The Redox-Active Manganese(III) Porphyrin, MnTnBuOE-2-PyP5+, Impairs the Migration and Invasion of Non-Small Cell Lung Cancer Cells, Either Alone or Combined with Cisplatin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Crystal Violet Staining Assay
2.4. MTS Reduction Assay
2.5. Cytotoxicity Assays of MnBuOE Combined with Cisplatin
2.6. Intracellular ROS Evaluation
2.7. Cell Migration Assays
2.7.1. Selection of MnBuOE and Cisplatin Concentrations for Migration/Invasion Assays
2.7.2. In Vitro Wound-Healing Assay
2.7.3. Chemotaxis Migration Assay
2.8. Chemoinvasion
2.9. Gene Expression
2.10. Statistical Analysis
3. Results
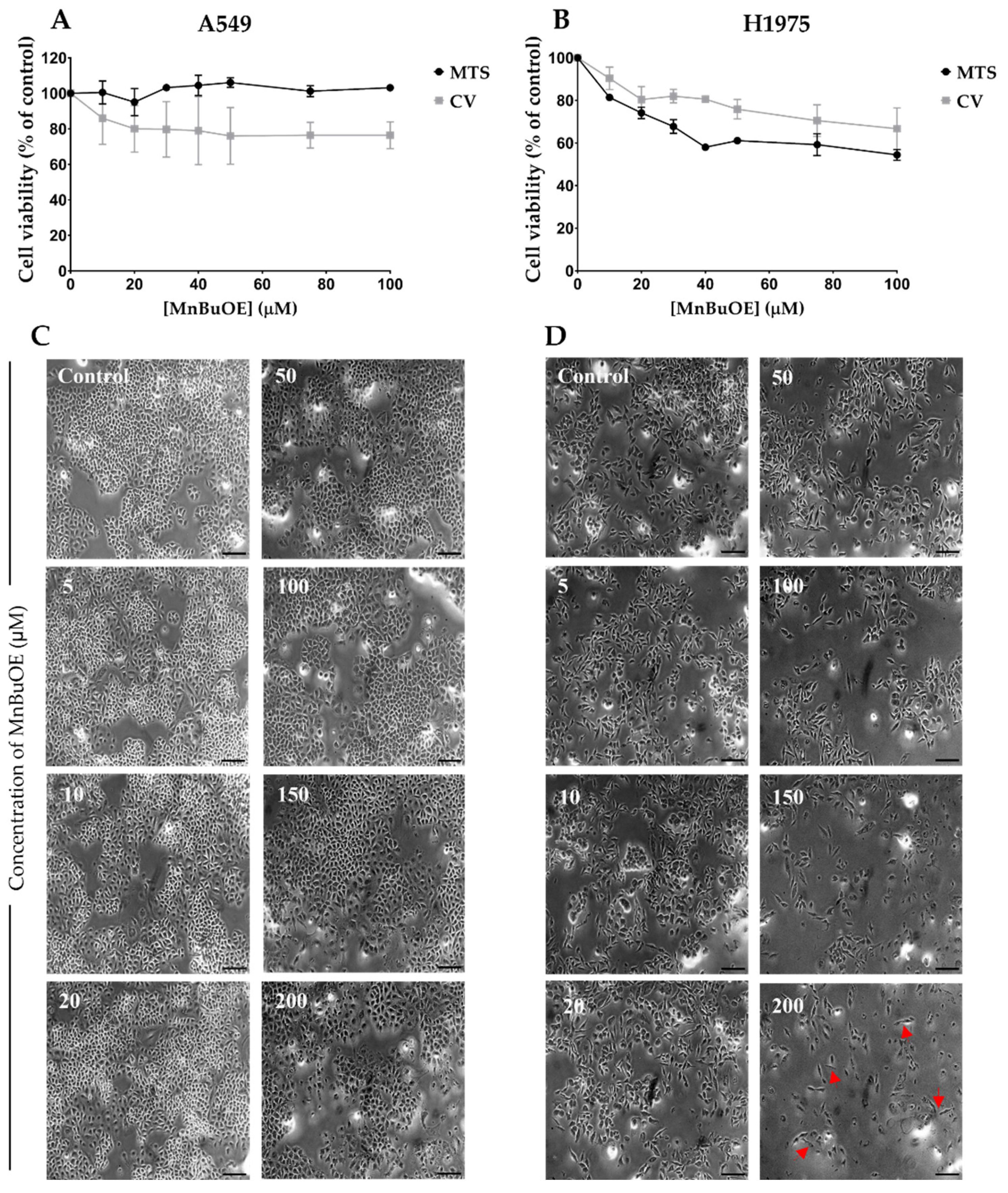
3.1. Effect of MnBuOE on Cell Viability in NSCLC Cells
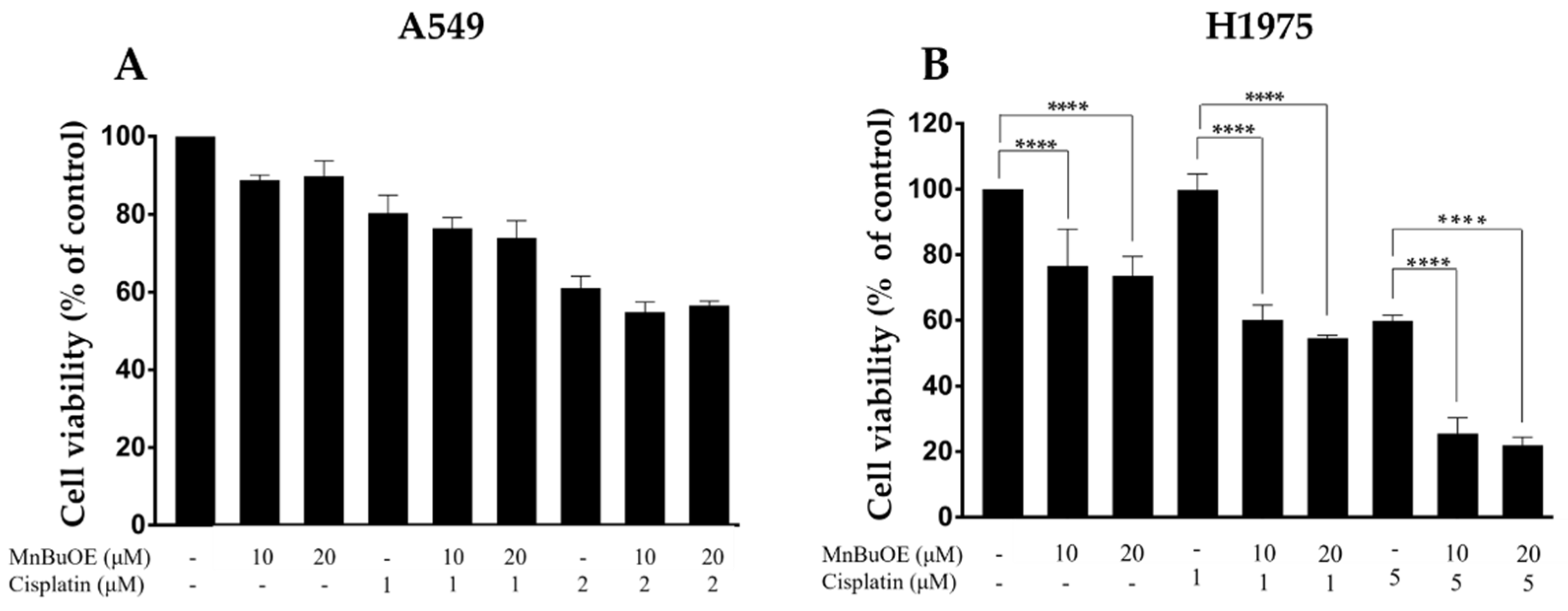
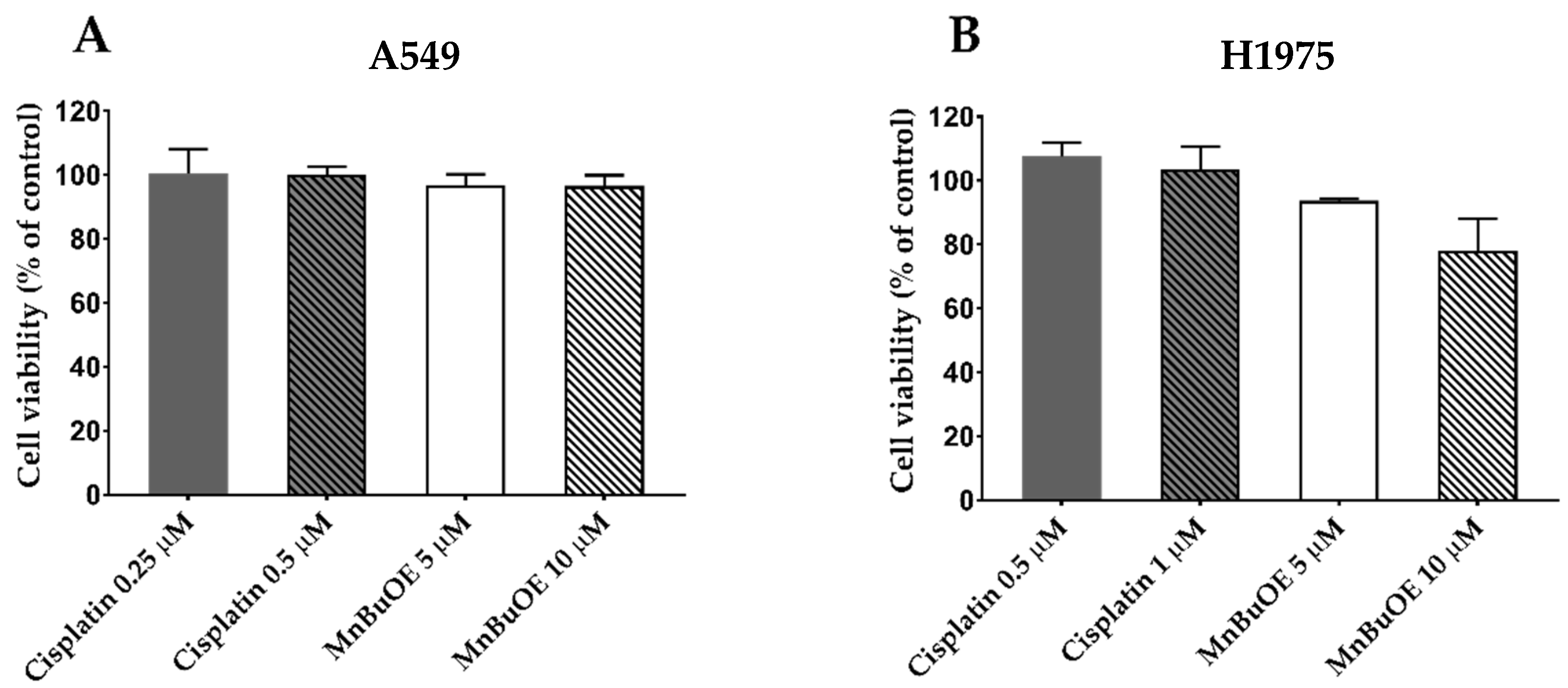
3.2. Impact of MnBuOE Combined with Cisplatin on the Viability of NSCLC Cells
3.3. MnBuOE per se and Combined with Cisplatin Increases ROS Levels
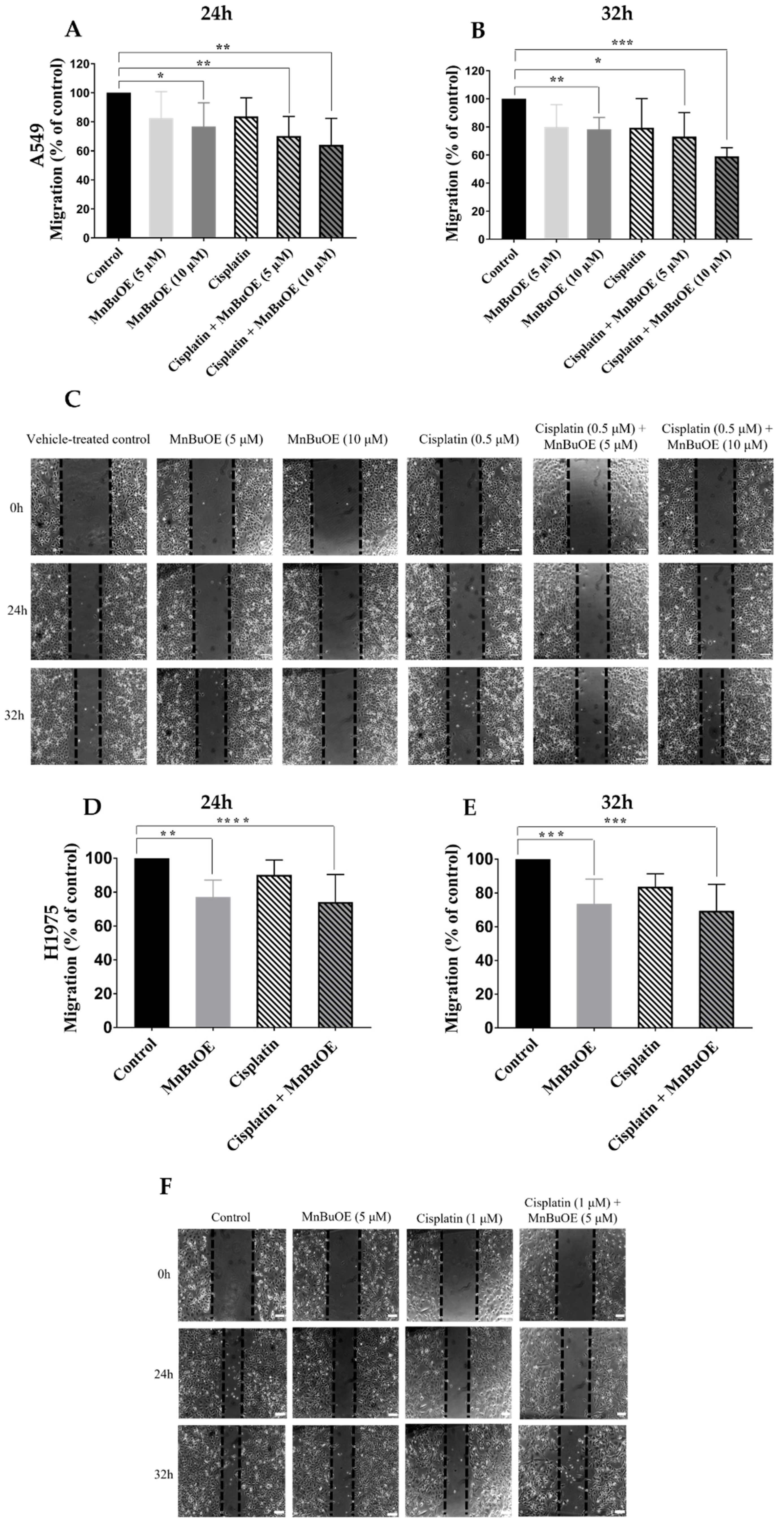
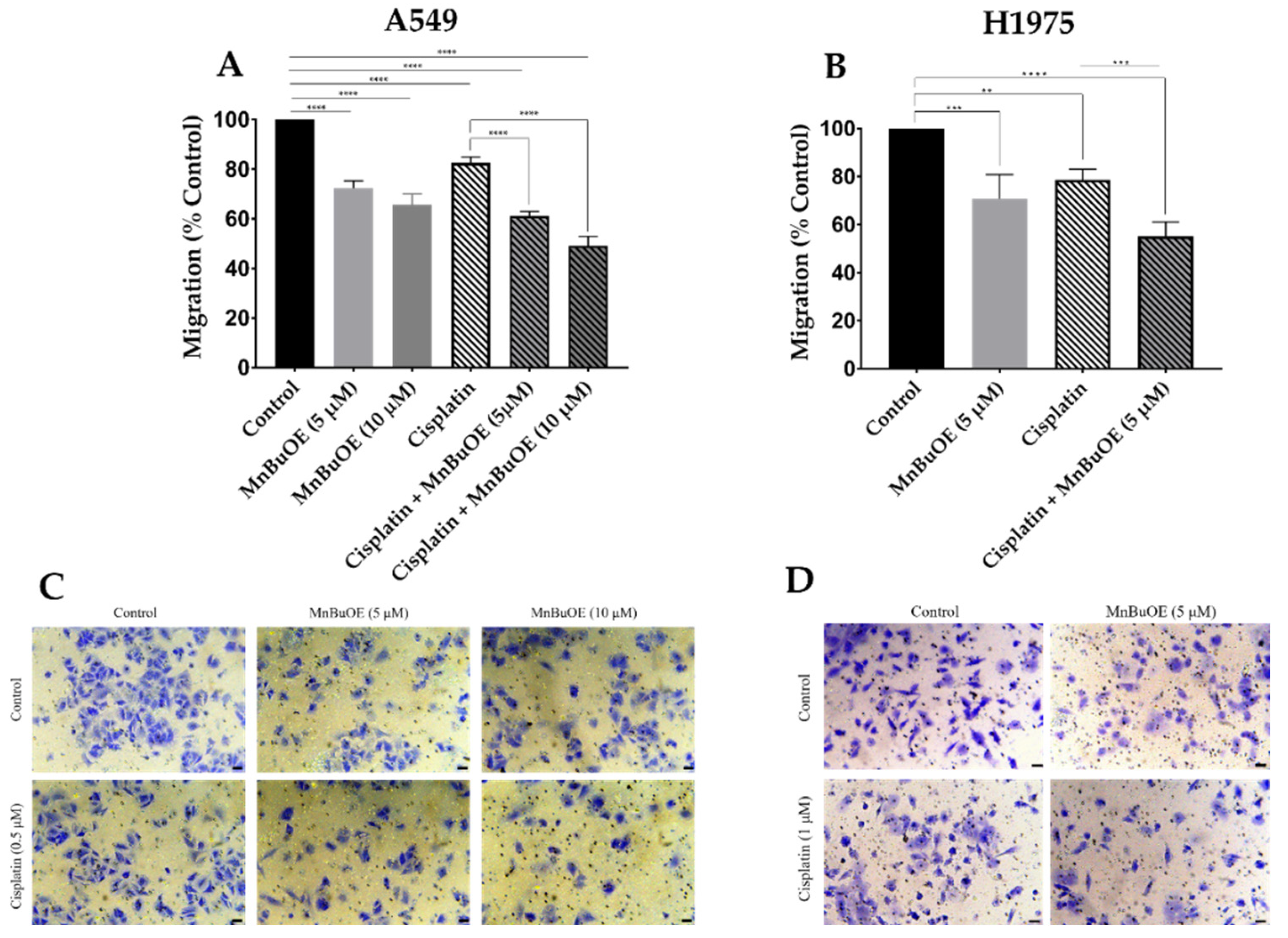
3.4. MnBuOE per se and Combined with Cisplatin Reduces Collective Migration of NSCLC Cells
3.5. MnBuOE Alone and in Combination with Cisplatin Reduces Chemotactic Cell Migration
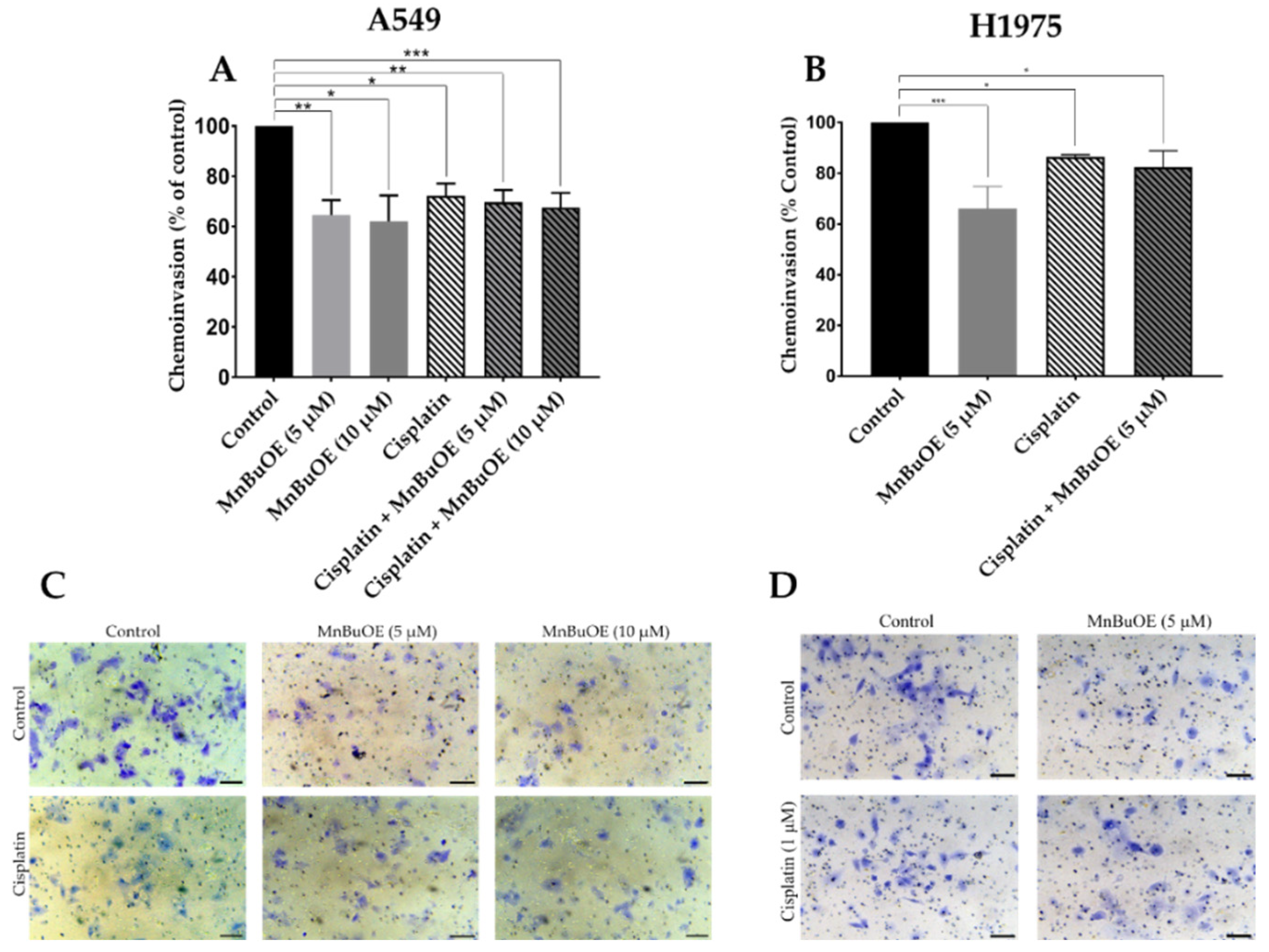
3.6. MnBuOE per se and Combined with Cisplatin Decreases Chemoinvasion of NSCLC Cells
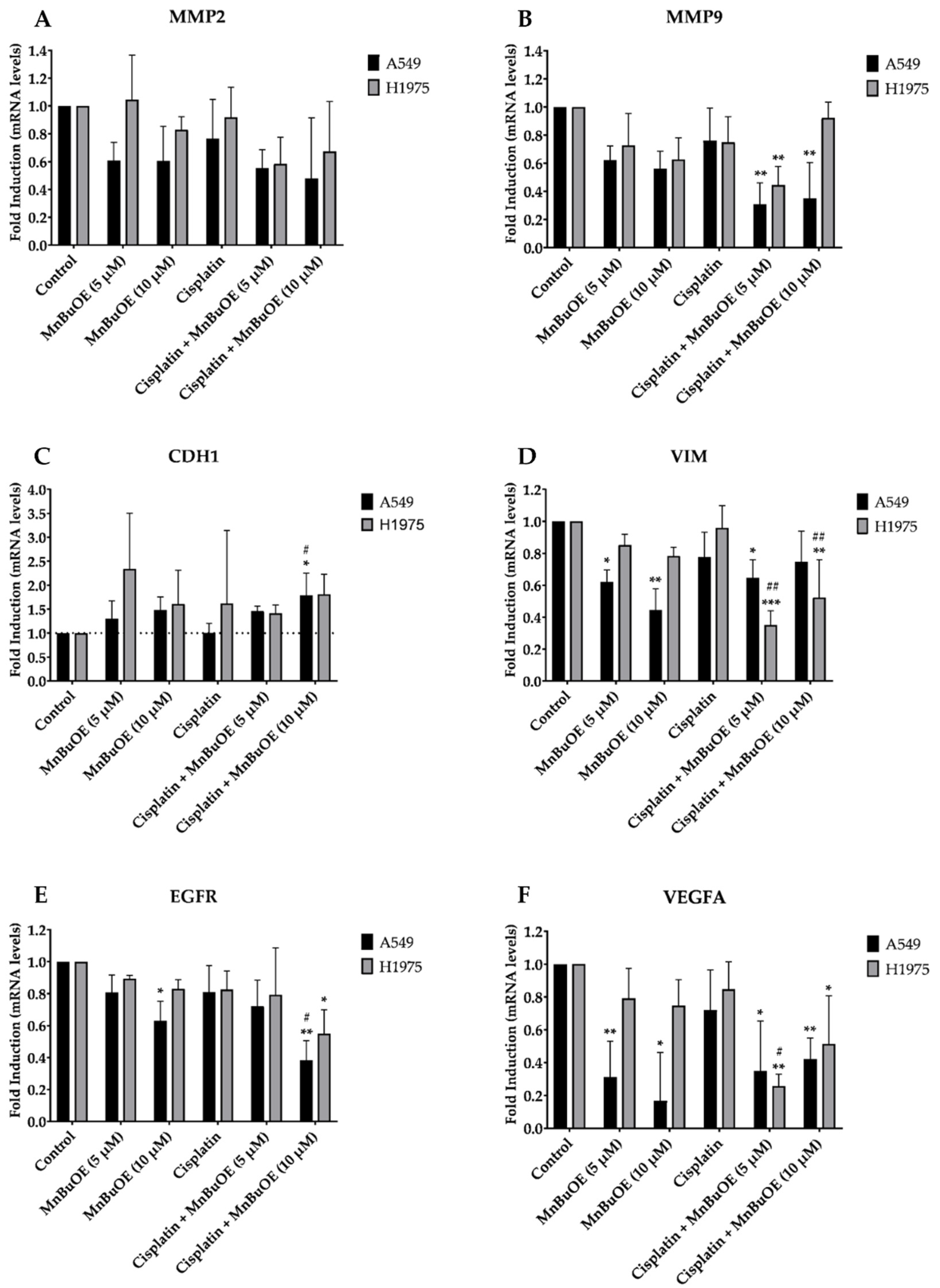
3.7. Effect of MnBuOE on the Expression of a Panel of Cell Migration-Related Genes in NSCLC Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Ebrahimi, S.O.; Reiisi, S.; Shareef, S. MiRNAs, Oxidative Stress, and Cancer: A Comprehensive and Updated Review. J. Cell. Physiol. 2020, 235, 8812–8825. [Google Scholar] [CrossRef]
- Avolio, R.; Matassa, D.S.; Criscuolo, D.; Landriscina, M.; Esposito, F. Modulation of Mitochondrial Metabolic Reprogramming and Oxidative Stress to Overcome Chemoresistance in Cancer. Biomolecules 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Gill, J.G.; Piskounova, E.; Morrison, S.J. Cancer, Oxidative Stress, and Metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Xu, X.; Li, M.; Hu, J.; Chen, Z.; Yu, J.; Dong, Y.; Sun, C.; Han, J. Somatic Mitochondrial DNA D-Loop Mutations in Meningioma Discovered: A Preliminary Data A Comprehensive Overview of Mitochondrial DNA 4977-Bp. J. Cancer Res. Ther. 2018, 14, 1525–1534. [Google Scholar] [CrossRef]
