Systemic Therapy for Metastatic Triple Negative Breast Cancer: Current Treatments and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
2. Immunotherapy
| Trial Name and Sample Size | Treatment Arms | Response Rate PD-L1+ (ITT) | Median PFS (Months) PD-L1+ (ITT) | Median OS (Months) PD-L1+ (ITT) |
|---|---|---|---|---|
| First Line (combination with chemotherapy) | ||||
| IMpassion130 [25] n = 369 PD-L1+ (ITT n = 902) | Nab-paclitaxel + placebo | 42.6% (45.9%) | 5.0 (5.5) | 18 (18.7) |
| Nab-paclitaxel + atezolizumab | 58.9% (56.0%) | 7.5 (7.2) | 25 (21) | |
| IMpassion131 [26] n = 292 PD-L1+ (ITT n = 651) | Paclitaxel + placebo | 55% (54%) | 5.7 (5.6) | 28.3 (22.8) |
| Paclitaxel + atezolizumab | 63% (47%) | 6.0 (5.7) | 22.1 (19.2) | |
| Keynote-355 [29] n = 323 PD-L1+ (ITT n = 847) | Chemo (paclitaxel, nab-paclitaxel or Gem/carbo) + placebo | 55% (47%) | 5.6 (5.6) | 16.1 (15.5) |
| Chemo + pembrolizumab | 63% (54%) | 9.7 (7.5) | 23.0 (17.2) | |
| ≥2nd line (monotherapy) | ||||
| Keynote-119 [15] n = 194 PD-L1+ (ITT n = 622) | Chemo (capecitabine, eribulin, gemcitabine or vinorelbine) | 9.2% (10.6%) | 3.4 (3.3) | 11.6 (10.8) |
| Pembrolizumab | 17.7% (9.6%) | 2.1 (2.1) | 12.1 (9.9) | |
| ≤2nd line—single arm | ||||
| ENHANCE 1 [34] n = 74 PD-L1+ (ITT n = 167) | Pembrolizumab + Eribulin (single arm) | 28.4% (23.4%) | 6.1 in 1st line, 4.1 in 2nd line (4.1) | 21.0 in 1st line; 14.0 in 2nd line (16.1) |
3. Antibody-Drug Conjugates
3.1. Sacituzumab Govitecan (SG)
3.2. Trastuzumab Deruxtecan (T-DXd)
3.3. Datopotamab Deruxtecan (Dato-DXd)
3.4. Patritumab Deruxtecan
3.5. Ladiratuzumab Vedotin (LV) (SGN-LIV1A)
3.6. Disitamab Vedotin (DV)
3.7. Enfortumab Vedotin (EV)
3.8. ADC Summary
4. Targeted Agents
4.1. PARP Inhibitors
4.2. HER2 Mutations
4.3. AKT Pathway
4.4. FGFR
4.5. Androgen Receptor
4.6. Targeted Agents Summary
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Kushi, L.H.; Weltzien, E.; Maring, B.; Kutner, S.E.; Fulton, R.S.; Lee, M.M.; Ambrosone, C.B.; Caan, B.J. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.W.; Fish, K.; Shema, S.J.; Clarke, C.A. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010, 12, R99. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Van De Vijver, M.J.; Jacquemier, J.; Anderson, T.J.; Osin, P.P.; McGuffog, L.; Easton, D.F. The pathology of familial breast cancer: Predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002, 20, 2310–2318. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanovic, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Debien, V.; De Caluwe, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Teschendorff, A.E.; Miremadi, A.; Pinder, S.E.; Ellis, I.O.; Caldas, C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007, 8, R157. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Winer, E.P.; Lipatov, O.; Im, S.A.; Goncalves, A.; Munoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Cortes, J.; Lipatov, O.; Im, S.A.; Goncalves, A.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; Ohtani, S.; et al. KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann. Oncol. 2019, 30, 859–860. [Google Scholar]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.B.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Cortes-Ciriano, I.; Lee, S.; Park, W.Y.; Kim, T.M.; Park, P.J. A molecular portrait of microsatellite instability across multiple cancers. Nat. Commun. 2017, 8, 15180. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.H.; Brogi, E.; Zeng, Z.; Akram, M.; Catalano, J.; Paty, P.B.; Norton, L.; Shia, J. DNA mismatch repair deficiency in breast carcinoma: A pilot study of triple-negative and non-triple-negative tumors. Am. J. Surg. Pathol. 2012, 36, 1700–1708. [Google Scholar] [CrossRef]
- Adams, S.; Diamond, J.R.; Hamilton, E.; Pohlmann, P.R.; Tolaney, S.M.; Chang, C.W.; Zhang, W.; Iizuka, K.; Foster, P.G.; Molinero, L.; et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer with 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol. 2019, 5, 334–342. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.; Gligorov, J.; Andre, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Roche Provides Update on Tecentriq US Indication for PD-L1 Positive, Metastatic Triple-Negative Breast Cancer. 2021. Available online: https://www.roche.com/media/releases/med-cor-2021-08-27 (accessed on 24 April 2023).
