Direct Electrical Stimulation: An Original Functional Atlas of the Human Brain. NeuroImage 2020, 205, 116237. [Google Scholar] [CrossRef]
- Hickok, G.; Poeppel, D. The Cortical Organization of Speech Processing. Nat. Rev. Neurosci. 2007, 8, 393–402. [Google Scholar] [CrossRef]
- Binder, J.R. Current Controversies on Wernicke’s Area and Its Role in Language. Curr. Neurol. Neurosci. Rep. 2017, 17, 58. [Google Scholar] [CrossRef]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef]
- Crinion, J.; Ashburner, J.; Leff, A.; Brett, M.; Price, C.; Friston, K. Spatial Normalization of Lesioned Brains: Performance Evaluation and Impact on FMRI Analyses. Neuroimage 2007, 37, 866–875. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Why Voxel-Based Morphometry Should Be Used. NeuroImage 2001, 14, 1238–1243. [Google Scholar] [CrossRef]
- Biswal, B.B.; Mennes, M.; Zuo, X.-N.; Gohel, S.; Kelly, C.; Smith, S.M.; Beckmann, C.F.; Adelstein, J.S.; Buckner, R.L.; Colcombe, S.; et al. Toward Discovery Science of Human Brain Function. Proc. Natl. Acad. Sci. USA 2010, 107, 4734–4739. [Google Scholar] [CrossRef]
- Bordin, V.; Bertani, I.; Mattioli, I.; Sundaresan, V.; McCarthy, P.; Suri, S.; Zsoldos, E.; Filippini, N.; Mahmood, A.; Melazzini, L.; et al. Integrating Large-Scale Neuroimaging Research Datasets: Harmonisation of White Matter Hyperintensity Measurements across Whitehall and UK Biobank Datasets. NeuroImage 2021, 237, 118189. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Majewska, A.K.; Newton, J.R.; Sur, M. Remodeling of Synaptic Structure in Sensory Cortical Areas In Vivo. J. Neurosci. 2006, 26, 3021–3029. [Google Scholar] [CrossRef]
- Fierstra, J.; van Niftrik, C.; Piccirelli, M.; Bozinov, O.; Pangalu, A.; Krayenbühl, N.; Valavanis, A.; Weller, M.; Regli, L. Diffuse Gliomas Exhibit Whole Brain Impaired Cerebrovascular Reactivity. Magn. Reson. Imaging 2018, 45, 78–83. [Google Scholar] [CrossRef]
- Vigneau, M.; Beaucousin, V.; Hervé, P.-Y.; Jobard, G.; Petit, L.; Crivello, F.; Mellet, E.; Zago, L.; Mazoyer, B.; Tzourio-Mazoyer, N. What Is Right-Hemisphere Contribution to Phonological, Lexico-Semantic, and Sentence Processing? Insights from a Meta-Analysis. NeuroImage 2011, 54, 577–593. [Google Scholar] [CrossRef]
