Definitions, Biology, and Current Therapeutic Landscape of Myelodysplastic/Myeloproliferative Neoplasms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Definitions
2.1. Chronic Myelomonocytic Leukemia
2.2. Atypical Chronic Myeloid Leukemia
2.3. Clonal Monocytosis of Undetermined Significance
2.4. Myelodysplastic/Myeloproliferative Neoplasm with Thrombocytosis and SF3B1 Mutation
2.5. Myelodysplastic/Myeloproliferative Neoplasm with Ring Sideroblasts and Thrombocytosis, Not Otherwise Specified
2.6. Myelodysplastic/Myeloproliferative Neoplasm, Not Otherwise Specified
3. Biology
3.1. Genetic Mutations
3.2. Chromosomal Abnormalities
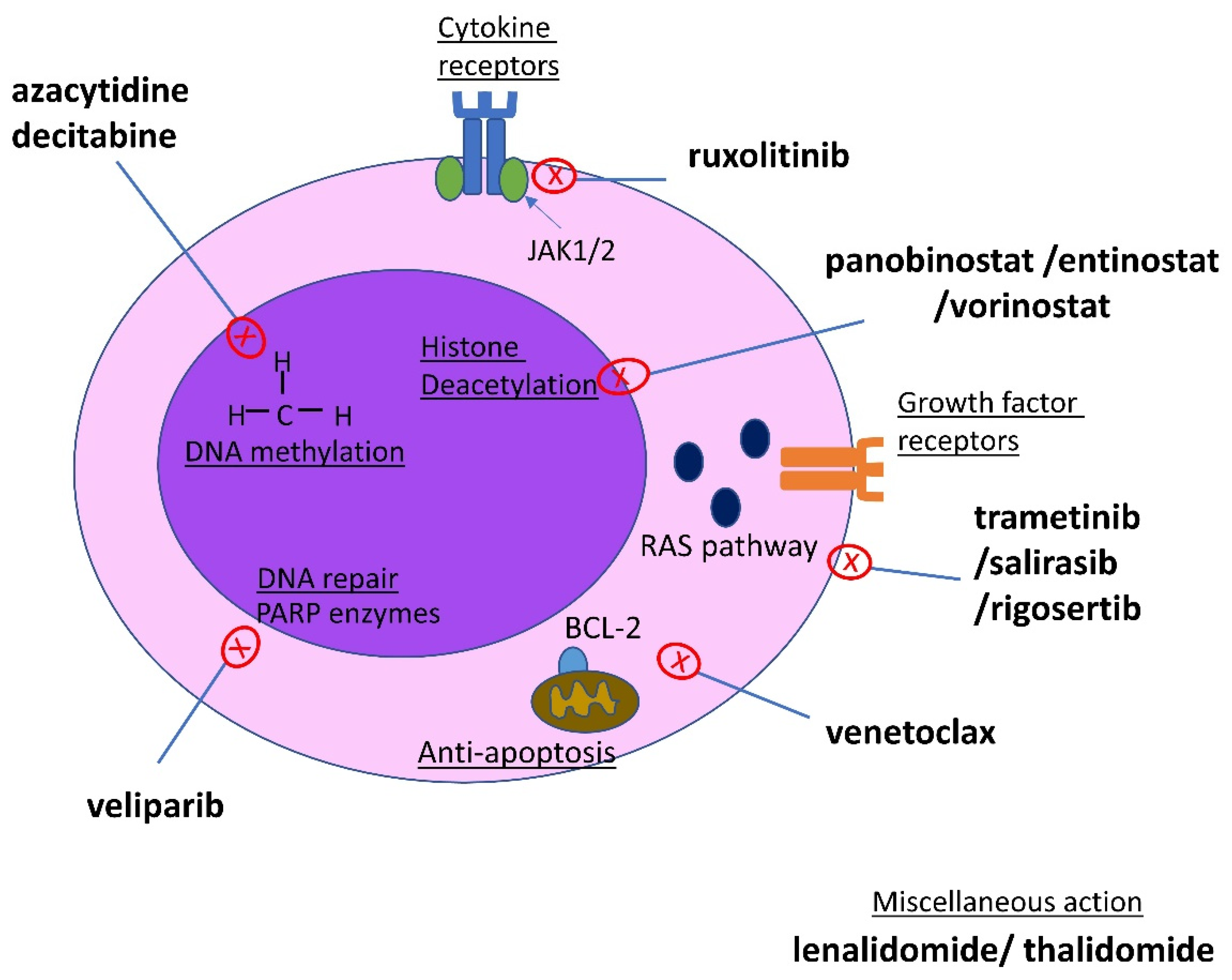
3.3. Current Therapeutic Strategies in MDS/MPN
3.3.1. Hypomethylating Agents
3.3.2. Ruxolitinib
3.3.3. Venetoclax
3.3.4. Immune-Modulatory Agents (IMiDs)
3.3.5. PARP Inhibitors
3.3.6. RAS Pathway Inhibition
3.3.7. Histone Deacetylases Inhibitors
3.3.8. Other Therapies
4. Future Directions
5. Allogeneic Transplantation
5.1. CMML
5.2. Studies of aCML, MDS-MPN-NOS, and MDS/MPN Combinations
5.3. Preparation Regimens and Bridging Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Roman, E.; Smith, A.; Appleton, S.; Crouch, S.; Kelly, R.; Kinsey, S.; Cargo, C.; Patmore, R. Myeloid malignancies in the real-world: Occurrence, progression and survival in the UK’s population-based Haematological Malignancy Research Network 2004–15. Cancer Epidemiol. 2016, 42, 186–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patnaik, M.M.; Tefferi, A. Chronic Myelomonocytic leukemia: 2020 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 97–115. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Sole, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Cytopenia levels for aiding establishment of the diagnosis of myelodysplastic syndromes. Blood 2016, 128, 2096–2097. [Google Scholar] [CrossRef] [Green Version]
- Palomo, L.; Meggendorfer, M.; Hutter, S.; Twardziok, S.; Ademà, V.; Fuhrmann, I.; Fuster-Tormo, F.; Xicoy, B.; Zamora, L.; Acha, P.; et al. Molecular landscape and clonal architecture of adult myelodysplastic/myeloproliferative neoplasms. Blood 2020, 136, 1851–1862. [Google Scholar] [CrossRef]
- Karantanos, T.; Gondek, L.P.; Varadhan, R.; Moliterno, A.R.; DeZern, A.E.; Jones, R.J.; Jain, T. Gender-related differences in the outcomes and genomic landscape of patients with myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes. Br. J. Haematol. 2021, 193, 1142–1150. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Tefferi, A. Chronic myelomonocytic leukemia: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 352–372. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Renneville, A.; Gelsi-Boyer, V.; Meggendorfer, M.; Morabito, M.; Berthon, C.; Adès, L.; Fenaux, P.; Beyne-Rauzy, O.; et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J. Clin. Oncol. 2013, 31, 2428–2436. [Google Scholar] [CrossRef]
- Gelsi-Boyer, V.; Trouplin, V.; Adélaïde, J.; Bonansea, J.; Cervera, N.; Carbuccia, N.; Lagarde, A.; Prebet, T.; Nezri, M.; Sainty, D.; et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br. J. Haematol. 2009, 145, 788–800. [Google Scholar] [CrossRef]
- Abdel-Wahab, O.; Mullally, A.; Hedvat, C.; Garcia-Manero, G.; Patel, J.; Wadleigh, M.; Malinge, S.; Yao, J.; Kilpivaara, O.; Bhat, R.; et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood 2009, 114, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahab, O.; Pardanani, A.; Patel, J.; Wadleigh, M.; Lasho, T.; Heguy, A.; Beran, M.; Gilliland, D.G.; Levine, R.L.; Tefferi, A. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia 2011, 25, 1200–1202. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahab, O.; Pardanani, A.; Rampal, R.; Lasho, T.L.; Levine, R.L.; Tefferi, A. DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leukemia 2011, 25, 1219–1220. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, V.; Kohlmann, A.; Eder, C.; Haferlach, C.; Kern, W.; Cross, N.C.; Haferlach, T.; Schnittger, S. Molecular profiling of chronic myelomonocytic leukemia reveals diverse mutations in >80% of patients with TET2 and EZH2 being of high prognostic relevance. Leukemia 2011, 25, 877–879. [Google Scholar] [CrossRef]
- Daver, N.; Strati, P.; Jabbour, E.; Kadia, T.; Luthra, R.; Wang, S.; Patel, K.; Ravandi, F.; Cortes, J.; Qin Dong, X.; et al. FLT3 mutations in myelodysplastic syndrome and chronic myelomonocytic leukemia. Am. J. Hematol. 2013, 88, 56–59. [Google Scholar] [CrossRef] [Green Version]
- Itzykson, R.; Solary, E. An evolutionary perspective on chronic myelomonocytic leukemia. Leukemia 2013, 27, 1441–1450. [Google Scholar] [CrossRef] [Green Version]
- Elena, C.; Galli, A.; Such, E.; Meggendorfer, M.; Germing, U.; Rizzo, E.; Cervera, J.; Molteni, E.; Fasan, A.; Schuler, E.; et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood 2016, 128, 1408–1417. [Google Scholar] [CrossRef] [Green Version]
- Patnaik, M.M.; Zahid, M.F.; Lasho, T.L.; Finke, C.; Ketterling, R.L.; Gangat, N.; Robertson, K.D.; Hanson, C.A.; Tefferi, A. Number and type of TET2 mutations in chronic myelomonocytic leukemia and their clinical relevance. Blood Cancer J. 2016, 6, e472. [Google Scholar] [CrossRef] [Green Version]
- Patnaik, M.M.; Lasho, T. Evidence-Based Minireview: Myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes: A focused review. Hematology 2020, 2020, 460–464. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Renneville, A.; Morabito, M.; Preudhomme, C.; Berthon, C.; Adès, L.; Fenaux, P.; Platzbecker, U.; Gagey, O.; et al. Clonal architecture of chronic myelomonocytic leukemias. Blood 2013, 121, 2186–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wudhikarn, K.; Loghavi, S.; Mangaonkar, A.A.; Al-Kali, A.; Binder, M.; Carr, R.; Reichard, K.; Finke, C.; Howard, M.; Gangat, N.; et al. SF3B1-mutant CMML defines a predominantly dysplastic CMML subtype with a superior acute leukemia-free survival. Blood Adv. 2020, 4, 5716–5721. [Google Scholar] [CrossRef] [PubMed]
