Therapeutic and Adverse Effect of Anti-PD1 Immunotherapy in Melanoma: A Retrospective, Single-Institute Study of 222 Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
2.3. Ethical Permission
3. Results
3.1. General Characteristic of the Studied Melanoma Patient Group Treated by Checkpoint Inhibitors
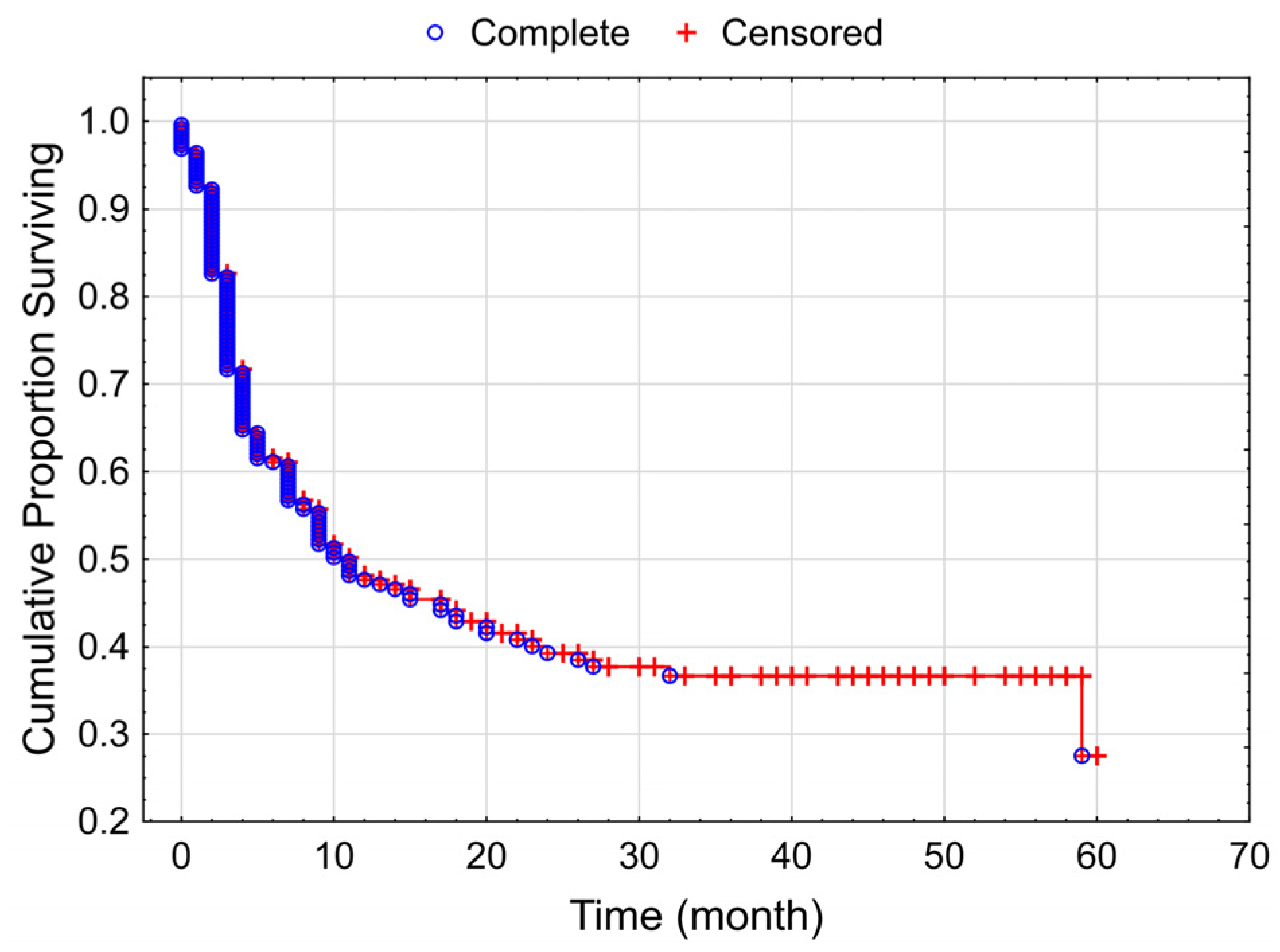
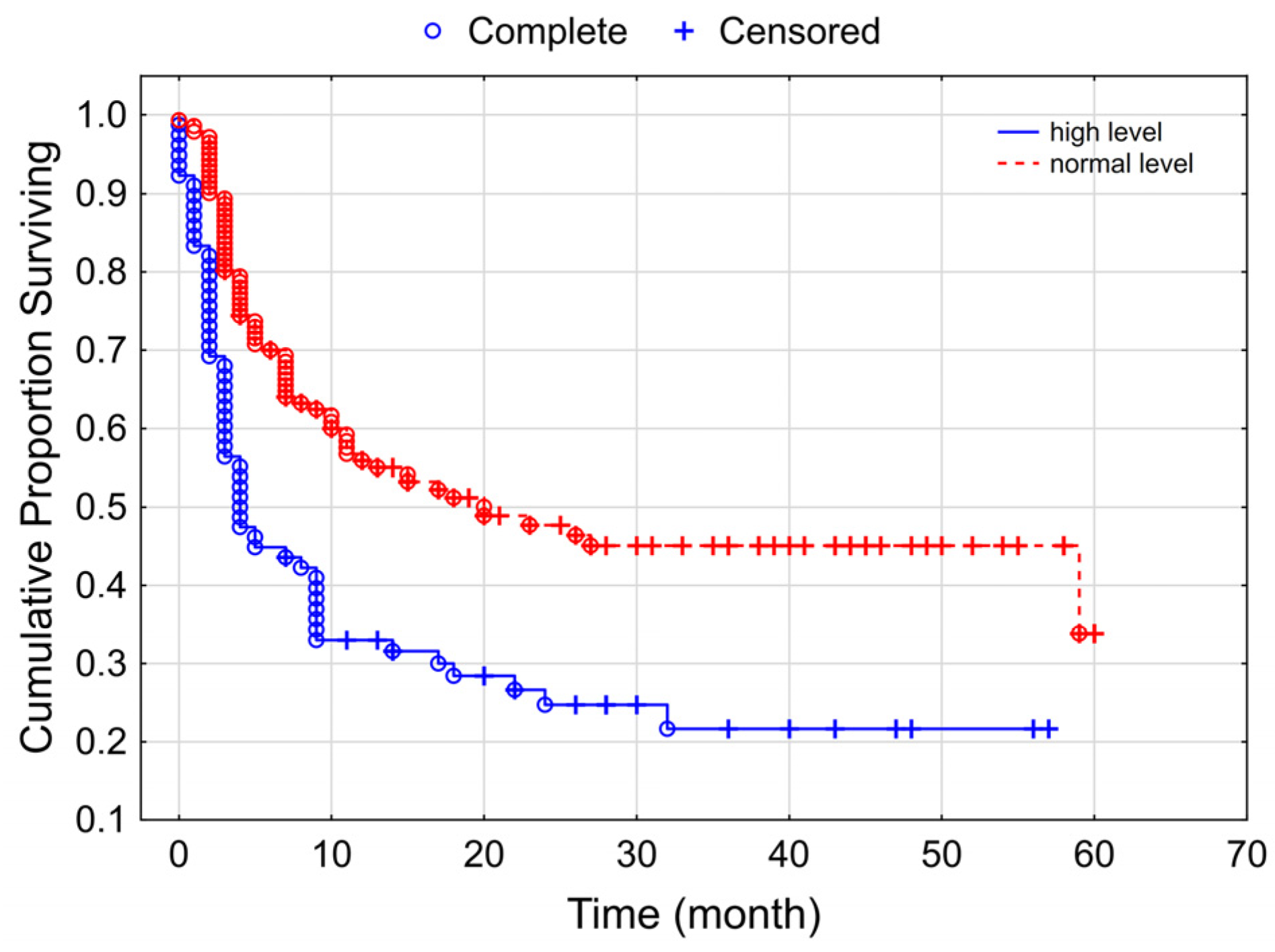
3.2. Progression-Free Survival of the Studied Melanoma Patient Group Receiving Checkpoint Inhibitor Therapy
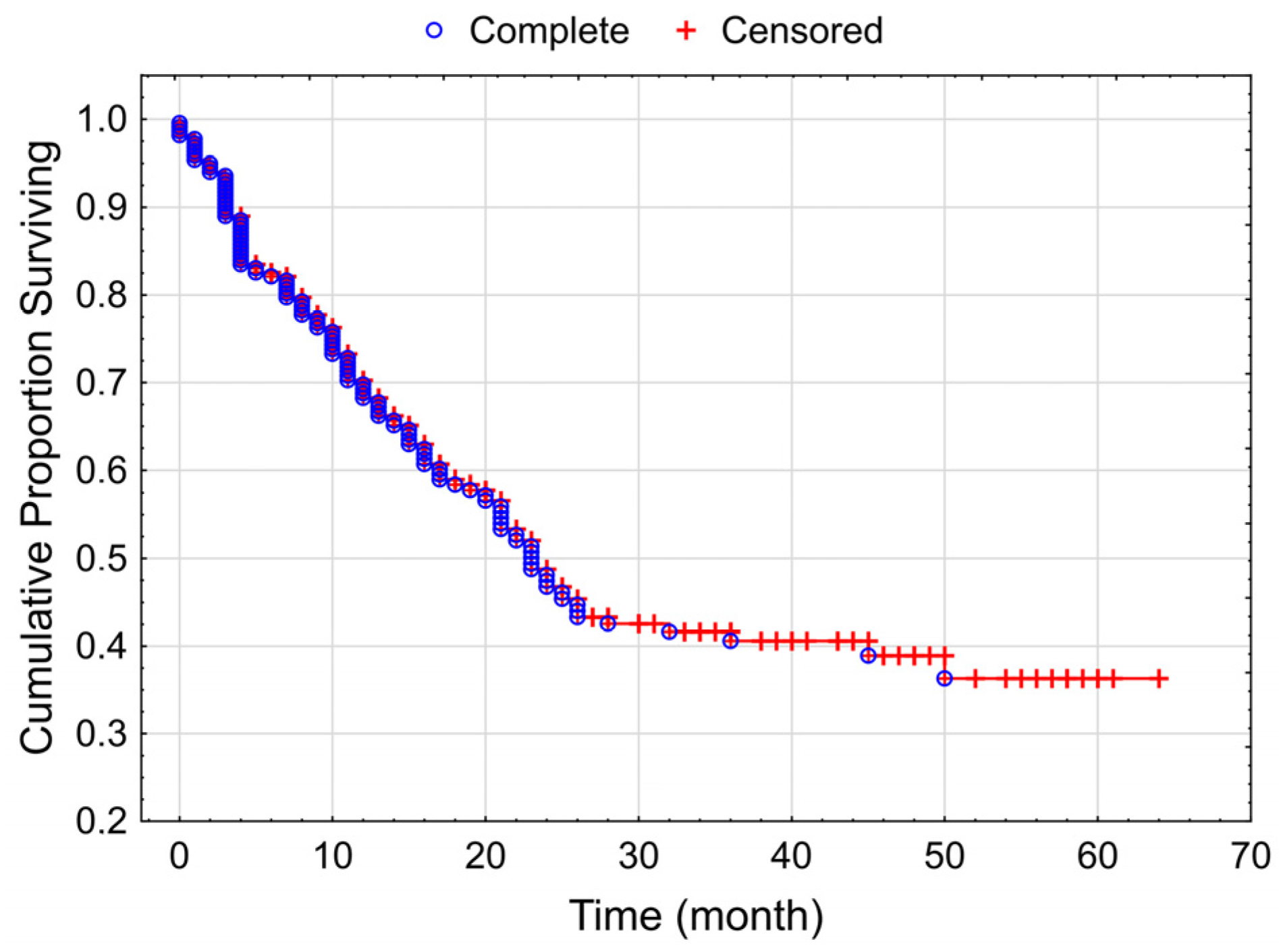
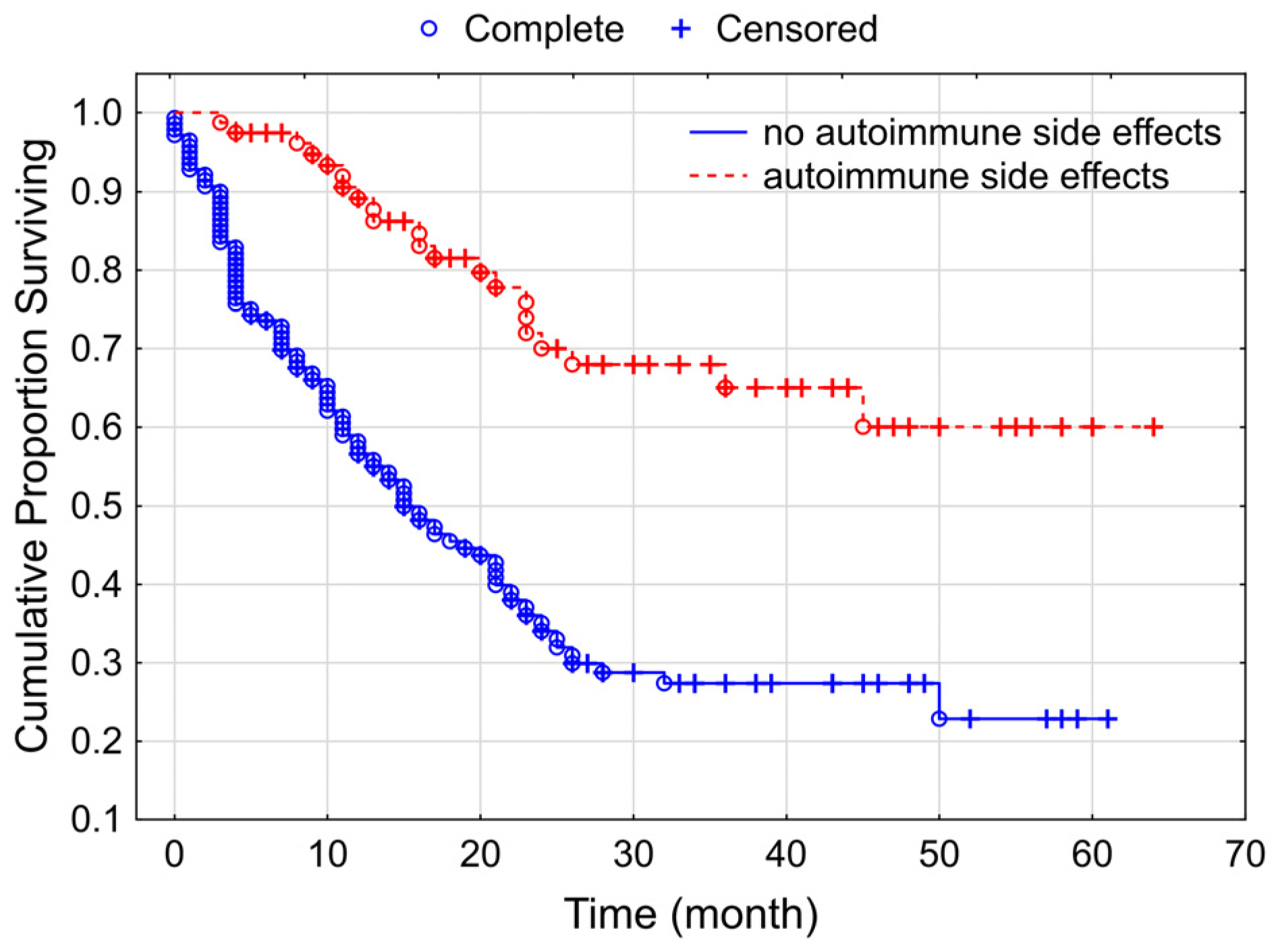
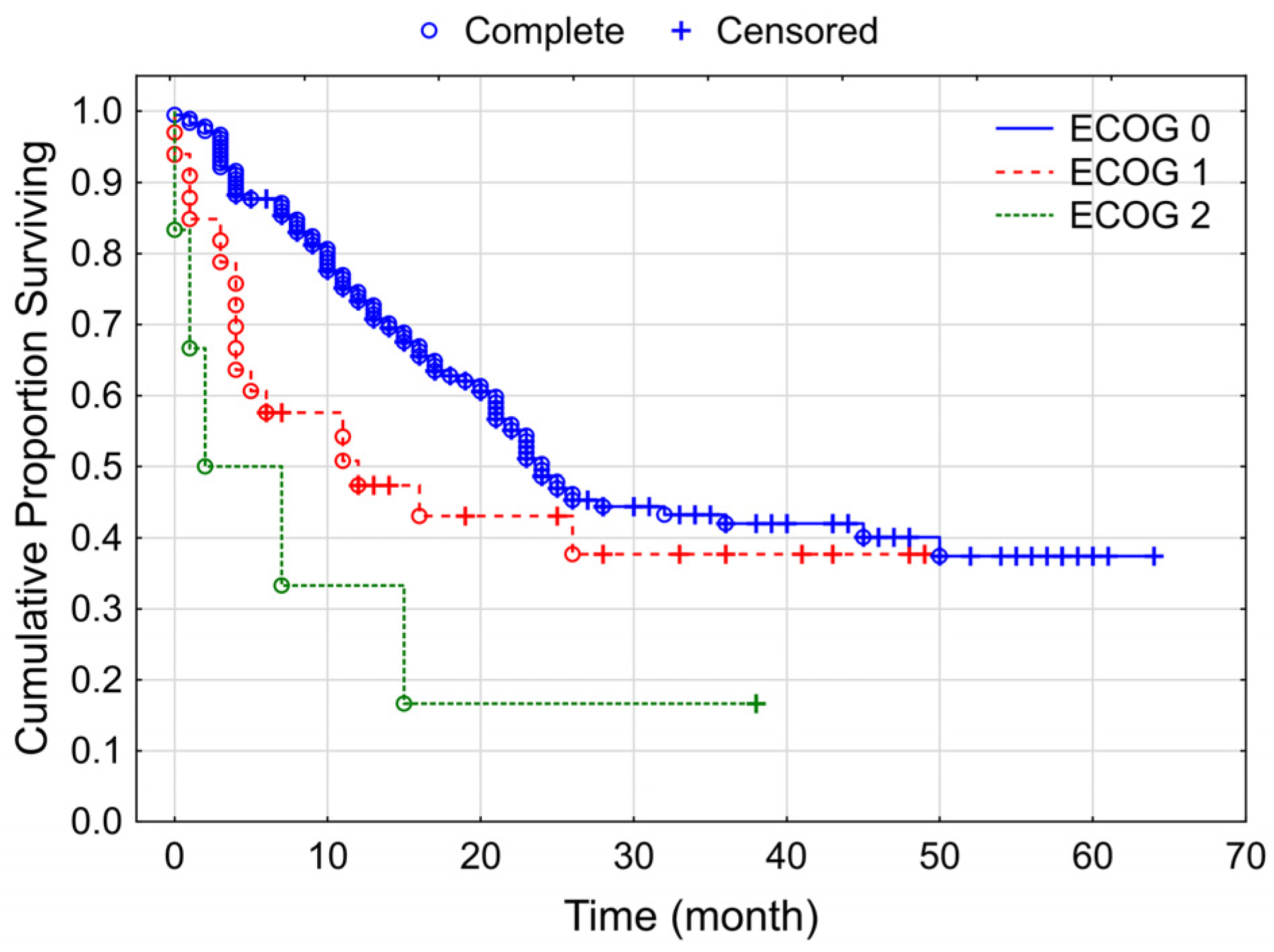
3.3. Overall Survival of the Studied Melanoma Patient Group Treated by Checkpoint Inhibitors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Erdmann, F.; Lortet-Tieulent, J.; Schuz, J.; Zeeb, H.; Greinert, R.; Breitbart, E.W.; Bray, F. International trends in the incidence of malignant melanoma 1953–2008—Are recent generations at higher or lower risk? Int. J. Cancer 2013, 132, 385–400. [Google Scholar] [CrossRef] [PubMed]