- Doskey, C.M.; Buranasudja, V.; Wagner, B.A.; Wilkes, J.G.; Du, J.; Cullen, J.J.; Buettner, G.R. Tumor Cells Have Decreased Ability to Metabolize H2O2: Implications for Pharmacological Ascorbate in Cancer Therapy. Redox Biol. 2016, 10, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Egea, J.; Fabregat, I.; Frapart, Y.M.; Ghezzi, P.; Görlach, A.; Kietzmann, T.; Kubaichuk, K.; Knaus, U.G.; Lopez, M.G.; Olaso-Gonzalez, G.; et al. European Contribution to the Study of ROS: A Summary of the Findings and Prospects for the Future from the COST Action BM1203 (EU-ROS). Redox Biol. 2017, 13, 94–162. [Google Scholar] [CrossRef] [Green Version]
- Wondrak, G.T. Redox-Directed Cancer Therapeutics: Molecular Mechanisms and Opportunities. Antioxid. Redox Signal. 2009, 11, 3013–3069. [Google Scholar] [CrossRef] [Green Version]
- Batinić-Haberle, I.; Rebouças, J.S.; Spasojević, I. Superoxide Dismutase Mimics: Chemistry, Pharmacology, and Therapeutic Potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [Green Version]
- Flórido, A.; Saraiva, N.; Cerqueira, S.; Almeida, N.; Parsons, M.; Batinic-Haberle, I.; Miranda, J.P.; Costa, J.G.; Carrara, G.; Castro, M.; et al. The Manganese(III) Porphyrin MnTnHex-2-PyP 5+ Modulates Intracellular ROS and Breast Cancer Cell Migration: Impact on Doxorubicin-Treated Cells. Redox Biol. 2019, 20, 367–378. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Huang, Z.; Duan, W.; Du, L.; Siamakpour-Reihani, S.; Cao, Z.; Sheng, H.; Spasojevic, I.; Alvarez Secord, A. H2O2-Driven Anticancer Activity of Mn Porphyrins and the Underlying Molecular Pathways. Oxid. Med. Cell. Longev. 2021, 2021, 6653790. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tome, M.E. Thiol Regulation by Mn Porphyrins, Commonly Known as SOD Mimics. Redox Biol. 2019, 25, 101139. [Google Scholar] [CrossRef] [PubMed]
- Tovmasyan, A.; Bueno-Janice, J.C.; Jaramillo, M.C.; Sampaio, R.S.; Reboucas, J.S.; Kyui, N.; Benov, L.; Deng, B.; Huang, T.T.; Tome, M.E.; et al. Radiation-Mediated Tumor Growth Inhibition Is Significantly Enhanced with Redox-Active Compounds That Cycle with Ascorbate. Antioxid. Redox Signal. 2018, 29, 1196–1214. [Google Scholar] [CrossRef]