- Gelsi-Boyer, V.; Trouplin, V.; Roquain, J.; Adélaïde, J.; Carbuccia, N.; Esterni, B.; Finetti, P.; Murati, A.; Arnoulet, C.; Zerazhi, H.; et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia. Br. J. Haematol. 2010, 151, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karantanos, T.; Tsai, H.L.; Gondek, L.P.; DeZern, A.E.; Ghiaur, G.; Dalton, W.B.; Gojo, I.; Prince, G.T.; Webster, J.; Ambinder, A.; et al. Genomic landscape of myelodysplastic/myeloproliferative neoplasm can predict response to hypomethylating agent therapy. Leuk. Lymphoma 2022, 63, 1942–1948. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Itzykson, R.; Lasho, T.L.; Kosmider, O.; Finke, C.M.; Hanson, C.A.; Knudson, R.A.; Ketterling, R.P.; Tefferi, A.; Solary, E. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: A two-center study of 466 patients. Leukemia 2014, 28, 2206–2212. [Google Scholar] [CrossRef]
- Cargo, C.; Cullen, M.; Taylor, J.; Short, M.; Glover, P.; Van Hoppe, S.; Smith, A.; Evans, P.; Crouch, S. The use of targeted sequencing and flow cytometry to identify patients with a clinically significant monocytosis. Blood 2019, 133, 1325–1334. [Google Scholar] [CrossRef] [Green Version]
- Fend, F.; Horn, T.; Koch, I.; Vela, T.; Orazi, A. Atypical chronic myeloid leukemia as defined in the WHO classification is a JAK2 V617F negative neoplasm. Leuk. Res. 2008, 32, 1931–1935. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Barraco, D.; Lasho, T.L.; Finke, C.M.; Reichard, K.; Hoversten, K.P.; Ketterling, R.P.; Gangat, N.; Tefferi, A. Targeted next generation sequencing and identification of risk factors in World Health Organization defined atypical chronic myeloid leukemia. Am. J. Hematol. 2017, 92, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Jeromin, S.; Haferlach, T.; Weissmann, S.; Meggendorfer, M.; Eder, C.; Nadarajah, N.; Alpermann, T.; Kohlmann, A.; Kern, W.; Haferlach, C.; et al. Refractory anemia with ring sideroblasts and marked thrombocytosis cases harbor mutations in SF3B1 or other spliceosome genes accompanied by JAK2V617F and ASXL1 mutations. Haematologica 2015, 100, e125–e127. [Google Scholar] [CrossRef] [Green Version]
- Montalban-Bravo, G.; Kanagal-Shamanna, R.; Darbaniyan, F.; Siddiqui, M.T.; Sasaki, K.; Wei, Y.; Yang, H.; Chien, K.S.; Naqvi, K.; Jabbour, E.; et al. Clinical, genomic, and transcriptomic differences between myelodysplastic syndrome/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T) and myelodysplastic syndrome with ring sideroblasts (MDS-RS). Am. J. Hematol. 2021, 96, E246–E249. [Google Scholar] [CrossRef]
- Esperanza, S.; José, C.; Dolors, C.; Francesc, S.; Teresa, V.; Elisa, L.; Rosa, C.; María, J.C.; Jesús, M.H.-R.; Juan, C.C.; et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica 2011, 96, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Wassie, E.A.; Itzykson, R.; Lasho, T.L.; Kosmider, O.; Finke, C.M.; Hanson, C.A.; Ketterling, R.P.; Solary, E.; Tefferi, A.; Patnaik, M.M. Molecular and prognostic correlates of cytogenetic abnormalities in chronic myelomonocytic leukemia: A Mayo Clinic-French Consortium Study. Am. J. Hematol. 2014, 89, 1111–1115. [Google Scholar] [CrossRef]
- Palomo, L.; Acha, P.; Solé, F. Genetic Aspects of Myelodysplastic/Myeloproliferative Neoplasms. Cancers 2021, 13, 2120. [Google Scholar] [CrossRef]
- Taylor, S.M.; Jones, P.A. Mechanism of action of eukaryotic DNA methyltransferase: Use of 5-azacytosine-containing DNA. J. Mol. Biol. 1982, 162, 679–692. [Google Scholar] [CrossRef]
- Leonhardt, H.; Page, A.W.; Weier, H.U.; Bestor, T.H. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 1992, 71, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Esteller, M. Aberrant DNA Methylation as a Cancer-Inducing Mechanism. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 629–656. [Google Scholar] [CrossRef] [Green Version]
- Palii, S.S.; Van Emburgh, B.O.; Sankpal, U.T.; Brown, K.D.; Robertson, K.D. DNA methylation inhibitor 5-Aza-2’-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol. Cell Biol. 2008, 28, 752–771. [Google Scholar] [CrossRef] [Green Version]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Kantarjian, H.; Issa, J.-P.J.; Rosenfeld, C.S.; Bennett, J.M.; Albitar, M.; DiPersio, J.; Klimek, V.; Slack, J.; de Castro, C.; Ravandi, F.; et al. Decitabine improves patient outcomes in myelodysplastic syndromes. Cancer 2006, 106, 1794–1803. [Google Scholar] [CrossRef]
- Xu, R.; Li, M.; Wu, P.; Deng, C.; Geng, S.; Huang, X.; Weng, J.; Du, X. Hypomethylating agents in the treatment of chronic myelomonocytic leukemia: A meta-analysis and systematic review. Hematology 2021, 26, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.W.; Pocock, C.; Boissinot, M.; Mills, J.; Brown, J.; Cauchy, P.; Cross, N.C.P.; Hartley, S.; Kell, J.; Szubert, A.; et al. A multi-centre phase 2 study of azacitidine in chronic myelomonocytic leukaemia. Leukemia 2014, 28, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Coston, T.; Pophali, P.; Vallapureddy, R.; Lasho, T.L.; Finke, C.M.; Ketterling, R.P.; Carr, R.; Binder, M.; Mangaonkar, A.A.; Gangat, N.; et al. Suboptimal response rates to hypomethylating agent therapy in chronic myelomonocytic leukemia; a single institutional study of 121 patients. Am. J. Hematol. 2019, 94, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Santini, V.; Allione, B.; Zini, G.; Gioia, D.; Lunghi, M.; Poloni, A.; Cilloni, D.; Sanna, A.; Masiera, E.; Ceccarelli, M.; et al. A phase II, multicentre trial of decitabine in higher-risk chronic myelomonocytic leukemia. Leukemia 2018, 32, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Fianchi, L.; Criscuolo, M.; Breccia, M.; Maurillo, L.; Salvi, F.; Musto, P.; Mansueto, G.; Gaidano, G.; Finelli, C.; Aloe-Spiriti, A.; et al. High rate of remissions in chronic myelomonocytic leukemia treated with 5-azacytidine: Results of an Italian retrospective study. Leuk. Lymphoma 2013, 54, 658–661. [Google Scholar] [CrossRef]
- Pleyer, L.; Germing, U.; Sperr, W.R.; Linkesch, W.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Schreder, M.; Pfeilstocker, M.; Lang, A.; et al. Azacitidine in CMML: Matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leuk. Res. 2014, 38, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Sekeres, M.A.; Watts, J.; Radinoff, A.; Sangerman, M.A.; Cerrano, M.; Lopez, P.F.; Zeidner, J.F.; Campelo, M.D.; Graux, C.; Liesveld, J.; et al. Randomized phase 2 trial of pevonedistat plus azacitidine versus azacitidine for higher-risk MDS/CMML or low-blast AML. Leukemia 2021, 35, 2119–2124. [Google Scholar] [CrossRef]