- Vingerhoets, G.; Borsel, J.V.; Tesink, C.; van den Noort, M.; Deblaere, K.; Seurinck, R.; Vandemaele, P.; Achten, E. Multilingualism: An FMRI Study. NeuroImage 2003, 20, 2181–2196. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.P.; Badzakova-Trajkov, G.; Waldie, K.E. Language Lateralisation in Late Proficient Bilinguals: A Lexical Decision FMRI Study. Neuropsychologia 2012, 50, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Denvil, D.; Capelle, L. Long Term Reshaping of Language, Sensory, and Motor Maps after Glioma Resection: A New Parameter to Integrate in the Surgical Strategy. J. Neurol. Neurosurg. Psychiatry 2002, 72, 511–516. [Google Scholar] [CrossRef]
- Duffau, H.; Capelle, L.; Denvil, D.; Sichez, N.; Gatignol, P.; Lopes, M.; Mitchell, M.-C.; Sichez, J.-P.; Van Effenterre, R. Functional Recovery after Surgical Resection of Low Grade Gliomas in Eloquent Brain: Hypothesis of Brain Compensation. J. Neurol. Neurosurg. Psychiatry 2003, 74, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.M.H.; Leff, A.P.; Prejawa, S.; Bruce, R.; Haigh, Z.; Lim, L.; Ramsden, S.; Oberhuber, M.; Ludersdorfer, P.; Crinion, J.; et al. Right Hemisphere Structural Adaptation and Changing Language Skills Years after Left Hemisphere Stroke. Brain J. Neurol. 2017, 140, 1718–1728. [Google Scholar] [CrossRef]
- Duchaine, B.; Yovel, G. A Revised Neural Framework for Face Processing. Annu. Rev. Vis. Sci. 2015, 1, 393–416. [Google Scholar] [CrossRef]
- Molenberghs, P.; Hayward, L.; Mattingley, J.B.; Cunnington, R. Activation Patterns during Action Observation Are Modulated by Context in Mirror System Areas. NeuroImage 2012, 59, 608–615. [Google Scholar] [CrossRef]
- Bonini, L. The Extended Mirror Neuron Network: Anatomy, Origin, and Functions. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2017, 23, 56–67. [Google Scholar] [CrossRef]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating Theory of Mind: A Meta-Analysis of Functional Brain Imaging Studies. Neurosci. Biobehav. Rev. 2014, 42, 9–34. [Google Scholar] [CrossRef]
- Vilasboas, T.; Herbet, G.; Duffau, H. Challenging the Myth of Right Nondominant Hemisphere: Lessons from Corticosubcortical Stimulation Mapping in Awake Surgery and Surgical Implications. World Neurosurg. 2017, 103, 449–456. [Google Scholar] [CrossRef]
- Foundas, A.L.; Leonard, C.M.; Gilmore, R.L.; Fennell, E.B.; Heilman, K.M. Pars Triangularis Asymmetry and Language Dominance. Proc. Natl. Acad. Sci. USA 1996, 93, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N.; Levitsky, W. Human Brain: Left-Right Asymmetries in Temporal Speech Region. Science 1968, 161, 186–187. [Google Scholar] [CrossRef]
