Recent Advances in Clinical Research for Skin Cancer Chemoprevention
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
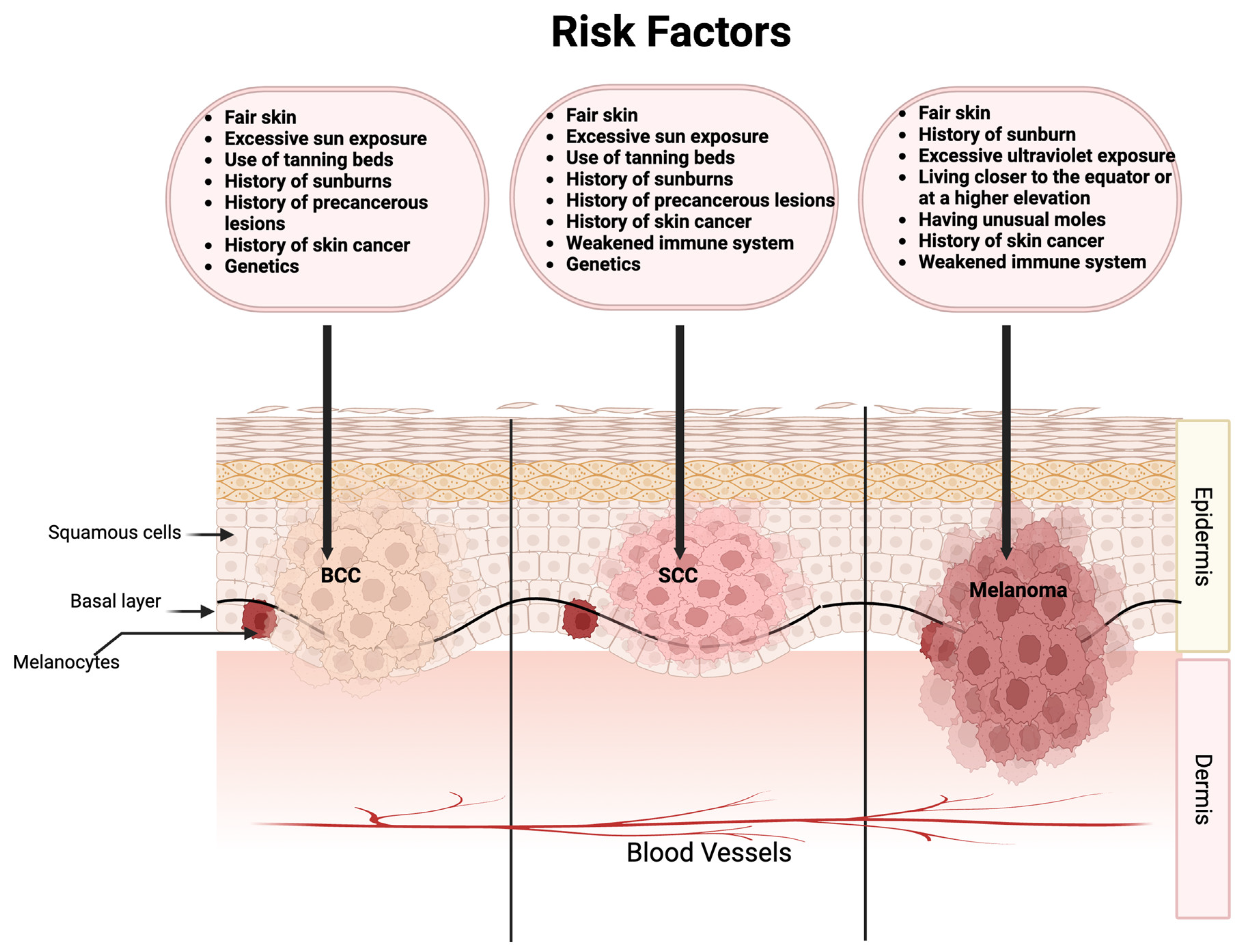
3. Cancer Types or Conditions of the Clinical Trials
4. Interventions
5. Populations
6. Endpoints
7. ClinicalTrials.Gov Outcomes
8. PubMed Database Outcomes
9. Examples of Chemoprevention Trials
9.1. Nicotinamide
9.2. NSAIDs
9.3. Retinoids
9.4. 5-Fluorouracil (5-FU)
9.5. Difluoromethylornithine (DFMO)
9.6. Polyunsaturated Fatty Acids (PUFAs)
9.7. Antidiabetic Drugs
10. Safety Issues
11. Limitation of the Current Review
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sable, K.A.; Shields, B.E. The Role of Dietary Antioxidants in Melanoma and Nonmelanoma Skin Cancer. Cutis 2023, 111, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Pop, T.D.; Diaconeasa, Z. Recent Advances in Phenolic Metabolites and Skin Cancer. Int. J. Mol. Sci. 2021, 22, 9707. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.C.F.; Sousa, J.J.S.; Pais, A. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Cala, C.M.; Xu, H. Photoimmunology. Dermatol. Clin. 2014, 32, 277–290. [Google Scholar] [CrossRef]
- Krakowski, A.C.; Hafeez, F.; Westheim, A.; Pan, E.Y.; Wilson, M. Advanced basal cell carcinoma: What dermatologists need to know about diagnosis. J. Am. Acad. Dermatol. 2022, 86, S1–S13. [Google Scholar] [CrossRef]
- Saeidi, V.; Doudican, N.; Carucci, J.A. Understanding the squamous cell carcinoma immune microenvironment. Front. Immunol. 2023, 14, 1084873. [Google Scholar] [CrossRef]
- Chandra, A.; Newman, A.; Mullens, D.; Lin, C.C. Human Papillomavirus (HPV)-Associated Squamous Cell Carcinoma In situ With Positive p16 and Ki-67 Immunohistochemical Stains in a Young Immunocompetent Patient. Cureus 2020, 12, e9673. [Google Scholar] [CrossRef]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11, e2021161S. [Google Scholar] [CrossRef]
- Singh, N.; McClure, E.M.; Akaike, T.; Park, S.Y.; Huynh, E.T.; Goff, P.H.; Nghiem, P. The Evolving Treatment Landscape of Merkel Cell Carcinoma. Curr. Treat. Options Oncol. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nardone, B.; Orrell, K.A.; Vakharia, P.P.; West, D.P. Skin cancer associated with commonly prescribed drugs: Tumor necrosis factor alpha inhibitors (TNF-alphaIs), angiotensin-receptor blockers (ARBs), phosphodiesterase type 5 inhibitors (PDE5Is) and statins -weighing the evidence. Expert Opin. Drug Saf. 2018, 17, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Lai, C.C.; Chen, Y.H.; Lai, E.C.; Hung, M.J.; Chi, C.C. Associations of thiazide use with skin cancers: A systematic review and meta-analysis. BMC Med. 2022, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Emerick, K.S. Skin-Cancer Chemoprevention in Transplant Recipients. N. Engl. J. Med. 2023, 388, 844–846. [Google Scholar] [CrossRef]
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The efficacy and safety of sunscreen use for the prevention of skin cancer. Cmaj 2020, 192, E1802–E1808. [Google Scholar] [CrossRef]
- Fonseca, M.; Rehman, M.; Soares, R.; Fonte, P. The Impact of Flavonoid-Loaded Nanoparticles in the UV Protection and Safety Profile of Topical Sunscreens. Biomolecules 2023, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Agus, D.B.; Jaffee, E.M.; Van Dang, C. Cancer Moonshot 2.0. Lancet Oncol. 2021, 22, 164–165. [Google Scholar] [CrossRef]
- Stratton, S.P.; Alberts, D.S.; Einspahr, J.G.; Sagerman, P.M.; Warneke, J.A.; Curiel-Lewandrowski, C.; Myrdal, P.B.; Karlage, K.L.; Nickoloff, B.J.; Brooks, C.; et al. A phase 2a study of topical perillyl alcohol cream for chemoprevention of skin cancer. Cancer Prev. Res. 2010, 3, 160–169. [Google Scholar] [CrossRef]