- De Vries, E.; Bray, F.I.; Coebergh, J.W.; Parkin, D.M. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: Rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int. J. Cancer 2003, 107, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Rutkowski, P.; Hassel, J.C.; McNeil, C.M.; Kalinka, E.A.; et al. Five-Year Outcomes With Nivolumab in Patients With Wild-Type BRAF Advanced Melanoma. J. Clin. Oncol. 2020, 38, 3937–3946. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Parrag, P.; Weber, A.; Liszkay, G.; Nagy, P.; Kasler, M.; Polgar, C.; Kenessey, I. A melanóma hazai morbiditási és mortalitási helyzete a XXI. század első két évtizedében. Magy. Onkol. 2022, 66, 94–99. [Google Scholar]
- Balatoni, T.; Ladanyi, A.; Frohlich, G.; Czirbesz, K.; Kovacs, P.; Panczel, G.; Bence, E.; Plotar, V.; Liszkay, G. Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Pathol. Oncol. Res. 2020, 26, 317–325. [Google Scholar] [CrossRef]
- Suo, A.; Chan, Y.; Beaulieu, C.; Kong, S.; Cheung, W.Y.; Monzon, J.G.; Smylie, M.; Walker, J.; Morris, D.; Cheng, T. Anti-PD1-Induced Immune-Related Adverse Events and Survival Outcomes in Advanced Melanoma. Oncologist 2020, 25, 438–446. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Ladanyi, A. Prognostic value of tumor-infiltrating immune cells in melanoma. Magy. Onkol. 2013, 57, 85–95. [Google Scholar]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Hodi, F.S.; Long, G.V. Nivolumab with or without Relatlimab in Untreated Advanced Melanoma. Reply. N. Engl. J. Med. 2022, 386, 1860–1861. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Cybulska-Stopa, B.; Piejko, K.; Ostaszewski, K.; Dziura, R.; Galus, L.; Ziolkowska, B.; Kempa-Kaminska, N.; Zietek, M.; Bal, W.; Kamycka, A.; et al. Long-term clinical evidence of comparable efficacy and toxicity of nivolumab and pembrolizumab in advanced melanoma treatment. Melanoma Res. 2023, 33, 208–217. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Park, J.J.; Mendis, S.; Rai, R.; Xu, W.; Lo, S.; Drummond, M.; Rowe, C.; Wong, A.; McArthur, G.; et al. Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br. J. Cancer 2017, 116, 1558–1563. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Plonka, P.M.; Raman, C.; Brozyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Chen, J.Y.; Slominski, A.T. How cancer hijacks the body’s homeostasis through the neuroendocrine system. Trends Neurosci. 2023, 46, 263–275. [Google Scholar] [CrossRef]