- Zalewska-Ziob, M.; Adamek, B.; Kasperczyk, J.; Romuk, E.; Hudziec, E.; Chwalińska, E.; Dobija-Kubica, K.; Rogoziński, P.; Bruliński, K. Activity of Antioxidant Enzymes in the Tumor and Adjacent Noncancerous Tissues of Non-Small-Cell Lung Cancer. Oxid. Med. Cell. Longev. 2019, 2019, 2901840. [Google Scholar] [CrossRef] [Green Version]
- Mapuskar, K.A.; Anderson, C.M.; Spitz, D.R.; Batinic-Haberle, I.; Allen, B.G.; Oberley-Deegan, R.E. Utilizing Superoxide Dismutase Mimetics to Enhance Radiation Therapy Response While Protecting Normal Tissues. Semin. Radiat. Oncol. 2019, 29, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, R. Potential Therapeutic Applications of MnSODs and SOD-Mimetics. Chem. A Eur. J. 2018, 24, 5032–5041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.G.; Saraiva, N.; Batinic-Haberle, I.; Castro, M.; Oliveira, N.G.; Fernandes, A.S. The SOD Mimic MnTnHex-2-Pyp5+ Reduces the Viability and Migration of 786-O Human Renal Cancer Cells. Antioxidants 2019, 8, 490. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.B.; Manguinhas, R.; Costa, J.G.; Saraiva, N.; Gil, N.; Rosell, R.; Camões, S.P.; Batinic-Haberle, I.; Spasojevic, I.; Castro, M.; et al. MnTnHex-2-PyP5+ Displays Anticancer Properties and Enhances Cisplatin Effects in Non-Small Cell Lung Cancer Cells. Antioxidants 2022, 11, 2198. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Roberts, E.R.H.; Vujaskovic, Z.; Leong, K.W.; Spasojevic, I. SOD Therapeutics: Latest Insights into Their Structure-Activity Relationships and Impact on the Cellular Redox-Based Signaling Pathways. Antioxid. Redox Signal. 2014, 20, 2372–2415. [Google Scholar] [CrossRef] [Green Version]
- Weitzel, D.H.; Tovmasyan, A.; Ashcraft, K.A.; Rajic, Z.; Weitner, T.; Liu, C.; Li, W.; Buckley, A.F.; Prasad, M.R.; Young, K.H.; et al. Radioprotection of the Brain White Matter by Mn(III) N-Butoxyethylpyridylporphyrin-Based Superoxide Dismutase Mimic MnTnBuOE-2-PyP5+. Mol. Cancer Ther. 2015, 14, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Azzarà, A.; Chiaramonte, A.; Filomeni, E.; Pinto, B.; Mazzoni, S.; Piaggi, S.; Guzzardi, M.; Bruschi, F.; Iozzo, P.; Scarpato, R. Increased Level of DNA Damage in SomeOrgans of Obese Zucker Rats by C-H2AX Analysis Alessia. Environ. Mol. Mutagen. 2010, 405, 391–405. [Google Scholar] [CrossRef]