- Anderson, J.M.; Moy, L.; Moy, R.L. Preventative Options and the Future of Chemoprevention for Cutaneous Tumors. Dermatol. Clin. 2023, 41, 231–238. [Google Scholar] [CrossRef]
- Tang, J.Y.; Aszterbaum, M.; Athar, M.; Barsanti, F.; Cappola, C.; Estevez, N.; Hebert, J.; Hwang, J.; Khaimskiy, Y.; Kim, A.; et al. Basal cell carcinoma chemoprevention with nonsteroidal anti-inflammatory drugs in genetically predisposed PTCH1+/− humans and mice. Cancer Prev. Res. 2010, 3, 25–34. [Google Scholar] [CrossRef]
- Kadakia, K.C.; Barton, D.L.; Loprinzi, C.L.; Sloan, J.A.; Otley, C.C.; Diekmann, B.B.; Novotny, P.J.; Alberts, S.R.; Limburg, P.J.; Pittelkow, M.R. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer 2012, 118, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.A.; Bingham, S.F.; Lew, R.A.; Hall, R.; Eilers, D.; Kirsner, R.; Naylor, M.; Kalivas, J.; Cole, G.; Marcolivio, K.; et al. Topical tretinoin therapy and all-cause mortality. Arch. Dermatol. 2009, 145, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dore, D.D.; Lapane, K.L.; Trivedi, A.N.; Mor, V.; Weinstock, M.A. Association between statin use and risk for keratinocyte carcinoma in the veterans affairs topical tretinoin chemoprevention trial. Ann. Intern. Med. 2009, 150, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.A.; Bingham, S.F.; Digiovanna, J.J.; Rizzo, A.E.; Marcolivio, K.; Hall, R.; Eilers, D.; Naylor, M.; Kirsner, R.; Kalivas, J.; et al. Tretinoin and the prevention of keratinocyte carcinoma (Basal and squamous cell carcinoma of the skin): A veterans affairs randomized chemoprevention trial. J. Investig. Dermatol. 2012, 132, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.A.; Thwin, S.S.; Siegel, J.A.; Marcolivio, K.; Means, A.D.; Leader, N.F.; Shaw, F.M.; Hogan, D.; Eilers, D.; Swetter, S.M.; et al. Chemoprevention of Basal and Squamous Cell Carcinoma With a Single Course of Fluorouracil, 5%, Cream: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bartels, P.; Yozwiak, M.; Einspahr, J.; Saboda, K.; Liu, Y.; Brooks, C.; Bartels, H.; Alberts, D.S. Chemopreventive efficacy of topical difluoromethylornithine and/or triamcinolone in the treatment of actinic keratoses analyzed by karyometry. Anal. Quant. Cytol. Histol. 2009, 31, 355–366. [Google Scholar] [PubMed]
- Jeter, J.M.; Curiel-Lewandrowski, C.; Stratton, S.P.; Myrdal, P.B.; Warneke, J.A.; Einspahr, J.G.; Bartels, H.G.; Yozwiak, M.; Bermudez, Y.; Hu, C.; et al. Phase IIB Randomized Study of Topical Difluoromethylornithine and Topical Diclofenac on Sun-Damaged Skin of the Forearm. Cancer Prev. Res. 2016, 9, 128–134. [Google Scholar] [CrossRef]
- Bailey, H.H.; Kim, K.; Verma, A.K.; Sielaff, K.; Larson, P.O.; Snow, S.; Lenaghan, T.; Viner, J.L.; Douglas, J.; Dreckschmidt, N.E.; et al. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of alpha-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev. Res. 2010, 3, 35–47. [Google Scholar] [CrossRef]
- Kreul, S.M.; Havighurst, T.; Kim, K.; Mendonca, E.A.; Wood, G.S.; Snow, S.; Borich, A.; Verma, A.; Bailey, H.H. A phase III skin cancer chemoprevention study of DFMO: Long-term follow-up of skin cancer events and toxicity. Cancer Prev. Res. 2012, 5, 1368–1374. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Massey, K.A.; Bennett, S.P.; Al-Aasswad, N.M.; Roshdy, K.; Gibbs, N.K.; Friedmann, P.S.; Nicolaou, A.; Rhodes, L.E. Randomized controlled trial of oral omega-3 PUFA in solar-simulated radiation-induced suppression of human cutaneous immune responses. Am. J. Clin. Nutr. 2013, 97, 646–652. [Google Scholar] [CrossRef]