- Gotlib, J. How I treat atypical chronic myeloid leukemia. Blood 2017, 129, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Hausmann, H.; Bhatt, V.R.; Yuan, J.; Maness, L.J.; Ganti, A.K. Activity of single-agent decitabine in atypical chronic myeloid leukemia. J. Oncol. Pharm. Pract. 2016, 22, 790–794. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, Z.; Ren, L.I.; Tao, D.; Tong, H. Decitabine for the treatment of atypical chronic myeloid leukemia: A report of two cases. Oncol. Lett. 2016, 11, 689–692. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Li, J.; Zhou, Z.; Zheng, D.; Liu, J.; Su, C. Efficacy and side-effects of decitabine in treatment of atypical chronic myeloid leukemia. Leuk. Lymphoma 2015, 56, 1911–1913. [Google Scholar] [CrossRef]
- Mao, L.; You, L.; Yang, M.; Li, Y.; Ye, X.; Tong, H. The first case of decitabine successfully in treatment of atypical chronic myeloid leukemia with CEBPA double mutation. Chemotherapy 2013, 2, 114. [Google Scholar]
- Mangaonkar, A.A.; Swoboda, D.M.; Coltro, G.; Lasho, T.L.; Novotny, P.J.; Pophali, P.; Carr, R.M.; Binder, M.; Finke, C.M.; Gangat, N.; et al. Clinicopathologic characteristics, prognostication and treatment outcomes for myelodysplastic/myeloproliferative neoplasm, unclassifiable (MDS/MPN-U): Mayo Clinic-Moffitt Cancer Center study of 135 consecutive patients. Leukemia 2020, 34, 656–661. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Kanagal-Shamanna, R.; Naqvi, K.; Sasaki, K.; Masarova, L.; Jabbour, E.; Pemmaraju, N.; Kadia, T.M.; Ravandi, F.; Daver, N.; et al. Clinical Outcomes with Hypomethylating Agents in Patients with Myelodysplastic Syndrome/Myeloproliferative Neoplasm with Ring Sideroblasts and Thrombocytosis (MDS/MPN-RS-T); A Case Series. Blood 2020, 136, 18–19. [Google Scholar] [CrossRef]
- Melody, M.; Al Ali, N.; Sallman, D.A.; Padron, E.; List, A.F.; Lancet, J.E.; Komrokji, R.S. Lenalidomide Is Effective Treatment Option for Patients with Refractory Anemia with Ring Sideroblasts and Thrombocytosis. Blood 2018, 132, 4383. [Google Scholar] [CrossRef]
- Komrokji, R.; Melody, M.; Al Ali, N.; Chan, O.; Klimek, V.; Ball, B.J.; Sekeres, M.A.; Lucas, G.; Maciejewski, J.P.; Sallman, D.A.; et al. Treatment outcomes for patients with myelodysplastic syndrome/myeloproliferative neoplasms with ring sideroblasts and thrombocytosis. Leuk. Lymphoma 2022, 63, 199–204. [Google Scholar] [CrossRef]
- Camiener, G.W.; Smith, C.G. Studies of the enzymatic deamination of cytosine arabinoside—I: Enzyme distribution and species specificity. Biochem. Pharmacol. 1965, 14, 1405–1416. [Google Scholar] [CrossRef]
- Savona, M.R.; Odenike, O.; Amrein, P.C.; Steensma, D.P.; DeZern, A.E.; Michaelis, L.C.; Faderl, S.; Harb, W.; Kantarjian, H.; Lowder, J.; et al. An oral fixed-dose combination of decitabine and cedazuridine in myelodysplastic syndromes: A multicentre, open-label, dose-escalation, phase 1 study. Lancet Haematol. 2019, 6, e194–e203. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Griffiths, E.A.; Steensma, D.P.; Roboz, G.J.; Wells, R.; McCloskey, J., II; Odenike, O.; DeZern, A.E.; Yee, K.; Busque, L.; et al. Oral cedazuridine/decitabine for MDS and CMML: A phase 2 pharmacokinetic/pharmacodynamic randomized crossover study. Blood 2020, 136, 674–683. [Google Scholar] [CrossRef]
- Savona, M.R.; McCloskey, J.K.; Griffiths, E.A.; Yee, K.; Zeidan, A.M.; Al-Kali, A.; Deeg, H.J.; Patel, P.; Sabloff, M.; Keating, M.-M.; et al. Efficacy of Oral Decitabine/Cedazuridine (ASTX727) in the CMML Subgroup from the Ascertain Phase 3 Study. Blood 2021, 138 (Suppl. S1), 3682. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Roboz, G.; Walsh, K.; Kantarjian, H.; Ritchie, E.; Kropf, P.; O’Connell, C.; Tibes, R.; Lunin, S.; Rosenblat, T.; et al. Guadecitabine (SGI-110) in patients with intermediate or high-risk myelodysplastic syndromes: Phase 2 results from a multicentre, open-label, randomised, phase 1/2 trial. Lancet Haematol. 2019, 6, e317–e327. [Google Scholar] [CrossRef] [PubMed]
- Merlevede, J.; Droin, N.; Qin, T.; Meldi, K.; Yoshida, K.; Morabito, M.; Chautard, E.; Auboeuf, D.; Fenaux, P.; Braun, T.; et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat. Commun. 2016, 7, 10767. [Google Scholar] [CrossRef] [Green Version]
- Duchmann, M.; Yalniz, F.F.; Sanna, A.; Sallman, D.; Coombs, C.C.; Renneville, A.; Kosmider, O.; Braun, T.; Platzbecker, U.; Willems, L.; et al. Prognostic Role of Gene Mutations in Chronic Myelomonocytic Leukemia Patients Treated with Hypomethylating Agents. EBioMedicine 2018, 31, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liapis, K.; Kotsianidis, I. Approaching First-Line Treatment in Patients with Advanced CMML: Hypomethylating Agents or Cytotoxic Treatment? Front. Oncol. 2021, 11, 801524. [Google Scholar] [CrossRef] [PubMed]
- Padron, E.; Painter, J.S.; Kunigal, S.; Mailloux, A.W.; McGraw, K.; McDaniel, J.M.; Kim, E.; Bebbington, C.; Baer, M.; Yarranton, G.; et al. GM-CSF-dependent pSTAT5 sensitivity is a feature with therapeutic potential in chronic myelomonocytic leukemia. Blood 2013, 121, 5068–5077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padron, E.; Dezern, A.; Andrade-Campos, M.; Vaddi, K.; Scherle, P.; Zhang, Q.; Ma, Y.; Balasis, M.E.; Tinsley, S.; Ramadan, H.; et al. A Multi-Institution Phase I Trial of Ruxolitinib in Patients with Chronic Myelomonocytic Leukemia (CMML). Clin. Cancer Res. 2016, 22, 3746–3754. [Google Scholar] [CrossRef] [Green Version]
- Abaza, Y.; Hidalgo-Lopez, J.E.; Verstovsek, S.; Jabbour, E.; Ravandi, F.; Borthakur, G.; Estrov, Z.; Alvarado, Y.; Burger, J.; Schneider, H.; et al. Phase I study of ruxolitinib in previously treated patients with low or intermediate-1 risk myelodysplastic syndrome with evidence of NF-kB activation. Leuk. Res. 2018, 73, 78–85. [Google Scholar] [CrossRef]
- Hunter, A.M.; Newman, H.; Dezern, A.E.; Steensma, D.P.; Niyongere, S.; Roboz, G.J.; Mo, Q.; Chan, O.; Gerds, A.; Sallman, D.A.; et al. Integrated Human and Murine Clinical Study Establishes Clinical Efficacy of Ruxolitinib in Chronic Myelomonocytic Leukemia. Clin. Cancer Res. 2021, 27, 6095–6105. [Google Scholar] [CrossRef]
- Maxson, J.E.; Gotlib, J.; Pollyea, D.A.; Fleischman, A.G.; Agarwal, A.; Eide, C.A.; Bottomly, D.; Wilmot, B.; McWeeney, S.K.; Tognon, C.E.; et al. Oncogenic CSF3R Mutations in Chronic Neutrophilic Leukemia and Atypical CML. N. Engl. J. Med. 2013, 368, 1781–1790. [Google Scholar] [CrossRef] [Green Version]
- Fleischman, A.G.; Maxson, J.E.; Luty, S.B.; Agarwal, A.; Royer, L.R.; Abel, M.L.; MacManiman, J.D.; Loriaux, M.M.; Druker, B.J.; Tyner, J.W. The CSF3R T618I mutation causes a lethal neutrophilic neoplasia in mice that is responsive to therapeutic JAK inhibition. Blood 2013, 122, 3628–3631. [Google Scholar] [CrossRef] [Green Version]