- Mesti, T.; Ceplak Mencin, V.; Mileva Boshkoska, B.; Ocvirk, J. Adverse events during immunotherapy in Slovenian patients with metastatic melanoma reveal a positive correlation with better treatment outcomes. Radiol. Oncol. 2021, 55, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef]
- Kelderman, S.; Heemskerk, B.; van Tinteren, H.; van den Brom, R.R.; Hospers, G.A.; van den Eertwegh, A.J.; Kapiteijn, E.W.; de Groot, J.W.; Soetekouw, P.; Jansen, R.L.; et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol. Immunother. 2014, 63, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Weber, J.S.; Infante, J.R.; Kim, K.B.; Daud, A.; Gonzalez, R.; Sosman, J.A.; Hamid, O.; Schuchter, L.; Cebon, J.; et al. Overall Survival and Durable Responses in Patients With BRAF V600-Mutant Metastatic Melanoma Receiving Dabrafenib Combined With Trametinib. J. Clin. Oncol. 2016, 34, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef] [PubMed]


| Patients | No (%) |
|---|---|
| Total | 222 (100%) |
| Age (years) (median, min–max) | 67 (27–90) |
| Follow-up time (in months) (median, min–max) | 16 (0–64) |
| Duration of therapy (in months) (median, min–max) | 7 (0–60) |
| Gender | |
| Male | 142 (63.9%) |
| Female | 80 (36.1%) |
| ECOG status | |
| 0 | 183 (82.4%) |
| 1 | 33 (14.9%) |
| 2 | 6 (2.7%) |
| Therapy | |
| Pembrolizumab | 173 (77.9%) |
| Nivolumab | 49 (22.1%) |
| Line of treatment | |
| First | 150 (67.6%) |
| Second or third | 72 (32.4%) |
| Stage | |
| Unresectable III | 15 (6.8%) |
| M1a | 21 (9.5%) |
| M1b | 62 (27.9%) |
| M1c | 89 (40.0%) |
| M1d | 35 (15.8%) |
| Brain metastasis | |
| No | 192 (86.5%) |
| Yes | 30 (13.5%) |
| LDH | |
| Normal value | 144 (64.9%) |
| Above normal level | 78 (35.1%) |
| BRAF mutation | |
| Negative | 172 (77.5%) |
| Positive | 50 (22.5%) |
| Therapeutic response | |
| Complete response | 25 (11.3%) |
| Partial response | 71 (32%) |
| Stable disease | 37 (16.7%) |
| Progressive disease | 73 (32.9%) |
| Not available | 16 (7.2%) |
| Side effect | |
| Autoimmune | 79 (35.6%) |
| Other | 45 (20.3%) |