- Curiel-Lewandrowski, C.; Tang, J.Y.; Einspahr, J.G.; Bermudez, Y.; Hsu, C.H.; Rezaee, M.; Lee, A.H.; Tangrea, J.; Parnes, H.L.; Alberts, D.S.; et al. Pilot study on the bioactivity of vitamin d in the skin after oral supplementation. Cancer Prev. Res. 2015, 8, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernandez-Penas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Martin, A.J.; Dalziell, R.A.; McKenzie, C.A.; Lowe, P.M.; Eris, J.M.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Bielski, V.A.; et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br. J. Dermatol. 2016, 175, 1073–1075. [Google Scholar] [CrossRef]
- Allen, N.C.; Martin, A.J.; Snaidr, V.A.; Eggins, R.; Chong, A.H.; Fernandez-Penas, P.; Gin, D.; Sidhu, S.; Paddon, V.L.; Banney, L.A.; et al. Nicotinamide for Skin-Cancer Chemoprevention in Transplant Recipients. N. Engl. J. Med. 2023, 388, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Martin, A.J.; Chen, A.C.; Scolyer, R.A.; Lyons, J.G.; McKenzie, C.A.; Madore, J.; Halliday, G.M.; Damian, D.L. A Reduction in Inflammatory Macrophages May Contribute to Skin Cancer Chemoprevention by Nicotinamide. J. Investig. Dermatol. 2019, 139, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Viner, J.L.; Pentland, A.P.; Cantrell, W.; Lin, H.Y.; Bailey, H.; Kang, S.; Linden, K.G.; Heffernan, M.; Duvic, M.; et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: A randomized, double-blind, placebo-controlled trial. J. Natl. Cancer Inst. 2010, 102, 1835–1844. [Google Scholar] [CrossRef]
- Yan, M.K.; Orchard, S.G.; Adler, N.R.; Wolfe, R.; McLean, C.; Rodriguez, L.M.; Woods, R.L.; Gibbs, P.; Chan, A.T.; Haydon, A.; et al. Effect of Aspirin on Melanoma Incidence in Older Persons: Extended Follow-up of a Large Randomized Double-blind Placebo-controlled Trial. Cancer Prev. Res. 2022, 15, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.P.; Lapane, K.L.; Weinstock, M.A.; Group, V.T. Association between non-steroidal anti-inflammatory drugs and keratinocyte carcinomas of the skin among participants in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Pharmacoepidemiol. Drug Saf. 2011, 20, 922–929. [Google Scholar] [CrossRef]
- Passarelli, M.N.; Barry, E.L.; Zhang, D.; Gangar, P.; Rees, J.R.; Bresalier, R.S.; McKeown-Eyssen, G.; Karagas, M.R.; Baron, J.A. Risk of basal cell carcinoma in a randomized clinical trial of aspirin and folic acid for the prevention of colorectal adenomas. Br. J. Dermatol. 2018, 179, 337–344. [Google Scholar] [CrossRef]
- Campione, E.; Diluvio, L.; Paterno, E.J.; Chimenti, S. Topical treatment of actinic keratoses with piroxicam 1% gel: A preliminary open-label study utilizing a new clinical score. Am. J. Clin. Dermatol. 2010, 11, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.Y.; Rizzo, A.E.; Cohen, T.S.; Dyer, R.K.; Korgavkar, K.; Bingham, S.F.; Weinstock, M.A.; Veterans Affairs Topical Tretinoin Chemoprevention (VATTC) Trial Group. Predictors of squamous cell carcinoma in high-risk patients in the VATTC trial. J. Investig. Dermatol. 2013, 133, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Phibbs, C.S.; Chow, A.; Weinstock, M.A.; Veterans Affairs Topical Tretinoin Chemoprevention (VATTC) Trial Group. Impact of topical fluorouracil cream on costs of treating keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis. J. Am. Acad. Dermatol. 2018, 79, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Means, A.D.; Leader, N.F.; Siegel, J.A.; Arron, S.T.; Walker, J.L.; Pomerantz, H.; Beatson, M.; Weinstock, M.A. Rate and Proportion of Malignant Skin Biopsies for Basal Cell and Squamous Cell Carcinoma on the Face and Ears After a Single Course of Topical 5-Fluorouracil: The Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial. Dermatol. Surg. 2021, 47, 541–543. [Google Scholar] [CrossRef]