- Dao, K.H.; Solti, M.B.; Maxson, J.E.; Winton, E.F.; Press, R.D.; Druker, B.J.; Tyner, J.W. Significant clinical response to JAK1/2 inhibition in a patient with CSF3R-T618I-positive atypical chronic myeloid leukemia. Leuk. Res. Rep. 2014, 3, 67–69. [Google Scholar] [CrossRef] [Green Version]
- Freedman, J.L.; Desai, A.V.; Bailey, L.C.; Aplenc, R.; Burnworth, B.; Zehentner, B.K.; Teachey, D.T.; Wertheim, G. Atypical Chronic Myeloid Leukemia in Two Pediatric Patients. Pediatr. Blood Cancer 2016, 63, 156–159. [Google Scholar] [CrossRef]
- Dao, K.T.; Gotlib, J.; Deininger, M.M.N.; Oh, S.T.; Cortes, J.E.; Collins, R.H., Jr.; Winton, E.F.; Parker, D.R.; Lee, H.; Reister, A.; et al. Efficacy of Ruxolitinib in Patients With Chronic Neutrophilic Leukemia and Atypical Chronic Myeloid Leukemia. J. Clin. Oncol. 2020, 38, 1006–1018. [Google Scholar] [CrossRef]
- Shanavas, M.; Popat, U.; Michaelis, L.C.; Fauble, V.; McLornan, D.; Klisovic, R.; Mascarenhas, J.; Tamari, R.; Arcasoy, M.O.; Davies, J.; et al. Outcomes of Allogeneic Hematopoietic Cell Transplantation in Patients with Myelofibrosis with Prior Exposure to Janus Kinase 1/2 Inhibitors. Biol. Blood Marrow Transplant. 2016, 22, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Montalban-Bravo, G.; Hammond, D.; DiNardo, C.D.; Konopleva, M.; Borthakur, G.; Short, N.J.; Ramos-Perez, J.; Guerra, V.; Kanagal-Shamanna, R.; Naqvi, K.; et al. Activity of venetoclax-based therapy in chronic myelomonocytic leukemia. Leukemia 2021, 35, 1494–1499. [Google Scholar] [CrossRef]
- Anderson, K.C. Lenalidomide and thalidomide: Mechanisms of action--similarities and differences. Semin. Hematol. 2005, 42 (Suppl. S4), S3–S8. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Marit, G.; Caillot, D.; Moreau, P.; Facon, T.; Stoppa, A.M.; Hulin, C.; Benboubker, L.; Garderet, L.; et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2012, 366, 1782–1791. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Reeves, J.A.; Feldman, E.J.; Dewald, G.W.; Bennett, J.M.; Deeg, H.J.; Dreisbach, L.; Schiffer, C.A.; Stone, R.M.; Greenberg, P.L.; et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood 2008, 111, 86–93. [Google Scholar] [CrossRef]
- List, A.; Dewald, G.; Bennett, J.; Giagounidis, A.; Raza, A.; Feldman, E.; Powell, B.; Greenberg, P.; Thomas, D.; Stone, R.; et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N. Engl. J. Med. 2006, 355, 1456–1465. [Google Scholar] [CrossRef] [Green Version]
- Burgstaller, S.; Stauder, R.; Kuehr, T.; Lang, A.; Machherndl-Spandl, S.; Mayrbaeurl, B.; Noesslinger, T.; Petzer, A.; Valent, P.; Greil, R.; et al. A phase I study of lenalidomide in patients with chronic myelomonocytic leukemia (CMML)–AGMT_CMML-1. Leuk. Lymphoma 2018, 59, 1121–1126. [Google Scholar] [CrossRef]
- Buckstein, R.; Kerbel, R.; Cheung, M.; Shaked, Y.; Chodirker, L.; Lee, C.R.; Lenis, M.; Davidson, C.; Cussen, M.-A.; Reis, M.; et al. Lenalidomide and metronomic melphalan for CMML and higher risk MDS: A phase 2 clinical study with biomarkers of angiogenesis. Leuk. Res. 2014, 38, 756–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekeres, M.A.; Othus, M.; List, A.F.; Odenike, O.; Stone, R.M.; Gore, S.D.; Litzow, M.R.; Buckstein, R.; Fang, M.; Roulston, D.; et al. Randomized Phase II Study of Azacitidine Alone or in Combination With Lenalidomide or with Vorinostat in Higher-Risk Myelodysplastic Syndromes and Chronic Myelomonocytic Leukemia: North American Intergroup Study SWOG S1117. J. Clin. Oncol. 2017, 35, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, M.; Hertzberg, M.; Benson, W.; Taylor, K.; Cunningham, I.; Stevenson, W.; Hiwase, D.; Eek, R.; Zantomio, D.; Jong, S.; et al. Azacitidine with or without lenalidomide in higher risk myelodysplastic syndrome & low blast acute myeloid leukemia. Haematologica 2019, 104, 700–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, D.I.; Feld, J.; El Jamal, S.M.; Mascarenhas, J.; Tremblay, D. Myelodysplastic syndrome/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis: Ringing in a new future. Leuk. Res. 2022, 115, 106820. [Google Scholar] [CrossRef] [PubMed]
- Huls, G.; Mulder, A.B.; Rosati, S.; van de Loosdrecht, A.A.; Vellenga, E.; de Wolf, J.T.M. Efficacy of single-agent lenalidomide in patients with JAK2 (V617F) mutated refractory anemia with ring sideroblasts and thrombocytosis. Blood 2010, 116, 180–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keen, R.; Pantin, J.; Savage, N.; Dainer, P.M. Treatment of Refractory Anemia with Ring Sideroblasts Associated with Marked Thrombocytosis with Lenalidomide in a Patient Testing Negative for 5q Deletion and JAK2 V617F and MPL W515K/L Mutations. Hematol Rep 2016, 8, 6592. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.; Culligan, D.; Vickers, M.A. Refractory Anemia with Ring Sideroblasts Associated with Marked Thrombocytosis Complicated by Massive Splenomegaly Treated with Lenalidomide Resulting in Resolution of Splenomegaly but Severe and Prolonged Pancytopenia. Case Rep. Hematol. 2013, 2013, 718480. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, K.; Sasaki, K.; Montalban-Bravo, G.; Teach, M.S.; Pierce, S.A.; Kantarjian, H.M.; Garcia-Manero, G. Characteristics and Role of Lenalidomide Therapy in Patients with Myelodysplastic/Myeloproliferative Neoplasm with Ring Sideroblasts and Thrombocytosis. Blood 2018, 132 (Suppl. S1), 5513. [Google Scholar] [CrossRef]
- Pratz, K.W.; Koh, B.D.; Patel, A.G.; Flatten, K.S.; Poh, W.; Herman, J.G.; Dilley, R.; Harrell, M.I.; Smith, B.D.; Karp, J.E.; et al. Poly (ADP-Ribose) Polymerase Inhibitor Hypersensitivity in Aggressive Myeloproliferative Neoplasms. Clin. Cancer Res. 2016, 22, 3894–3902. [Google Scholar] [CrossRef] [Green Version]
- Dréan, A.; Lord, C.J.; Ashworth, A. PARP inhibitor combination therapy. Crit. Rev. Oncol./Hematol. 2016, 108, 73–85. [Google Scholar] [CrossRef]
- Karanika, S.; Karantanos, T.; Li, L.; Corn, P.G.; Thompson, T.C. DNA damage response and prostate cancer: Defects, regulation and therapeutic implications. Oncogene 2015, 34, 2815–2822. [Google Scholar] [CrossRef] [Green Version]