| Side Effect | No (%) |
|---|---|
| Hypothyroidism | 22 (9.91%) |
| Vitiligo | 18 (8.12%) |
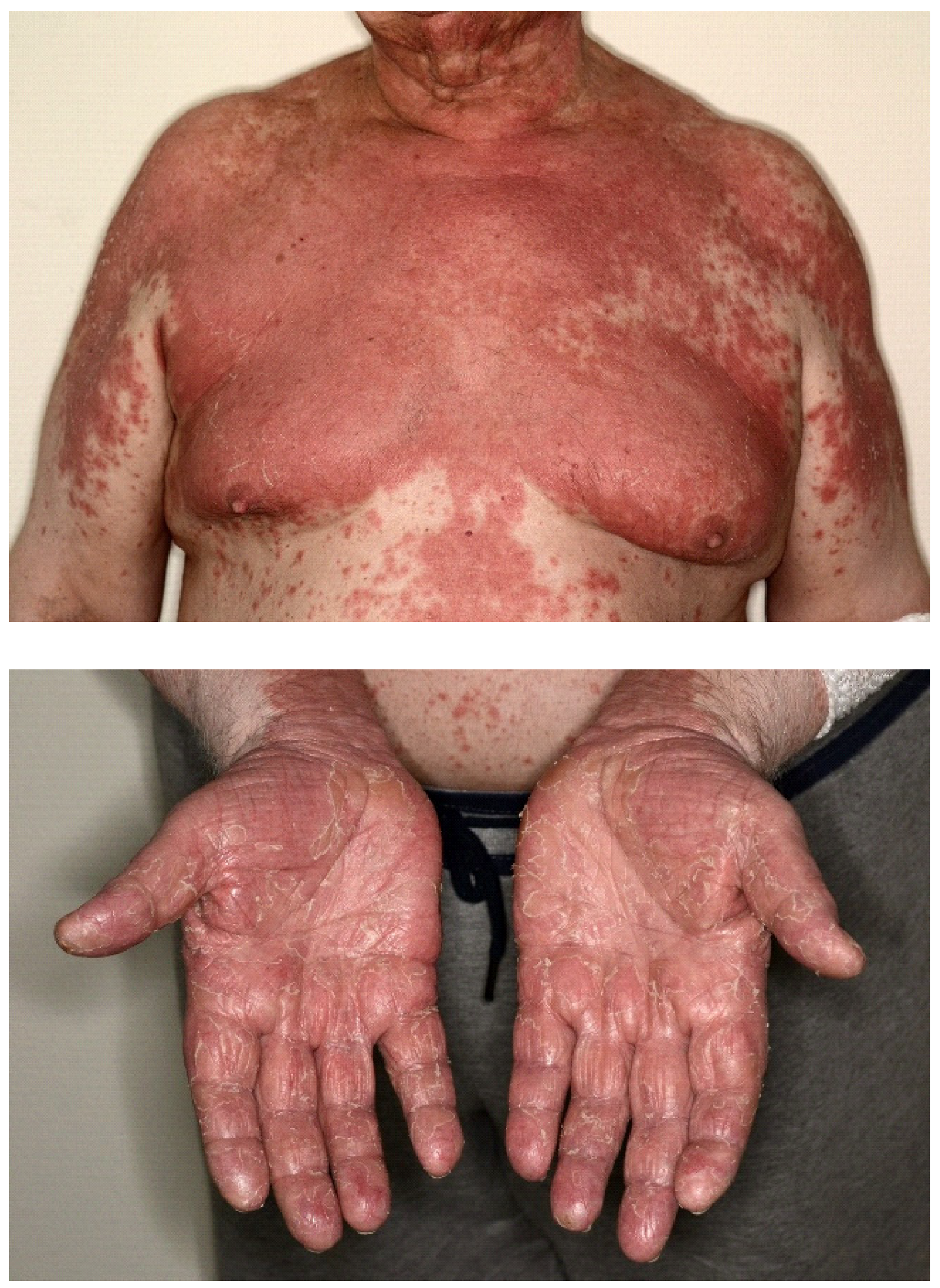
| Skin symptoms | 13 (5.86%) |
| Pneumonitis | 9 (4.05%) |
| Colitis | 7 (3.15%) |
| Arthralgia, artritis | 5 (2.25%) |
| Hypophysitis | 3 (1.35%) |
| Pancreatitis | 3 (1.35%) |
| Ocular inflammation | 2 (0.90%) |
| Hyperthyreosis | 2 (0.90%) |
| Drug-induced diabetes | 1 (0.45%) |
| Hemolytic anemia | 1 (0.45%) |
| Systemic autoimmune disease | 1 (0.45%) |
| Parameters | RR 1 (95% CI) | p |
|---|---|---|
| Age | 0.995 (0.979–1.011) | 0.543 |
| Gender (reference: female) | 0.779 (0.532–1.141) | 0.2 |
| Line of therapy (reference: second or later) | 0.722 (0.425–1.228) | 0.229 |
| ECOG (reference: 2) | ||
| 0 | 0.786 (0.282–2.191) | 0.445 |
| 1 | 0.976 (0.328–2.908) | 0.778 |
| LDH (reference: high) | 0.553 (0.377–0.813) | 0.003 |
| BRAF (reference: negative) | 1.135 (0.597–2.159) | 0.7 |
| M-stage (reference: N) | ||
| M1a | 1.068 (0.402–2.84) | 0.737 |
| M1b | 1.867 (0.828–4.208) | 0.228 |
| M1c | 1.848 (0.858–3.98) | 0.153 |
| M1d | 0.935 (0.367–2.383) | 0.957 |