- Beatson, M.; Means, A.D.; Leader, N.F.; Robinson-Bostom, L.; Weinstock, M.A. Characteristics of Keratinocyte Carcinomas and Patients with Keratinocyte Carcinomas Following a Single 2-4 Week Course of Topical 5-fluorouracil on the Face and Ears. Acta Derm.-Venereol. 2019, 99, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.U.; Ahmed, I.; Matin, R.N.; Homer, V.; Lear, J.T.; Ismail, F.; Whitmarsh, T.; Green, A.C.; Thomson, J.; Milligan, A.; et al. Topical treatment of actinic keratoses in organ transplant recipients: A feasibility study for SPOT (Squamous cell carcinoma Prevention in Organ transplant recipients using Topical treatments). Br. J. Dermatol. 2022, 187, 324–337. [Google Scholar] [CrossRef]
- Cuevas Sanchez, P.; Espinoza, W.; Perez, C.; Angulo, J.; Gimenez-Gallego, G. Topical treatment of actinic keratoses with potassium dobesilate 5% cream. a preliminary open-label study. Eur. J. Med. Res. 2011, 16, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Misitzis, A.; Stratigos, A.; Mastorakos, G.; Weinstock, M. Antidiabetic Treatment in Patients at High Risk for a Subsequent Keratinocyte Carcinoma. J. Drugs Dermatol. 2022, 21, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Chiou, A.S.; Mackay-Wiggan, J.M.; Aszterbaum, M.; Chanana, A.M.; Lee, W.; Lindgren, J.A.; Raphael, M.A.; Thompson, B.J.; Bickers, D.R.; et al. Tazarotene: Randomized, double-blind, vehicle-controlled, and open-label concurrent trials for basal cell carcinoma prevention and therapy in patients with basal cell nevus syndrome. Cancer Prev. Res. 2014, 7, 292–299. [Google Scholar] [CrossRef]
- Linden, K.G.; Leachman, S.A.; Zager, J.S.; Jakowatz, J.G.; Viner, J.L.; McLaren, C.E.; Barr, R.J.; Carpenter, P.M.; Chen, W.P.; Elmets, C.A.; et al. A randomized, double-blind, placebo-controlled phase II clinical trial of lovastatin for various endpoints of melanoma pathobiology. Cancer Prev. Res. 2014, 7, 496–504. [Google Scholar] [CrossRef]
- Tahata, S.; Singh, S.V.; Lin, Y.; Hahm, E.R.; Beumer, J.H.; Christner, S.M.; Rao, U.N.; Sander, C.; Tarhini, A.A.; Tawbi, H.; et al. Evaluation of Biodistribution of Sulforaphane after Administration of Oral Broccoli Sprout Extract in Melanoma Patients with Multiple Atypical Nevi. Cancer Prev. Res. 2018, 11, 429–438. [Google Scholar] [CrossRef]
- Mantel, A.; Carpenter-Mendini, A.; VanBuskirk, J.; Pentland, A.P. Aldo-keto reductase 1C3 is overexpressed in skin squamous cell carcinoma (SCC) and affects SCC growth via prostaglandin metabolism. Exp. Dermatol. 2014, 23, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Halliday, G.M.; Surjana, D.; Damian, D.L. Nicotinamide prevents ultraviolet radiation-induced cellular energy loss. Photochem. Photobiol. 2010, 86, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Olbricht, S.; Ruiz, E.S.; Hartman, R.I. Nicotinamide for Keratinocyte Carcinoma Chemoprevention: A Nationwide Survey of Mohs Surgeons. Dermatol. Surg. 2021, 47, 452–453. [Google Scholar] [CrossRef]
- Zhao, Y. Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2016, 374, 789. [Google Scholar] [CrossRef]
- Thompson, B.C.; Halliday, G.M.; Damian, D.L. Nicotinamide enhances repair of arsenic and ultraviolet radiation-induced DNA damage in HaCaT keratinocytes and ex vivo human skin. PLoS ONE 2015, 10, e0117491. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Pozzi, V.; Sartini, D.; Salvolini, E.; Brisigotti, V.; Molinelli, E.; Campanati, A.; Offidani, A.; Emanuelli, M. Beyond Nicotinamide Metabolism: Potential Role of Nicotinamide N-Methyltransferase as a Biomarker in Skin Cancers. Cancers 2021, 13, 4943. [Google Scholar] [CrossRef]