- McElroy, T.; Brown, T.; Kiffer, F.; Wang, J.; Byrum, S.D.; Oberley-Deegan, R.E.; Allen, A.R. Assessing the Effects of Redox Modifier MnTnBuOE-2-PyP5+ on Cognition and Hippocampal Physiology Following Doxorubicin, Cyclophosphamide, and Paclitaxel Treatment. Int. J. Mol. Sci. 2020, 21, 1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leu, D.; Spasojevic, I.; Nguyen, H.; Deng, B.; Tovmasyan, A.; Weitner, T.; Sampaio, R.S.; Batinic-Haberle, I.; Huang, T.T. CNS Bioavailability and Radiation Protection of Normal Hippocampal Neurogenesis by a Lipophilic Mn Porphyrin-Based Superoxide Dismutase Mimic, MnTnBuOE-2-PyP5+. Redox Biol. 2017, 12, 864–871. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Boss, M.K.; Tovmasyan, A.; Roy Choudhury, K.; Fontanella, A.N.; Young, K.H.; Palmer, G.M.; Birer, S.R.; Landon, C.D.; Park, W.; et al. Novel Manganese-Porphyrin Superoxide Dismutase-Mimetic Widens the Therapeutic Margin in a Preclinical Head and Neck Cancer Model. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 892–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmacek, E.A.; Chatterjee, A.; Tong, Q.; Lin, C.; Oberley-Deegan, R.E. MnTnBuOE-2-PyP Protects Normal Colorectal Fibroblasts from Radiation Damage and Simultaneously Enhances Radio/Chemotherapeutic Killing of Colorectal Cancer Cells. Oncotarget 2016, 7, 34532–34545. [Google Scholar] [CrossRef] [Green Version]
- Chaiswing, L.; Yarana, C.; St. Clair, W.; Tovmasyan, A.; Batinic-Haberle, I.; Spasojevic, I.; St. Clair, D. A Redox-Active Mn Porphyrin, MnTnBuOE-2-PyP5+, Synergizes with Carboplatin in Treatment of Chemoresistant Ovarian Cell Line. Oxid. Med. Cell. Longev. 2022, 2022, 9664636. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA. Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomarker. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Niu, F.Y.; Zhou, Q.; Yang, J.J.; Zhong, W.Z.; Chen, Z.H.; Deng, W.; He, Y.Y.; Chen, H.J.; Zeng, Z.; Ke, E.E.; et al. Distribution and Prognosis of Uncommon Metastases from Non-Small Cell Lung Cancer. BMC Cancer 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Song, J.; Feng, P.; Wang, Y.; Long, W.; Liu, M.; Li, L. Elevated Serum Apolipoprotein E Is Associated with Metastasis and Poor Prognosis of Non-Small Cell Lung Cancer. Tumor Biol. 2016, 37, 10715–10721. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.R.; Silva, M.M.; Quinet, A.; Cabral-Neto, J.B.; Menck, C.F.M. DNA Repair Pathways and Cisplatin Resistance: An Intimate Relationship. Clinics 2018, 73, e478s. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Rossi, A.; Di Maio, M. Platinum-Based Chemotherapy in Advanced Non-Small-Cell Lung Cancer: Optimal Number of Treatment Cycles. Expert Rev. Anticancer Ther. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Griesinger, F.; Korol, E.E.; Kayaniyil, S.; Varol, N.; Ebner, T.; Goring, S.M. Efficacy and Safety of First-Line Carboplatin-versus Cisplatin-Based Chemotherapy for Non-Small Cell Lung Cancer: A Meta-Analysis. Lung Cancer 2019, 135, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Manguinhas, R.; Fernandes, A.S.; Costa, J.G.; Saraiva, N.; Camões, S.P.; Gil, N.; Rosell, R.; Castro, M.; Miranda, J.P.; Oliveira, N.G. Impact of the Ape1 Redox Function Inhibitor E3330 in Non-Small Cell Lung Cancer Cells Exposed to Cisplatin: Increased Cytotoxicity and Impairment of Cell Migration and Invasion. Antioxidants 2020, 9, 550. [Google Scholar] [CrossRef]
- Guerreiro, P.S.; Corvacho, E.; Costa, J.G.; Saraiva, N.; Fernandes, A.S.; Castro, M.; Miranda, J.P.; Oliveira, N.G. The APE1 Redox Inhibitor E3330 Reduces Collective Cell Migration of Human Breast Cancer Cells and Decreases Chemoinvasion and Colony Formation When Combined with Docetaxel. Chem. Biol. Drug Des. 2017, 90, 561–571. [Google Scholar] [CrossRef]
- Guerreiro, Í.; Vidovic, B.; Costa, J.G.; Martins, M.; Ferreira, S.; Oliveira, N.G.; Saraiva, N.; Fernandes, A.S. The Dietary Isothiocyanate Erucin Reduces Kidney Cell Motility by Disturbing Tubulin Polymerization. Mol. Nutr. Food Res. 2023, 67, 2200581. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Serejo, J.; Gaspar, J.; Cabral, F.; Bettencourt, A.F.; Rueff, J.; Castro, M.; Costa, J.; Oliveira, N.G. Oxidative Injury in V79 Chinese Hamster Cells: Protective Role of the Superoxide Dismutase Mimetic MnTM-4-PyP. Cell Biol. Toxicol. 2010, 26, 91–101. [Google Scholar] [CrossRef]
- Cipriano, M.; Pinheiro, P.F.; Sequeira, C.O.; Rodrigues, J.S.; Oliveira, N.G.; Antunes, A.M.M.; Castro, M.; Marques, M.M.; Pereira, S.A.; Miranda, J.P. Nevirapine Biotransformation Insights: An Integrated in Vitro Approach Unveils the Biocompetence and Glutathiolomic Profile of a Human Hepatocyte-like Cell 3d Model. Int. J. Mol. Sci. 2020, 21, 3998. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; Faria-Pereira, A.; Camões, S.P.; Serras, A.S.; Morais, V.A.; Ruas, J.L.; Miranda, J.P. Improving Human Mesenchymal Stem Cell-Derived Hepatic Cell Energy Metabolism by Manipulating Glucose Homeostasis and Glucocorticoid Signaling. Front. Endocrinol. 2023, 13, 1043543. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Chen, J.; Xue, M.; Pan, X. ELTD1 Promotes Invasion and Metastasis by Activating MMP2 in Colorectal Cancer. Int. J. Biol. Sci. 2021, 17, 3048–3058. [Google Scholar] [CrossRef]
- Jin, Z.; Li, H.; Hong, X.; Ying, G.; Lu, X.; Zhuang, L.; Wu, S. TRIM14 Promotes Colorectal Cancer Cell Migration and Invasion through the SPHK1/STAT3 Pathway. Cancer Cell Int. 2018, 18, 202. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Liu, Z.; Tang, X. Overexpression of SLFN5 Induced the Epithelial-Mesenchymal Transition in Human Lung Cancer Cell Line A549 through β-Catenin/Snail/E-Cadherin Pathway. Eur. J. Pharmacol. 2019, 862, 172630. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, X.; Wang, M.; Zhao, X.; Xue, L. X-Linked Inhibitor of Apoptosis Protein Accelerates Migration by Inducing Epithelial-Mesenchymal Transition through TGF-β Signaling Pathway in Esophageal Cancer Cells. Cell Biosci. 2019, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Han, X.; Zheng, S.; Liu, Q.; Tuerxun, A.; Zhang, Q.; Yang, L.; Lu, X. CALM1 Promotes Progression and Dampens Chemosensitivity to EGFR Inhibitor in Esophageal Squamous Cell Carcinoma. Cancer Cell Int. 2021, 21, 121. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Y.; Cheng, F.; Hu, Y.; Yang, S.; Rao, J.; Wang, X. Mir-1301 Inhibits Hepatocellular Carcinoma Cell Migration, Invasion, and Angiogenesis by Decreasing Wnt/β-Catenin Signaling through Targeting Bcl9. Cell Death Dis. 2017, 8, e2999. [Google Scholar] [CrossRef] [Green Version]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In Vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Reiswich, V.; Schmidt, C.E.; Lennartz, M.; Höflmayer, D.; Hube-Magg, C.; Weidemann, S.; Fraune, C.; Büscheck, F.; Möller, K.; Bernreuther, C.; et al. GATA3 Expression in Human Tumors: A Tissue Microarray Study on 16,557 Tumors. Pathobiology 2023, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Umelo, I.A.; De Wever, O.; Kronenberger, P.; Noor, A.; Teugels, E.; Chen, G.; Bracke, M.; Grève, J. De Combined