| Autoimmune side effects (reference: yes) | 4.269 (2.685–6.788) | <0.000001 |
| Parameters | RR 1 (95% CI) | p |
|---|---|---|
| Age | 1 (0.984–1.018) | 0.958 |
| Gender (reference: female) | 0.633 (0.425–0.942) | 0.024 |
| Line of therapy (reference: second or later) | 0.632 (0.378–1.057) | 0.081 |
| ECOG (reference: 2) | 0.327 (0.127–0.843) | |
| 0 | 0.453 (0.163–1.259) | 0.013 |
| 1 | 0.55 (0.362–0.836) | 0.487 |
| LDH (reference: high) | 1.146 (0.606–2.167) | 0.005 |
| BRAF (reference: negative) | 2.21 (0.791–6.174) | 0.676 |
| M-stage (reference: N) | 2.205 (0.909–5.35) | |
| M1a | 2.451 (1.079–5.566) | 0.322 |
| M1b | 1.779 (0.639–4.952) | 0.694 |
| M1c | 3.547 (2.183–5.762) | 0.259 |
| M1d | 1 (0.984–1.018) | 0.576 |
| Autoimmune side effects (reference: yes) | 0.633 (0.425–0.942) | <0.000001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eikenes, G.; Liszkay, G.; Balatoni, T.; Czirbesz, K.; Hunyadi, K.; Kozéki, Z.; Kispál, M.T.; Baranyai, F.; Danyi, T.; Bőcs, K.; et al. Therapeutic and Adverse Effect of Anti-PD1 Immunotherapy in Melanoma: A Retrospective, Single-Institute Study of 222 Patients. Cancers 2023, 15, 3966. https://doi.org/10.3390/cancers15153966