- Thomas, G.J.; Herranz, P.; Cruz, S.B.; Parodi, A. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: Potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol. Ther. 2019, 32, e12800. [Google Scholar] [CrossRef]
- Whittle, B.J. COX-1 and COX-2 products in the gut: Therapeutic impact of COX-2 inhibitors. Gut 2000, 47, 320–325. [Google Scholar] [CrossRef]
- Frankel, L.; Ardeljan, A.D.; Takabe, K.; Rashid, O.M. The Association Between Aspirin and Basal Cell Carcinoma: A Clinical and Financial Analysis. World J. Oncol. 2022, 13, 343–349. [Google Scholar] [CrossRef]
- Martin, G.M.; Stockfleth, E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J. Drugs Dermatol. 2012, 11, 600–608. [Google Scholar] [PubMed]
- Ramchatesingh, B.; Martinez Villarreal, A.; Arcuri, D.; Lagace, F.; Setah, S.A.; Touma, F.; Al-Badarin, F.; Litvinov, I.V. The Use of Retinoids for the Prevention and Treatment of Skin Cancers: An Updated Review. Int. J. Mol. Sci. 2022, 23, 12622. [Google Scholar] [CrossRef] [PubMed]
- DiGiovanna, J.J. Retinoid chemoprevention in the high-risk patient. J. Am. Acad. Dermatol. 1998, 39, S82–S85. [Google Scholar] [CrossRef] [PubMed]
- Schauder, D.M.; Kim, J.; Nijhawan, R.I. Evaluation of the Use of Capecitabine for the Treatment and Prevention of Actinic Keratoses, Squamous Cell Carcinoma, and Basal Cell Carcinoma: A Systematic Review. JAMA Dermatol. 2020, 156, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.L.; Havighurst, T.; Lozar, T.; Jones, T.D.; Kim, K.; Bailey, H.H. Ototoxicity of Long-Term alpha-Difluoromethylornithine for Skin Cancer Prevention. Laryngoscope 2023, 133, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.Y.; Hawk, E.T. When “Effective” Prevention Agents Fail to Elicit Anticipated Effects: Challenges in Trial Design. Cancer Prev. Res. 2016, 9, 125–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Misitzis, A.; Stratigos, A.J.; Beatson, M.; Mastorakos, G.; Dellavalle, R.P.; Weinstock, M.A.; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial Group. The association of metformin use with keratinocyte carcinoma development in high-risk patients. Dermatol. Ther. 2020, 33, e14402. [Google Scholar] [CrossRef]
- Olson, J.M.; Ameer, M.A.; Goyal, A. Vitamin A Toxicity; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Giacalone, S.; Spigariolo, C.B.; Bortoluzzi, P.; Nazzaro, G. Oral nicotinamide: The role in skin cancer chemoprevention. Dermatol. Ther. 2021, 34, e14892. [Google Scholar] [CrossRef]
| ID | Condition | Intervention | Subject | Endpoint | Size | Results |
|---|---|---|---|---|---|---|
| NCT00023621 | BCC | Oral celecoxib | Patients with history of BCC | Rate of BCC | 60 | Celecoxib significantly reduced BCC number and burden [20]. |
| NCT00644384 | NMSC | Oral acitretin | With history of NMSC | Rate of new NMSC; surrogate biomarkers | 130 | No results published |
| NCT00003611 | NMSC | Oral acitretin | With history of skin cancers with organ transplantation | Rate of new NMSC; surrogate biomarkers | 70 | Acitretin showed benefit but not significant; the patients who received acitretin reported significantly more mucositis and skin toxicities compared to the patients who received placebo [21]. |
| NCT00007631 | NMSC | Topical tretinoin | With history of NMSC | Rate of NMSC | 1131 | High-dose topical tretinoin was ineffective at reducing risk of NMSC [22,23,24]. |