- Wada, J.A.; Clarke, R.; Hamm, A. Cerebral Hemispheric Asymmetry in Humans. Cortical Speech Zones in 100 Adults and 100 Infant Brains. Arch. Neurol. 1975, 32, 239–246. [Google Scholar] [CrossRef]
- Jehna, M.; Becker, J.; Zaar, K.; von Campe, G.; Mahdy Ali, K.; Reishofer, G.; Payer, F.; Synowitz, M.; Fazekas, F.; Enzinger, C.; et al. Symmetry of the Arcuate Fasciculus and Its Impact on Language Performance of Patients with Brain Tumors in the Language-Dominant Hemisphere. J. Neurosurg. 2017, 127, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-Z.; Mathias, S.R.; Guadalupe, T.; ENIGMA Laterality Working Group; Glahn, D.C.; Franke, B.; Crivello, F.; Tzourio-Mazoyer, N.; Fisher, S.E.; Thompson, P.M.; et al. Mapping Cortical Brain Asymmetry in 17,141 Healthy Individuals Worldwide via the ENIGMA Consortium. Proc. Natl. Acad. Sci. USA 2018, 115, E5154–E5163. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Schijven, D.; Carrion-Castillo, A.; Joliot, M.; Mazoyer, B.; Fisher, S.E.; Crivello, F.; Francks, C. The Genetic Architecture of Structural Left–Right Asymmetry of the Human Brain. Nat. Hum. Behav. 2021, 5, 1226–1239. [Google Scholar] [CrossRef]
- Karolis, V.R.; Corbetta, M.; Thiebaut de Schotten, M. The Architecture of Functional Lateralisation and Its Relationship to Callosal Connectivity in the Human Brain. Nat. Commun. 2019, 10, 1417. [Google Scholar] [CrossRef]
- Okada, N.; Fukunaga, M.; Yamashita, F.; Koshiyama, D.; Yamamori, H.; Ohi, K.; Yasuda, Y.; Fujimoto, M.; Watanabe, Y.; Yahata, N.; et al. Abnormal Asymmetries in Subcortical Brain Volume in Schizophrenia. Mol. Psychiatry 2016, 21, 1460–1466. [Google Scholar] [CrossRef]
- Reynolds, J.E.; Long, X.; Grohs, M.N.; Dewey, D.; Lebel, C. Structural and Functional Asymmetry of the Language Network Emerge in Early Childhood. Dev. Cogn. Neurosci. 2019, 39, 100682. [Google Scholar] [CrossRef]
- Stockert, A.; Wawrzyniak, M.; Klingbeil, J.; Wrede, K.; Kümmerer, D.; Hartwigsen, G.; Kaller, C.P.; Weiller, C.; Saur, D. Dynamics of Language Reorganization after Left Temporo-Parietal and Frontal Stroke. Brain 2020, 143, 844–861. [Google Scholar] [CrossRef]

| Left Tumors (n = 18) | Right Tumors (n = 13) | |
|---|---|---|
| Handedness | ||
| Right | 16 | 4 |
| Left | 1 | 7 |
| Ambidextrous | 1 | 2 |
| Tumor type WHO | ||
| Anaplastic astrocytoma | 3 | 2 |
| Glioblastoma multiforme | 6 | 5 |
| Metastatic | 2 | 0 |
| Oligoastrocytoma | 3 | 1 |
| Oligodendroglioma | 3 | 2 |
| No data | 1 | 3 |
| Tumor grade | ||
| Low grade | 6 | 7 |
| High grade | 11 | 4 |
| No data | 1 | 2 |