- Pratz, K.W.; Rudek, M.A.; Gojo, I.; Litzow, M.R.; McDevitt, M.A.; Ji, J.; Karnitz, L.M.; Herman, J.G.; Kinders, R.J.; Smith, B.D.; et al. A Phase I Study of Topotecan, Carboplatin and the PARP Inhibitor Veliparib in Acute Leukemias, Aggressive Myeloproliferative Neoplasms, and Chronic Myelomonocytic Leukemia. Clin. Cancer Res. 2017, 23, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Gojo, I.; Beumer, J.H.; Pratz, K.W.; McDevitt, M.A.; Baer, M.R.; Blackford, A.L.; Smith, B.D.; Gore, S.D.; Carraway, H.E.; Showel, M.M.; et al. A Phase 1 Study of the PARP Inhibitor Veliparib in Combination with Temozolomide in Acute Myeloid Leukemia. Clin. Cancer Res. 2017, 23, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Burgess, M.R.; Hwang, E.; Firestone, A.J.; Huang, T.; Xu, J.; Zuber, J.; Bohin, N.; Wen, T.; Kogan, S.C.; Haigis, K.M.; et al. Preclinical efficacy of MEK inhibition in Nras-mutant AML. Blood 2014, 124, 3947–3955. [Google Scholar] [CrossRef]
- Borthakur, G.; Popplewell, L.; Boyiadzis, M.; Foran, J.; Platzbecker, U.; Vey, N.; Walter, R.B.; Olin, R.; Raza, A.; Giagounidis, A.; et al. Activity of the oral mitogen-activated protein kinase kinase inhibitor trametinib in RAS-mutant relapsed or refractory myeloid malignancies. Cancer 2016, 122, 1871–1879. [Google Scholar] [CrossRef] [Green Version]
- Badar, T.; Cortes, J.E.; Ravandi, F.; O’Brien, S.; Verstovsek, S.; Garcia-Manero, G.; Kantarjian, H.; Borthakur, G. Phase I study of S-trans, trans-farnesylthiosalicylic acid (salirasib), a novel oral RAS inhibitor in patients with refractory hematologic malignancies. Clin. Lymphoma Myeloma Leuk. 2015, 15, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Athuluri-Divakar, S.K.; Vasquez-Del Carpio, R.; Dutta, K.; Baker, S.J.; Cosenza, S.C.; Basu, I.; Gupta, Y.K.; Reddy, M.V.; Ueno, L.; Hart, J.R.; et al. A Small Molecule RAS-Mimetic Disrupts RAS Association with Effector Proteins to Block Signaling. Cell 2016, 165, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Manero, G.; Fenaux, P.; Al-Kali, A.; Baer, M.R.; Sekeres, M.A.; Roboz, G.J.; Gaidano, G.; Scott, B.L.; Greenberg, P.; Platzbecker, U.; et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): A randomised, controlled, phase 3 trial. Lancet Oncol. 2016, 17, 496–508. [Google Scholar] [CrossRef]
- Navada, S.C.; Garcia-Manero, G.; OdchimarReissig, R.; Pemmaraju, N.; Alvarado, Y.; Ohanian, M.N.; John, R.B.; Demakos, E.P.; Zbyszewski, P.S.; Maniar, M.; et al. Rigosertib in combination with azacitidine in patients with myelodysplastic syndromes or acute myeloid leukemia: Results of a phase 1 study. Leuk. Res. 2020, 94, 106369. [Google Scholar] [CrossRef]
- Khanna, V.; Pierce, S.T.; Dao, K.H.; Tognon, C.E.; Hunt, D.E.; Junio, B.; Tyner, J.W.; Druker, B.J. Durable Disease Control with MEK Inhibition in a Patient with NRAS-mutated Atypical Chronic Myeloid Leukemia. Cureus 2015, 7, e414. [Google Scholar] [CrossRef] [Green Version]
- Moe-Behrens, G.H.; Pandolfi, P.P. Targeting aberrant transcriptional repression in acute myeloid leukemia. Rev. Clin. Exp. Hematol. 2003, 7, 139–159. [Google Scholar] [PubMed]
- Griffiths, E.A.; Gore, S.D. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin. Hematol. 2008, 45, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiskus, W.; Rao, R.; Fernandez, P.; Herger, B.; Yang, Y.; Chen, J.; Kolhe, R.; Mandawat, A.; Wang, Y.; Joshi, R.; et al. Molecular and biologic characterization and drug sensitivity of pan-histone deacetylase inhibitor–resistant acute myeloid leukemia cells. Blood 2008, 112, 2896–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, P.; Wei, A.; Mithraprabhu, S.; Cummings, N.; Liu, H.B.; Perugini, M.; Reed, K.; Avery, S.; Patil, S.; Walker, P.; et al. Dual epigenetic targeting with panobinostat and azacitidine in acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood Cancer J. 2014, 4, e170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, Y.; Munakata, W.; Ogura, M.; Uchida, T.; Taniwaki, M.; Kobayashi, T.; Shimada, F.; Yonemura, M.; Matsuoka, F.; Tajima, T.; et al. Phase I study of panobinostat and 5-azacitidine in Japanese patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Int. J. Hematol. 2018, 107, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Sekeres, M.A.; Egyed, M.; Breccia, M.; Graux, C.; Cavenagh, J.D.; Salman, H.; Illes, A.; Fenaux, P.; DeAngelo, D.J.; et al. A phase 1b/2b multicenter study of oral panobinostat plus azacitidine in adults with MDS, CMML or AML with ⩽30% blasts. Leukemia 2017, 31, 2799–2806. [Google Scholar] [CrossRef] [Green Version]
- Prebet, T.; Sun, Z.; Figueroa, M.E.; Ketterling, R.; Melnick, A.; Greenberg, P.L.; Herman, J.; Juckett, M.; Wang, E.S.; Smith, M.R.; et al. Prolonged Administration of Azacitidine With or Without Entinostat for Myelodysplastic Syndrome and Acute Myeloid Leukemia With Myelodysplasia-Related Changes: Results of the US Leukemia Intergroup Trial E1905. J. Clin. Oncol. 2014, 32, 1242–1248. [Google Scholar] [CrossRef] [Green Version]
- Patnaik, M.M.; Sallman, D.A.; Mangaonkar, A.A.; Heuer, R.; Hirvela, J.; Zblewski, D.; Al-Kali, A.; Binder, M.; Balasis, M.E.; Newman, H.; et al. Phase 1 study of lenzilumab, a recombinant anti–human GM-CSF antibody, for chronic myelomonocytic leukemia. Blood 2020, 136, 909–913. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Ali, H.; Gupta, V.; Schiller, G.J.; Lee, S.; Yacoub, A.; Talpaz, M.; Sardone, M.; Wysowskyj, H.; Shemesh, S.; et al. Results from Ongoing Phase 1/2 Clinical Trial of Tagraxofusp (SL-401) in Patients with Relapsed/Refractory Chronic Myelomonocytic Leukemia (CMML). Blood 2018, 132, 1821. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Ali, H.; Wang, E.S.; Yacoub, A.; Gupta, V.; Lee, S.; Schiller, G.J.; Foran, J.M.; Tefferi, A.; Brooks, C.L.; et al. Tagraxofusp (SL-401) in Patients with Chronic Myelomonocytic Leukemia (CMML): Updated Results of an Ongoing Phase 1/2 Trial. Blood 2021, 138, 538. [Google Scholar] [CrossRef]
- Soucy, T.A.; Smith, P.G.; Milhollen, M.A.; Berger, A.J.; Gavin, J.M.; Adhikari, S.; Brownell, J.E.; Burke, K.E.; Cardin, D.P.; Critchley, S.; et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009, 458, 732–736. [Google Scholar] [CrossRef]
- Smith, P.G.; Traore, T.; Grossman, S.; Narayanan, U.; Carew, J.S.; Lublinksky, A.; Kuranda, M.; Milhollen, M. Azacitidine/Decitabine Synergism with the NEDD8-Activating Enzyme Inhibitor MLN4924 in Pre-Clinical AML Models. Blood 2011, 118. [Google Scholar] [CrossRef]