| NCT00847912 | NMSC | 5-FU topical | Veterans with history of NMSC | Rate of NMSC | 954 | Risk of SCC reduction was seen in the first year only; risk of BCC reduction in the first year was not significant [25]. |
| NCT00021294 | NMSC | Topical DFMO combined with triamcinolone | Patients with AK | Rate of NMSC | 102 | The low-dose topical drug interventions were effective in reducing skin biopsy nuclear abnormality [26]. |
| NCT00601640 | Other | DFMO combined with diclofenac | Individuals with skin sun damage | Nuclear marker | 156 | The addition of topical DFMO to topical diclofenac did not enhance its activity [27]. |
| NCT00204789 | Other | Oral DFMO | Organ transplant recipients | Safety; targets of DFMO | 52 | No significant effect for DFMO [28]; oral DFMO at 500 mg/m2/day was safe and tolerable and resulted in significant inhibition of phorbol ester-induced skin ODC activity [29]. |
| NCT01032343 | Skin immunity | Omega-3 polyunsaturated fatty acids (PUFA) | Healthy volunteers | Nickel contact hypersensitivity | 79 | Oral PUFAs abrogated photoimmunosuppression in human skin, providing additional support for their chemopreventive role [30]. |
| NCT01447355 | Other | Oral cholecalciferol (vitamin D) | Healthy subjects with insufficient serum levels of 25-hydroxyvitamine D | Changes in vitamin D receptor expression; skin differentiation biomarkers; safety and tolerability | 25 | High-dose cholecalciferol supplementation raised serum VD metabolite levels and CYP24 mRNA and caspase-14 levels in the skin [31]. |
| NCT00002811 | AK | Liposomal T4N5 lotion | Rate of AK | 30 | No results published | |
| NCT00089180 | NMSC | Liposomal T4N5 lotion | Renal transplant recipients with history of NMSC | Rate of NMSC | 100 | No results published |
| NCT03769285 | NMSC | Oral nicotinamide | Solid organ transplant recipients | Rate of NMSC | 120 | Ongoing |
| NCT04091022 | NMSC | Topical diclofenac and topical DFMO | With history of NMSC | Rate of NMSC | 138 | No results published |
| NCT02636569 | NMSC | Topical diclofenac | History of NMSC | Biomarkers in skin biopsies | 24 | No results published |
| NCT03210740 | NMSC | AM001 Cream (topical Potassium dobesilate) | Patients with AK | Clearance of AK | 30 | No results published |
| NCT02347813 | SCC | Oral pioglitazone | Patients with history of frequent occurrence of SCC | Rate of SCC | 12 | No results published |
| NCT05159752 NCT05370235 | Other | Afamelanotide | XP patients | Safety and efficacy on skin damage | 6 | Recruiting |
| PMID | Condition | Intervention | Subject | Endpoint | Size | Results |
|---|---|---|---|---|---|---|
| 26488693 | NMSC | Oral nicotinamide | With history of NMSC | New NMSC | 386 | Oral nicotinamide was safe and effective in reducing the rates of new NMSC and actinic keratoses in high-risk patients [32]. |
| 27061568 | NMSC | Oral nicotinamide | Organ transplant recipients with history of NMSCs | Rate of NMSC | 22 | Oral nicotinamide was associated with a statistically nonsignificant relative difference in the rate of NMSCs and statistically nonsignificant reduction in AK [33]. |
| 36856616 | NMSC | Oral nicotinamide | Organ transplant recipients with history of NMSCs | Rate of NMSC | 158 | Oral nicotinamide did not lead to lower numbers of NMSC or AK in immunosuppressed solid-organ transplant recipients [34]. |
| 30244097 | Skin immunity | Oral nicotinamide | Immunocompetent patients who had NMSCs | Immunological markers | 78 | The study found significant decrease in the number of macrophages in keratinocytes that arose in patients receiving nicotinamide compared to placebo [35]. |