| Previous surgery | ||
| Yes | 4 | 3 |
| No | 12 | 9 |
| No data | 2 | 1 |
| Language impairment | ||
| Yes | 12 | 8 |
| No | 4 | 5 |
| No data | 2 | 0 |
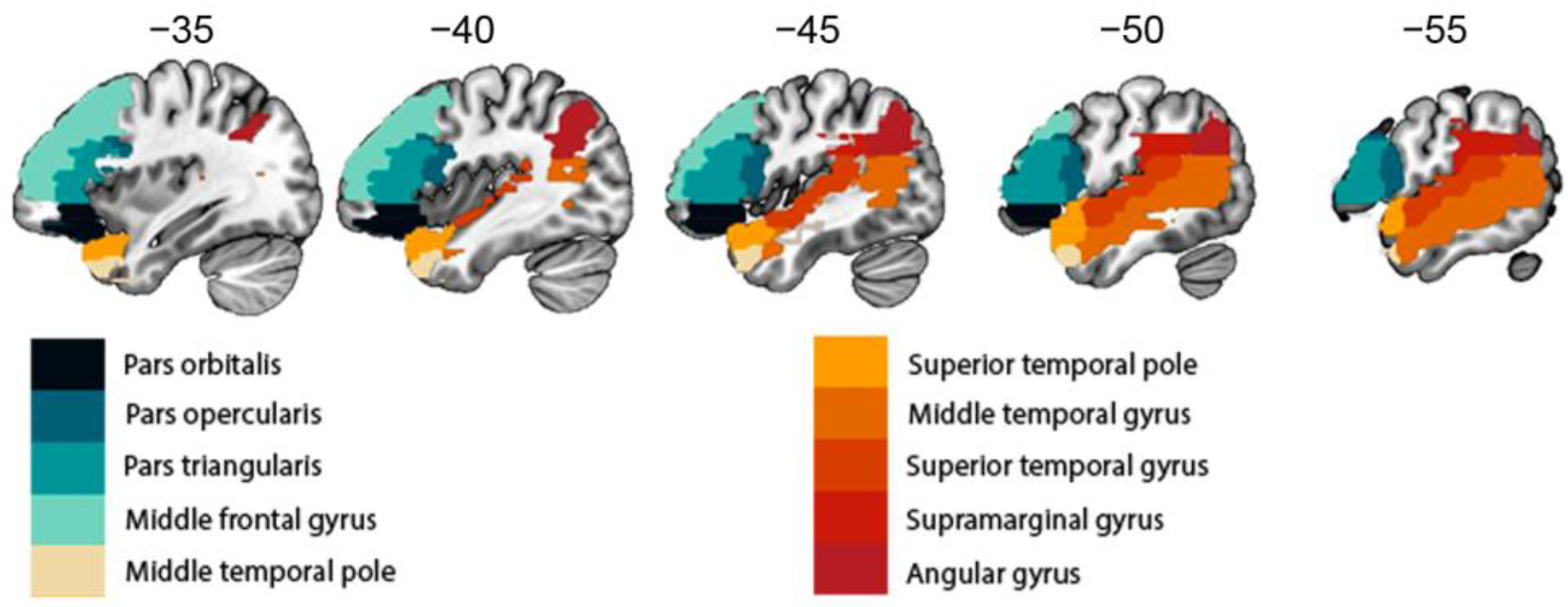
| Region | Left Tumor Group | Right Tumor Group | Healthy Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T | p | Cohen’s d | T | p | Cohen’s d | T | p | Cohen’s d | |
| Pars orbitalis | −1.11 | 0.282 | −0.26 | −4.50 | <0.001 * | −1.25 | 3.50 | <0.001 * | 0.42 |
| Pars opercularis | 6.29 | <0.001 * | 1.48 | 0.55 | 0.590 | 0.15 | 55.24 | <0.001 * | 6.56 |
| Pars triangularis | −4.33 | 0.001 * | −1.02 | −3.65 | <0.003 * | −1.01 | −36.11 | <0.001 * | −4.29 |
| Mid frontal gyrus | 1.55 | 0.139 | 0.37 | 0.22 | 0.827 | 0.06 | 21.84 | <0.001 * | 2.59 |
| Mid temporal pole | 4.13 | <0.001 * | 0.97 | 0.93 | 0.369 | 0.26 | 77.36 | <0.001 * | 9.18 |
| Sup temporal pole | 1.88 | 0.078 | 0.44 | −1.46 | 0.171 | −0.40 | 10.45 | <0.001 * | 1.25 |
| MTG | 1.13 | 0.275 | 0.27 | −4.67 | <0.001 * | −1.30 | −40.38 | <0.001 * | −4.79 |
| STG | 5.40 | <0.001 * | 1.27 | −1.66 | 0.123 | −0.46 | 76.76 | <0.001 * | 9.11 |
| SM | 6.51 | <0.001 * | 1.53 | −0.25 | 0.811 | 0.07 | 90.24 | <0.001 * | 10.71 |
| Angular gyrus | 4.85 | <0.001 * | 1.14 | 4.89 | <0.001 * | 1.36 | 92.25 | <0.001 * | 10.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manso-Ortega, L.; De Frutos-Sagastuy, L.; Gisbert-Muñoz, S.; Salamon, N.; Qiao, J.; Walshaw, P.; Quiñones, I.; Połczyńska, M.M. Grey Matter Reshaping of Language-Related Regions Depends on Tumor Lateralization. Cancers 2023, 15, 3852. https://doi.org/10.3390/cancers15153852
Manso-Ortega L, De Frutos-Sagastuy L, Gisbert-Muñoz S, Salamon N, Qiao J, Walshaw P, Quiñones I, Połczyńska MM. Grey Matter Reshaping of Language-Related Regions Depends on Tumor Lateralization. Cancers. 2023; 15(15):3852. https://doi.org/10.3390/cancers15153852
Chicago/Turabian StyleManso-Ortega, Lucía, Laura De Frutos-Sagastuy, Sandra Gisbert-Muñoz, Noriko Salamon, Joe Qiao, Patricia Walshaw, Ileana Quiñones, and Monika M. Połczyńska. 2023. "Grey Matter Reshaping of Language-Related Regions Depends on Tumor Lateralization" Cancers 15, no. 15: 3852. https://doi.org/10.3390/cancers15153852
APA StyleManso-Ortega, L., De Frutos-Sagastuy, L., Gisbert-Muñoz, S., Salamon, N., Qiao, J., Walshaw, P., Quiñones, I., & Połczyńska, M. M. (2023). Grey Matter Reshaping of Language-Related Regions Depends on Tumor Lateralization. Cancers, 15(15), 3852. https://doi.org/10.3390/cancers15153852