- Swords, R.T.; Coutre, S.; Maris, M.B.; Zeidner, J.F.; Foran, J.M.; Cruz, J.; Erba, H.P.; Berdeja, J.G.; Tam, W.; Vardhanabhuti, S.; et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood 2018, 131, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Adès, L.; Girshova, L.; Doronin, V.A.; Díez-Campelo, M.; Valcárcel, D.; Kambhampati, S.; Viniou, N.A.; Woszczyk, D.; De Paz Arias, R.; Symeonidis, A.; et al. Pevonedistat plus azacitidine vs azacitidine alone in higher-risk MDS/chronic myelomonocytic leukemia or low-blast-percentage AML. Blood Adv. 2022, 6, 5132–5145. [Google Scholar] [CrossRef]
- Lü, S.; Wang, J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J. Hematol. Oncol. 2014, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Warrell, R.P., Jr.; Coonley, C.J.; Gee, T.S. Homoharringtonine: An effective new drug for remission induction in refractory nonlymphoblastic leukemia. J. Clin. Oncol. 1985, 3, 617–621. [Google Scholar] [CrossRef]
- Alvandi, F.; Kwitkowski, V.E.; Ko, C.W.; Rothmann, M.D.; Ricci, S.; Saber, H.; Ghosh, D.; Brown, J.; Pfeiler, E.; Chikhale, E.; et al. U.S. Food and Drug Administration approval summary: Omacetaxine mepesuccinate as treatment for chronic myeloid leukemia. Oncologist 2014, 19, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Cortes, J.; Lipton, J.H.; Rea, D.; Digumarti, R.; Chuah, C.; Nanda, N.; Benichou, A.C.; Craig, A.R.; Michallet, M.; Nicolini, F.E.; et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood 2012, 120, 2573–2580. [Google Scholar] [CrossRef] [Green Version]
- Short, N.J.; Jabbour, E.; Naqvi, K.; Patel, A.; Ning, J.; Sasaki, K.; Nogueras-Gonzalez, G.M.; Bose, P.; Kornblau, S.M.; Takahashi, K.; et al. A phase II study of omacetaxine mepesuccinate for patients with higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia after failure of hypomethylating agents. Am. J. Hematol. 2019, 94, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Daver, N.; Vega-Ruiz, A.; Kantarjian, H.M.; Estrov, Z.; Ferrajoli, A.; Kornblau, S.; Verstovsek, S.; Garcia-Manero, G.; Cortes, J.E. A phase II open-label study of the intravenous administration of homoharringtonine in the treatment of myelodysplastic syndrome. Eur. J. Cancer Care 2013, 22, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Kurzrock, R.; Bueso-Ramos, C.E.; Kantarjian, H.; Freireich, E.; Tucker, S.L.; Siciliano, M.; Pilat, S.; Talpaz, M. BCR rearrangement-negative chronic myelogenous leukemia revisited. J. Clin. Oncol. 2001, 19, 2915–2926. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H.; Cortes, J.; Thomas, D.; Garcia-Manero, G.; Ferrajoli, A.; Faderl, S.; Richie, M.A.; Beran, M.; Giles, F.; et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: Final result of a phase 2 study. Cancer 2007, 110, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Moyo, T.K.; Mendler, J.H.; Itzykson, R.; Kishtagari, A.; Solary, E.; Seegmiller, A.C.; Gerds, A.T.; Ayers, G.D.; Dezern, A.E.; Nazha, A.; et al. The ABNL-MARRO 001 study: A phase 1-2 study of randomly allocated active myeloid target compound combinations in MDS/MPN overlap syndromes. BMC Cancer 2022, 22, 1013. [Google Scholar] [CrossRef]
- Zang, D.Y.; Deeg, H.J.; Gooley, T.; Anderson, J.E.; Anasetti, C.; Sanders, J.; Myerson, D.; Storb, R.; Appelbaum, F. Treatment of chronic myelomonocytic leukaemia by allogeneic marrow transplantation. Br. J. Haematol. 2000, 110, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Zabelina, T.; Guardiola, P.; Runde, V.; Sierra, J.; Van Biezen, A.; Niederwieser, D.; Zander, A.R.; De Witte, T. Allogeneic stem cell transplantation of adult chronic myelomonocytic leukaemia. A report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Br. J. Haematol. 2002, 118, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kerbauy, D.M.B.; Chyou, F.; Gooley, T.; Sorror, M.L.; Scott, B.; Pagel, J.M.; Myerson, D.; Appelbaum, F.R.; Storb, R.; Deeg, H.J. Allogeneic Hematopoietic Cell Transplantation for Chronic Myelomonocytic Leukemia. Biol. Blood Marrow Transplant. 2005, 11, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Shinde, S.S.; Damlaj, M.; Hefazi Rorghabeh, M.; Hashmi, S.K.; Litzow, M.R.; Hogan, W.J.; Gangat, N.; Elliott, M.A.; Al-Kali, A.; et al. Allogeneic hematopoietic stem cell transplant in adult patients with myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN) overlap syndromes. Leuk. Lymphoma 2017, 58, 872–881. [Google Scholar] [CrossRef]
- Park, S.; Labopin, M.; Yakoub-Agha, I.; Delaunay, J.; Dhedin, N.; Deconinck, E.; Michallet, M.; Robin, M.; De Revel, T.; Bernard, M.; et al. Allogeneic stem cell transplantation for chronic myelomonocytic leukemia: A report from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Eur. J. Haematol. 2013, 90, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, P.; Lim, Z.Y.; Nagi, W.; Kenyon, M.; Mijovic, A.; Ireland, R.; Marsh, J.; Ho, A.Y.L.; Mufti, G.J.; Pagliuca, A. Allogeneic haematopoietic SCT for chronic myelomonocytic leukaemia: A single-centre experience. Bone Marrow Transplant. 2010, 45, 1502–1507. [Google Scholar] [CrossRef] [Green Version]
- Itonaga, H.; Aoki, K.; Aoki, J.; Ishikawa, T.; Ishiyama, K.; Uchida, N.; Sakura, T.; Ohashi, K.; Kurokawa, M.; Ozawa, Y.; et al. Prognostic Impact of Donor Source on Allogeneic Hematopoietic Stem Cell Transplantation Outcomes in Adults with Chronic Myelomonocytic Leukemia: A Nationwide Retrospective Analysis in Japan. Biol. Blood Marrow Transplant. 2018, 24, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Pophali, P.; Matin, A.; Mangaonkar, A.A.; Carr, R.; Binder, M.; Al-Kali, A.; Begna, K.H.; Reichard, K.K.; Alkhateeb, H.; Shah, M.V.; et al. Prognostic impact and timing considerations for allogeneic hematopoietic stem cell transplantation in chronic myelomonocytic leukemia. Blood Cancer J. 2020, 10, 121. [Google Scholar] [CrossRef]
- Gagelmann, N.; Badbaran, A.; Beelen, D.W.; Salit, R.B.; Stölzel, F.; Rautenberg, C.; Becker, H.; Radujkovic, A.; Panagiota, V.; Bogdanov, R.; et al. A prognostic score including mutation profile and clinical features for patients with CMML undergoing stem cell transplantation. Blood Adv. 2021, 5, 1760–1769. [Google Scholar] [CrossRef]
- Symeonidis, A.; van Biezen, A.; de Wreede, L.; Piciocchi, A.; Finke, J.; Beelen, D.; Bornhäuser, M.; Cornelissen, J.; Volin, L.; Mufti, G.; et al. Achievement of complete remission predicts outcome of allogeneic haematopoietic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Br. J. Haematol. 2015, 171, 239–246. [Google Scholar] [CrossRef]