| 20051370 | BCC | Oral celecoxib | PTCH1(+/−) patients with BCNS | Rate of BCC | 60 | Celecoxib decreased the development of new BCCs in all subjects, but it did not reach statistical significance [20]. |
| 21115882 | NMSC | Oral celecoxib | History of AK | Incidence of SCC, BCC, and AK | 240 | Celecoxib might be effective for prevention of SCCs and BCCs in individuals who had extensive actinic damage and were at high risk for development of NMSC [36]. |
| 35395069 | Melanoma | Oral aspirin | Elderly | Rate of melanoma | 19,114 | Aspirin was not associated with a reduced risk of invasive melanoma in older individuals [37]. |
| 21688346 | NMSC | NSAIDs | Veterans with higher risk of NMSC | Rate of SCC and BCC | 728 | This study did not identify a negative association between NSAIDs and keratinocyte carcinomas [38]. |
| 29570772 | BCC | Aspirin and/or folic acid orally | Diagnosed with colorectal adenomas | Rate of BCC | 1121 | Neither aspirin nor folic acid treatment had a statistically significant effect on risk of BCC. Subgroup analysis suggested that chemopreventive NSAIDs may be specific to those at high risk for BCC [39]. |
| 20000874 | NMSC | Topical piroxicam | With history of AK | Actinic Keratosis Erythema Scale Atrophy score | 31 | The use of piroxicam 1% gel for 90 days induced complete regression in 48% of evaluated actinic keratoses [40]. |
| 23348836 | SCC | 5-FU; angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | Veterans with history of NMSC | Rate of SCC | 1131 | Key risk factors for additional SCCs in patients with multiple prior NMSC was identified [41]. |
| 29505863 | NMSC | Topical 5-FU | Veterans with high risk of NMSC | Cost | 932 | There was a significant cost savings for patients treated with 5-FU [42]. |
| 33795573 | NMSC | Topical 5-FU | History of AK | Rate of NMSC | 932 | A single 2- to 4-week course of topical 5-FU to the face and ears decreased overall biopsy rates for 1 year. SCC biopsy yield was decreased in the first year after treatment. There was a nonsignificant trend toward increased BCC biopsy yield [43]. |
| 30896781 | BCC | Topical 5-FU | Veterans with high risk of NMSC | Rate of BCC | 932 | 5-FU might be effective for the prevention of superficial subtype of BCCs even though there was no effect on BCCs overall [44]. |
| 34988975 | SCC | Topical 5-FU | Organ transplant recipients | Rate of | 40 | Trials of topical AK treatments for SCC chemoprevention are feasible [45]. |
| 21463984 | NMSC | Topical potassium dobesilate | History of AK | Lesions of actinic keratosis | 30 | The use of potassium dobesilate 5% cream for 16 weeks induced complete regression in 70% of evaluated actinic keratoses [46]. |
| 35533029 | NMSC | Metformin and sulfonylureas | Diabetic patients with history of NMSC | Rate of NMSC | 932 | Diabetic patients at high risk for KC might benefit from the use of metformin versus sulfonylureas [47]. |
| 24441673 | BCC | Topical tazarotene (a retinoid) | Patients who have BCNS | Rate of BCC | 34 | The study provided no evidence for either chemopreventive or chemotherapeutic effect of tazarotene against BCCs in patients with BCNS [48]. |
| 24614012 | Melanoma | Oral lovastatin for 6 months | Subjects with at least two clinically atypical nevi | Biomarkers of melanoma | 80 | There were no effects. Further research into pathogenesis of melanoma and other chemopreventitive agent is needed [49]. |