Eikenes G, Liszkay G, Balatoni T, Czirbesz K, Hunyadi K, Kozéki Z, Kispál MT, Baranyai F, Danyi T, Bőcs K, et al. Therapeutic and Adverse Effect of Anti-PD1 Immunotherapy in Melanoma: A Retrospective, Single-Institute Study of 222 Patients. Cancers. 2023; 15(15):3966. https://doi.org/10.3390/cancers15153966
Chicago/Turabian StyleEikenes, Grethe, Gabriella Liszkay, Tímea Balatoni, Kata Czirbesz, Karen Hunyadi, Zsófia Kozéki, Mihály Tamás Kispál, Fanni Baranyai, Tímea Danyi, Katalin Bőcs, and et al. 2023. "Therapeutic and Adverse Effect of Anti-PD1 Immunotherapy in Melanoma: A Retrospective, Single-Institute Study of 222 Patients" Cancers 15, no. 15: 3966. https://doi.org/10.3390/cancers15153966
APA StyleEikenes, G., Liszkay, G., Balatoni, T., Czirbesz, K., Hunyadi, K., Kozéki, Z., Kispál, M. T., Baranyai, F., Danyi, T., Bőcs, K., & Kenessey, I. (2023). Therapeutic and Adverse Effect of Anti-PD1 Immunotherapy in Melanoma: A Retrospective, Single-Institute Study of 222 Patients. Cancers, 15(15), 3966. https://doi.org/10.3390/cancers15153966