- Cahu, X.; Chevallier, P.; Clavert, A.; Suarez, F.; Michallet, M.; Vincent, L.; Vigouroux, S.; Blaise, D.; Mariette, C.; Bilger, K.; et al. Allo-SCT for Philadelphia-negative myeloproliferative neoplasms in blast phase: A study from the Societe Française de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). Bone Marrow Transplant. 2014, 49, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Eissa, H.; Gooley, T.A.; Sorror, M.L.; Nguyen, F.; Scott, B.L.; Doney, K.; Loeb, K.R.; Martin, P.J.; Pagel, J.M.; Radich, J.P.; et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia: Relapse-free survival is determined by karyotype and comorbidities. Biol. Blood Marrow Transplant. 2011, 17, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.D.; Ahn, K.W.; Hu, Z.H.; Hamadani, M.; Nishihori, T.; Wirk, B.; Beitinjaneh, A.; Rizzieri, D.; Grunwald, M.R.; Sabloff, M.; et al. Allogeneic Hematopoietic Cell Transplantation for Adult Chronic Myelomonocytic Leukemia. Biol. Blood Marrow Transplant. 2017, 23, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Such, E.; Germing, U.; Malcovati, L.; Cervera, J.; Kuendgen, A.; Della Porta, M.G.; Nomdedeu, B.; Arenillas, L.; Luño, E.; Xicoy, B.; et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood 2013, 121, 3005–3015. [Google Scholar] [CrossRef] [Green Version]
- Gagelmann, N.; Bogdanov, R.; Stölzel, F.; Rautenberg, C.; Panagiota, V.; Becker, H.; Radujkovic, A.; Luft, T.; Christopeit, M.; Finke, J.; et al. Long-Term Survival Benefit after Allogeneic Hematopoietic Cell Transplantation for Chronic Myelomonocytic Leukemia. Transplant. Cell. Ther. 2021, 27, 95.e1–95.e4. [Google Scholar] [CrossRef]
- Robin, M.; de Wreede, L.C.; Padron, E.; Bakunina, K.; Fenaux, P.; Koster, L.; Nazha, A.; Beelen, D.W.; Rampal, R.K.; Sockel, K.; et al. Role of allogeneic transplantation in chronic myelomonocytic leukemia: An international collaborative analysis. Blood 2022, 140, 1408–1418. [Google Scholar] [CrossRef]
- Woo, J.; Choi, D.R.; Storer, B.E.; Yeung, C.; Halpern, A.B.; Salit, R.B.; Sorror, M.L.; Woolston, D.W.; Monahan, T.; Scott, B.L.; et al. Impact of clinical, cytogenetic, and molecular profiles on long-term survival after transplantation in patients with chronic myelomonocytic leukemia. Haematologica 2020, 105, 652–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocheni, S.; Kröger, N.; Zabelina, T.; Zander, A.R.; Bacher, U. Outcome of allo-SCT for chronic myelomonocytic leukemia. Bone Marrow Transplant. 2009, 43, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, S.; Di Mario, A.; Salutari, P.; Rutella, S.; Chiusolo, P.; Rumi, C.; Menichella, G.; D’Onofrio, G.; Leone, G. Chemotherapy and recombinant human granulocyte colony-stimulating factor primed donor leukocyte infusion for treatment of relapse after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995, 16, 483–485. [Google Scholar] [PubMed]
- Elliott, M.A.; Tefferi, A.; Hogan, W.J.; Letendre, L.; Gastineau, D.A.; Ansell, S.M.; Dispenzieri, A.; Gertz, M.A.; Hayman, S.R.; Inwards, D.J.; et al. Allogeneic stem cell transplantation and donor lymphocyte infusions for chronic myelomonocytic leukemia. Bone Marrow Transplant. 2006, 37, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Kapaun, P.; Kabisch, H.; Held, K.R.; Walter, T.A.; Hegewisch, S.; Zander, A.R. Atypical chronic myelogenous leukemia in a patient with trisomy 8 mosaicism syndrome. Ann. Hematol. 1993, 66, 57–58. [Google Scholar] [CrossRef]
- Mittal, P.; Saliba, R.M.; Giralt, S.A.; Shahjahan, M.; Cohen, A.I.; Karandish, S.; Onida, F.; Beran, M.; Champlin, R.E.; de Lima, M. Allogeneic transplantation: A therapeutic option for myelofibrosis, chronic myelomonocytic leukemia and Philadelphia-negative/BCR-ABL-negative chronic myelogenous leukemia. Bone Marrow Transplant. 2004, 33, 1005–1009. [Google Scholar] [CrossRef]
- Koldehoff, M.; Beelen, D.W.; Trenschel, R.; Steckel, N.K.; Peceny, R.; Ditschkowski, M.; Ottinger, H.; Elmaagacli, A.H. Outcome of hematopoietic stem cell transplantation in patients with atypical chronic myeloid leukemia. Bone Marrow Transplant. 2004, 34, 1047–1050. [Google Scholar] [CrossRef] [Green Version]
- Itonaga, H.; Ota, S.; Ikeda, T.; Taji, H.; Amano, I.; Hasegawa, Y.; Ichinohe, T.; Fukuda, T.; Atsuta, Y.; Tanizawa, A.; et al. Allogeneic hematopoietic stem cell transplantation for the treatment of BCR-ABL1-negative atypical chronic myeloid leukemia and chronic neutrophil leukemia: A retrospective nationwide study in Japan. Leuk. Res. 2018, 75, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Onida, F.; de Wreede, L.C.; van Biezen, A.; Eikema, D.J.; Byrne, J.L.; Iori, A.P.; Schots, R.; Jungova, A.; Schetelig, J.; Finke, J.; et al. Allogeneic stem cell transplantation in patients with atypical chronic myeloid leukaemia: A retrospective study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Br. J. Haematol. 2017, 177, 759–765. [Google Scholar] [CrossRef]
- Gratwohl, A. The EBMT risk score. Bone Marrow Transplant. 2012, 47, 749–756. [Google Scholar] [CrossRef]
- Kurosawa, S.; Shimomura, Y.; Tachibana, T.; Ishiyama, K.; Ota, S.; Kobayashi, T.; Uchida, N.; Fukushima, K.; Ashida, T.; Matsuoka, K.-i.; et al. Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Myelodysplastic/Myeloproliferative Neoplasms-Unclassifiable: A Retrospective Nationwide Study of the Japan Society for Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 1607–1611. [Google Scholar] [CrossRef]
- Jain, T.; Tsai, H.L.; Elmariah, H.; Vachhani, P.; Karantanos, T.; Wall, S.A.; Gondek, L.P.; Bashey, A.; Keyzner, A.; Tamari, R.; et al. Haploidentical donor hematopoietic cell transplantation for myelodysplastic/myeloproliferative overlap neoplasms: Results from a North American collaboration. Haematologica 2023. [Google Scholar] [CrossRef]
- Fu, Y.; Schroeder, T.; Zabelina, T.; Badbaran, A.; Bacher, U.; Kobbe, G.; Ayuk, F.; Wolschke, C.; Schnittger, S.; Kohlmann, A.; et al. Postallogeneic monitoring with molecular markers detected by pretransplant next-generation or Sanger sequencing predicts clinical relapse in patients with myelodysplastic/myeloproliferative neoplasms. Eur. J. Haematol. 2014, 92, 189–194. [Google Scholar] [CrossRef]
- Langabeer, S.E.; McCarron, S.L.; Haslam, K.; O’Donovan, M.T.; Conneally, E. The CSF3R T618I mutation as a disease-specific marker of atypical CML post allo-SCT. Bone Marrow Transplant. 2014, 49, 843–844. [Google Scholar] [CrossRef]