| 29691233 | Melanoma | Sulforaphane with administration of oral broccoli sprout extract | Patients had at least 2 atypical nevi and a prior history of melanoma | Safety; plasma and skin drug levels; biomarkers | 17 | Oral BSE-SFN was well-tolerated at daily doses up to 200 µmol and achieved dose-dependent levels in plasma and skin. Efficacy studies may be performed in the future [50]. |
| 20103724 | Unspecified | Topical perillyl alcohol (POH) | Individuals with sun-damaged skin. | Skin histopathologic scores and nuclear chromatin pattern | 89 | Karyometric analyses could detect a modest effect of POH in sun-damaged skin. Improved delivery into the epidermis may be necessary [18]. |
| Drug Class | Mechanism of Action | Drug | Route of Administration | Dose/Strength and Duration |
|---|---|---|---|---|
| Chemotherapy | Antimetabolite | 5-Fluorouracil | Topical | 5%; 4 weeks [42] |
| 5%; 2–4 weeks [44] | ||||
| 5%; 4 weeks [43] | ||||
| Retinoids | PAR inhibition | Acitretin | Oral | 25 mg; 2 years [21] |
| Retinoids | PAR inhibition | Tretinoin | Topical | 0.1%; 1.5–5.5 years [24] |
| Retinoids | PAR inhibition | Tazarotene | Topical | 0.1%; 3 years [48] |
| NSAID | Anti-inflammation | Celecoxib | Oral | 200 mg; 2 years [20] |
| 200 mg; 9 months [36] | ||||
| NSAID | Anti-inflammation | Piroxicam | Topical | 1%; 12 weeks [40] |
| NSAID | Anti-inflammation | Diclofenac | Topical | 3%; 6 months [27] |
| NSAID | Anti-inflammation | Aspirin | Oral | 81 or 325 mg; 3 years [39] |
| 100 mg; 5–7 years [37] | ||||
| DFMO | Ornithine decarboxylase (ODC) inhibitor | Eflornithine | Topical | 10%; 6 months [27] |
| 10%; 6 months [26] | ||||
| Vitamin B3 | DNA repair/inhibiting immunosuppression | Nicotinamide | Oral | 500 mg; 12 months [32] |
| 500 mg; 6 months [33] | ||||
| 500 mg; 12 months [35] | ||||
| Vitamin D | Skin cell differentiation | Cholecalciferol | Oral | 50,000 IU; 8–9 weeks [31] |
| Bacteriophage T4 endonuclease 5 | DNA repair | T4N5 | Topical | 12-month; no results |
| Statin | Anti-inflammation | Lovastatin | Oral | 40–80 mg; 6 months [49] |
| Anti-diabetic medication | PPARγ agonist | Pioglitazone | Oral | 15–30 mg; 5½ months [51] |
| Anti-diabetic medication | Multiple | Metformin | Oral | 2.8 years [47] |
| Anti-diabetic medication | Unknown | Sulfonylurea | Oral | 2.8 years [47] |
| Limonene derivative | Unknown | Perillyl alcohol | Topical | 0.3% or 0.76%; 12 weeks [18] |
| Psoriasis medication | FGF inhibitor | Potassium dobesilate | Topical | 5%; 16 weeks [46] |
| Dietary supplement | Anti-inflammation | Omega-3 | Oral | 5 mg; 3 months [30] |
| Dietary supplement | Anti-inflammation | Sulforaphane | Oral | 50, 100, or 200 μmol; 28 days [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tow, R.; Hanoun, S.; Andresen, B.; Shahid, A.; Wang, J.; Kelly, K.M.; Meyskens, F.L., Jr.; Huang, Y. Recent Advances in Clinical Research for Skin Cancer Chemoprevention. Cancers 2023, 15, 3819. https://doi.org/10.3390/cancers15153819
Tow R, Hanoun S, Andresen B, Shahid A, Wang J, Kelly KM, Meyskens FL Jr., Huang Y. Recent Advances in Clinical Research for Skin Cancer Chemoprevention. Cancers. 2023; 15(15):3819. https://doi.org/10.3390/cancers15153819
Chicago/Turabian StyleTow, Ruby, Samuel Hanoun, Bradley Andresen, Ayaz Shahid, Jeffrey Wang, Kristen M. Kelly, Frank L. Meyskens, Jr., and Ying Huang. 2023. "Recent Advances in Clinical Research for Skin Cancer Chemoprevention" Cancers 15, no. 15: 3819. https://doi.org/10.3390/cancers15153819
APA StyleTow, R., Hanoun, S., Andresen, B., Shahid, A., Wang, J., Kelly, K. M., Meyskens, F. L., Jr., & Huang, Y. (2023). Recent Advances in Clinical Research for Skin Cancer Chemoprevention. Cancers, 15(15), 3819. https://doi.org/10.3390/cancers15153819