Inhibition of Rho-Associated Protein Kinase and EGFR Suppresses the Invasive Phenotype in EGFR-Dependent Lung Cancer Cells. Lung Cancer 2015, 90, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.X.; Wang, J.; Song, B.; Wei, H.; Lv, W.P.; Tian, L.M.; Li, M.; Lv, S. Establishment and Biological Characteristics of Acquired Gefitinib Resistance in Cell Line NCI-H1975/Gefinitib-Resistant with Epidermal Growth Factor Receptor T790M Mutation. Mol. Med. Rep. 2015, 11, 2767–2774. [Google Scholar] [CrossRef] [Green Version]
- Hanke, N.T.; Imler, E.; Marron, M.T.; Seligmann, B.E.; Garland, L.L.; Baker, A.F. Characterization of Carfilzomib-Resistant Non-Small Cell Lung Cancer Cell Lines. J. Cancer Res. Clin. Oncol. 2018, 144, 1317–1327. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, L.; Liu, Y. Propofol Improves Sensitivity of Lung Cancer Cells to Cisplatin and Its Mechanism. Med. Sci. Monit. 2020, 26, e919786-1. [Google Scholar] [CrossRef] [Green Version]
- Yulyana, Y.; Tovmasyan, A.; Ho, I.A.W.; Sia, K.C.; Newman, J.P.; Ng, W.H.; Guo, C.M.; Hui, K.M.; Batinic-Haberle, I.; Lam, P.Y.P. Redox-Active Mn Porphyrin-Based Potent SOD Mimic, MnTnBuOE-2-PyP5+, Enhances Carbenoxolone-Mediated TRAIL-Induced Apoptosis in Glioblastoma Multiforme. Stem Cell Rev. Rep. 2016, 12, 140–155. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.S.; Gaspar, J.; Cabral, M.F.; Rueff, J.; Castro, M.; Batinic-Haberle, I.; Costa, J.; Oliveira, N.G. Protective Role of Ortho-Substituted Mn(III) N-Alkylpyridylporphyrins against the Oxidative Injury Induced by Tert-Butylhydroperoxide. Free Radic. Res. 2010, 44, 430–440. [Google Scholar] [CrossRef]
- Peshavariya, H.M.; Dusting, G.J.; Selemidis, S. Analysis of Dihydroethidium Fluorescence for the Detection of Intracellular and Extracellular Superoxide Produced by NADPH Oxidase. Free Radic. Res. 2007, 41, 699–712. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, P.; Yan, R.; Wang, Q.; Fang, E.; Wu, H.; Li, S.; Tan, H.; Zhou, X.; Ma, X.; et al. MnTE-2-PyP Attenuates TGF-β-Induced Epithelial-Mesenchymal Transition of Colorectal Cancer Cells by Inhibiting the Smad2/3 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn Porphyrin-Based Redox-Active Therapeutics. In Oxidative Stress in Applied Basic Research and Clinical Practice; Springer International Publishing: Cham, Switzerland, 2016; pp. 165–212. ISBN 9783319307053. [Google Scholar] [CrossRef]


| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Reference |
|---|---|---|---|
| β-ACTIN | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT | [43] |
| MMP2 | TGACTTTCTTGGATCGGGTCG | AAGCACCACATCAGATGACTG | [44] |
| MMP9 | TGTACCGCTATGGTTACACTCG | GGCAGGGACAGTTGCTTCT | [45] |
| CDH1 | CTGAGAACGAGGCTAACG | GTCCACCATCATCATTCAATAT | [46] |
| VIM | AGTCCACTGAGTACCGGAGAC | CATTTCACGCATCTGGCGTTC | [47] |
| EGFR | AGGCACGAGTAACAAGCTCAC | ATGAGGACATAACCAGCCACC | [48] |
| VEGFA | AGGGCAGAATCATCACGAAGT | AGGGTCTCGATTGGATGGCA | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, R.B.; Manguinhas, R.; Costa, J.G.; Saraiva, N.; Gil, N.; Rosell, R.; Camões, S.P.; Batinic-Haberle, I.; Spasojevic, I.; Castro, M.; et al. The Redox-Active Manganese(III) Porphyrin, MnTnBuOE-2-PyP5+, Impairs the Migration and Invasion of Non-Small Cell Lung Cancer Cells, Either Alone or Combined with Cisplatin. Cancers 2023, 15, 3814. https://doi.org/10.3390/cancers15153814
Soares RB, Manguinhas R, Costa JG, Saraiva N, Gil N, Rosell R, Camões SP, Batinic-Haberle I, Spasojevic I, Castro M, et al. The Redox-Active Manganese(III) Porphyrin, MnTnBuOE-2-PyP5+, Impairs the Migration and Invasion of Non-Small Cell Lung Cancer Cells, Either Alone or Combined with Cisplatin. Cancers. 2023; 15(15):3814. https://doi.org/10.3390/cancers15153814
Chicago/Turabian StyleSoares, Rita B., Rita Manguinhas, João G. Costa, Nuno Saraiva, Nuno Gil, Rafael Rosell, Sérgio P. Camões, Ines Batinic-Haberle, Ivan Spasojevic, Matilde Castro, and et al. 2023. "The Redox-Active Manganese(III) Porphyrin, MnTnBuOE-2-PyP5+, Impairs the Migration and Invasion of Non-Small Cell Lung Cancer Cells, Either Alone or Combined with Cisplatin" Cancers 15, no. 15: 3814. https://doi.org/10.3390/cancers15153814
APA StyleSoares, R. B., Manguinhas, R., Costa, J. G., Saraiva, N., Gil, N., Rosell, R., Camões, S. P., Batinic-Haberle, I., Spasojevic, I., Castro, M., Miranda, J. P., Guedes de Pinho, P., Fernandes, A. S., & Oliveira, N. G. (2023). The Redox-Active Manganese(III) Porphyrin, MnTnBuOE-2-PyP5+, Impairs the Migration and Invasion of Non-Small Cell Lung Cancer Cells, Either Alone or Combined with Cisplatin. Cancers, 15(15), 3814. https://doi.org/10.3390/cancers15153814