- Pan, X.; Gao, M.; Sun, Y.; Zhou, Y.; Wang, K.; Wang, Y.; Xu, L.; Zhang, X.; Huang, X.; Zhao, X.S. Significance of WT1 and multiparameter flow cytometry assessment in patients with chronic myelomonocytic leukemia receiving allogeneic hematopoietic stem cell transplantation. Int. J. Lab. Hematol. 2022, 44, 510–517. [Google Scholar] [CrossRef]
- Radujkovic, A.; Hegenbart, U.; Müller-Tidow, C.; Herfarth, K.; Dreger, P.; Luft, T. High leukemia-free survival after TBI-based conditioning and mycophenolate mofetil-containing immunosuppression in patients allografted for chronic myelomonocytic leukemia: A single-center experience. Ann. Hematol. 2020, 99, 855–866. [Google Scholar] [CrossRef]
- Wedge, E.; Sengeløv, H.; Hansen, J.W.; Andersen, N.S.; Schjødt, I.; Petersen, S.L.; Kornblit, B.; Grønbæk, K.; Friis, L.S. Improved Outcomes after Allogenic Hematopoietic Stem Cell Transplantation with Fludarabine/Treosulfan for Patients with Myelodysplastic Syndromes. Biol. Blood Marrow Transplant. 2020, 26, 1091–1098. [Google Scholar] [CrossRef]
- Monaco, F.; Scott, B.L.; Chauncey, T.R.; Petersen, F.B.; Storer, B.E.; Baron, F.; Flowers, M.E.; Deeg, H.J.; Maloney, D.G.; Storb, R.; et al. Total body irradiation dose escalation decreases risk of progression and graft rejection after hematopoietic cell transplantation for myelodysplastic syndromes or myeloproliferative neoplasms. Haematologica 2019, 104, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, J.; Chhabra, S.; Kohrt, H.E.; Lavori, P.; Laport, G.G.; Arai, S.; Johnston, L.; Miklos, D.B.; Shizuru, J.A.; Weng, W.K.; et al. Total lymphoid irradiation-antithymocyte globulin conditioning and allogeneic transplantation for patients with myelodysplastic syndromes and myeloproliferative neoplasms. Biol. Blood Marrow Transplant. 2014, 20, 837–843. [Google Scholar] [CrossRef] [Green Version]
- Kongtim, P.; Popat, U.; Jimenez, A.; Gaballa, S.; El Fakih, R.; Rondon, G.; Chen, J.; Bueso-Ramos, C.; Borthakur, G.; Pemmaraju, N.; et al. Treatment with Hypomethylating Agents before Allogeneic Stem Cell Transplant Improves Progression-Free Survival for Patients with Chronic Myelomonocytic Leukemia. Biol. Blood Marrow Transplant. 2016, 22, 47–53. [Google Scholar] [CrossRef] [Green Version]
| Agent | Mechanism of Action | Response | Reference |
|---|---|---|---|
| HMA (Azacitidine and Decitabine) | Irreversibly bind DNA transferase, cause direct DNA damage [34,35,36,37] | ORR: 17–75% CR: 7–45% | [38,39,40,41,42,43,44,45,46,47] |
| Ruxolitinib | JAK1/2 Inhibitor | ORR: 0–38% | [66,67,68] |
| Lenalidomide | Immune-modulatory agent, anti-angiogenic properties, cytokine repression, activation apoptotic pathways [76] | ORR: 25–69% | [80,81,82,83] |
| Venetoclax | BCL-2 inhibitor | ORR: 67%CR: 4% | [75] |
| BMT | Infusion of bone marrow stem cells post-preparation regimen | 3 yr DFS: 39%; RR: 25% [124] 3 yr OS, NRM, Relapse incidence: 31%, 31%, 47% [130] 4 yr NRM, RR, DFS, and OS: 41%, 32%, 27%, and 33% [134] RFS 4 yr: 41% [126] 5 yr DFS: 18%, RR: 49% [125] 10 yr NRM, RR, PFS: 34%, 27%, and 29–38% [136,141] | [124,125,126,128,130,134,135,136,137,139,140,141] |
| Agent | Target/Mechanism | Disease | Type of Study | Response | Other Outcomes | Reference |
|---|---|---|---|---|---|---|
| Veliparib + topotecan and carboplatin | PARP inhibition + chemotherapy | High-risk MPN, CMML, AML | Phase I | ORR 67% | Median OS 15.8 months | [92] |
| Veliparib + temozolomide | PARP inhibition + chemotherapy | AML arising from CMML | Phase I | One patient had CR, two patients had SD | Two patients had counts normalization and clearance of circulating blasts | [93] |
| Trametinib | MEK1/MEK2 inhibition | Relapsed/Refractory CMML | Phase I/II | ORR 27% | [95] | |
| Salirasib | RAS inhibitor | CMML | Phase I | One of two CMML patients showed platelets improvement | [96] | |
| Rigosertib | RAS inhibition | CMML | Phase III | ORR 0%, SD 35% | No difference in OS compared to supportive care | [98] |
| Rigosertib + Azacitidine | RAS inhibition + HMA | 1 CMML patient | Phase I/II | SD | [99] | |
| Panobinostat + Azacitidine | HDAC inhibition + HMA | 4 CMML patients | Phase I | SD 100% | [105] | |
| Panobinostat + Azacitidine | HDAC inhibition + HMA | 17 CMML patients | Phase Ib/IIb | CR 29% compared to 10.3% with azacitidine alone | Similar safety profile | [106] |
| Entinostat + Azacitidine | HDAC inhibition + HMA | 5 CMML patients | Phase II | Response 32% with azacitidine alone compared to 27% with combination | OS 22 months with azacitidine alone compared to 14.7 months with combination, antagonistic effect based on methylation profile | [107] |
| Lenzilumab | Antibody against GM-CSF | CMML | Phase I | Clinical benefit 33% | No grade III or IV adverse events | [108] |
| Tagraxofusp | Anti-CD123 drug conjugate | Relapsed/Refractory CMML | Phase I/II | CR 11% | Splenic reduction 42% | [110] |
| Pevonedistat + Azacitidine | NEDD88 inhibitor + HMA | 17 CMML patients | Phase II | ORR 77.5% with combination compared to 75% with azacitidine alone | [47] | |
| Pevonedistat + Azacitidine | NEDD88 inhibitor + HMA | 27 CMML patients | Phase III | ORR 44% with the combination compared to 35% with azacitidine alone | Similar OS and EFS between arms | [114] |
| Omacetaxine mepesuccinate | Semi-synthetic form of HHT, plant alkaloid preventing protein synthesis | 8 CMML patients, progressed on HMA | Phase II | ORR 33% | Median OS 7.5 months, grade III adverse events 26% | [119] |
| PEG-IFN-alpha | Inhibition of protein synthesis and cytotoxicity | 5 aCML patients | Phase II | CR 40% | Significant toxicity | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerke, M.B.; Christodoulou, I.; Karantanos, T. Definitions, Biology, and Current Therapeutic Landscape of Myelodysplastic/Myeloproliferative Neoplasms. Cancers 2023, 15, 3815. https://doi.org/10.3390/cancers15153815
Gerke MB, Christodoulou I, Karantanos T. Definitions, Biology, and Current Therapeutic Landscape of Myelodysplastic/Myeloproliferative Neoplasms. Cancers. 2023; 15(15):3815. https://doi.org/10.3390/cancers15153815
Chicago/Turabian StyleGerke, Margo B., Ilias Christodoulou, and Theodoros Karantanos. 2023. "Definitions, Biology, and Current Therapeutic Landscape of Myelodysplastic/Myeloproliferative Neoplasms" Cancers 15, no. 15: 3815. https://doi.org/10.3390/cancers15153815
APA StyleGerke, M. B., Christodoulou, I., & Karantanos, T. (2023). Definitions, Biology, and Current Therapeutic Landscape of Myelodysplastic/Myeloproliferative Neoplasms. Cancers, 15(15), 3815. https://doi.org/10.3390/cancers15153815