- Franzoi, M.A.; de Azambuja, E. Atezolizumab in metastatic triple-negative breast cancer: IMpassion130 and 131 trials—How to explain different results? ESMO Open 2020, 5, e001112. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Rugo, H.S.; Loi, S.; Adams, S.; Schmid, P.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.C.; Winer, E.P.; Kockx, M.; et al. Performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): Post-hoc analysis of IMpassion130. Ann. Oncol. 2019, 30, 858–859. [Google Scholar]
- Emens, L.A.; Molinero, L.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Dieras, V.; Iwata, H.; Barrios, C.H.; Nechaeva, M.; Nguyen-Duc, A.; et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-Negative Breast Cancer: Biomarker Evaluation of the IMpassion130 Study. J. Natl. Cancer Inst. 2021, 113, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Rozenblit, M.; Huang, R.; Danziger, N.; Hegde, P.; Alexander, B.; Ramkissoon, S.; Blenman, K.; Ross, J.S.; Rimm, D.L.; Pusztai, L. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J. Immunother. Cancer 2020, 8, e001558. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vennapusa, B.; Chang, C.W.; Tran, D.; Nakamura, R.; Sumiyoshi, T.; Hegde, P.; Molinero, L. Prevalence Study of PD-L1 SP142 Assay in Metastatic Triple-negative Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Kalinsky, K.; Kaklamani, V.G.; D’Adamo, D.R.; Aktan, G.; Tsai, M.L.; O’Regan, R.M.; Kaufman, P.A.; Wilks, S.T.; Andreopoulou, E.; et al. Eribulin Plus Pembrolizumab in Patients with Metastatic Triple-Negative Breast Cancer (ENHANCE 1): A Phase Ib/II Study. Clin. Cancer Res. 2021, 27, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.H.; Goncalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef]
- Schmid, P.; Abraham, J.; Chan, S.; Wheatley, D.; Brunt, A.M.; Nemsadze, G.; Baird, R.D.; Park, Y.H.; Hall, P.S.; Perren, T.; et al. Capivasertib Plus Paclitaxel Versus Placebo Plus Paclitaxel As First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial. J. Clin. Oncol. 2020, 38, 423–433. [Google Scholar] [CrossRef]
- Dent, R.; Oliveira, M.; Isakoff, S.J.; Im, S.A.; Espie, M.; Blau, S.; Tan, A.R.; Saura, C.; Wongchenko, M.J.; Xu, N.; et al. Final results of the double-blind placebo-controlled randomized phase 2 LOTUS trial of first-line ipatasertib plus paclitaxel for inoperable locally advanced/metastatic triple-negative breast cancer. Breast Cancer Res. Treat. 2021, 189, 377–386. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Goldman, J.W.; Hurvitz, S.A.; Guerrero-Zotano, A.; Unni, N.; Brufsky, A.; Park, H.; Waisman, J.R.; Yang, E.S.H.; Spanggaard, I.; et al. Neratinib plus fulvestrant plus trastzuzumab (N plus F plus T) for hormone receptor-positive (HR+), HER2-negative, HER2-mutant metastatic breast cancer (MBC): Outcomes and biomarker analysis from the SUMMIT trial. J. Clin. Oncol. 2022, 40, 1028. [Google Scholar]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoi, H.; Grellety, T.; Tredan, O.; Saghatchian, M.; Dalenc, F.; Mailliez, A.; L’Haridon, T.; Cottu, P.; Abadie-Lacourtoisie, S.; You, B.; et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann. Oncol. 2016, 27, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, G.A.; Moroose, R.; Santin, A.D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Sacituzumab Govitecan-Hziy for Metastatic Triple Negative Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sacituzumab-govitecan-hziy-metastatic-triple-negative-breast-cancer (accessed on 16 May 2023).
- Dieras, V.; Weaver, R.; Tolaney, S.M.; Bardia, A.; Punie, K.; Brufsky, A.; Rugo, H.S.; Kalinsky, K.; Traina, T.; Klein, L.; et al. Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Res. 2021, 81, PD13-07. [Google Scholar] [CrossRef]
- Vejjasilpa, K.; Nasongkla, N.; Manaspon, C.; Larbcharoensub, N.; Boongird, A.; Hongeng, S.; Israsena, N. Antitumor efficacy and intratumoral distribution of SN-38 from polymeric depots in brain tumor model. Exp. Biol. Med. 2015, 240, 1640–1647. [Google Scholar] [CrossRef]
- Vredenburgh, J.J.; Desjardins, A.; Reardon, D.A.; Friedman, H.S. Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol. 2009, 11, 80–91. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Dieras, V.; Deluche, E.; Lusque, A.; Pistilli, B.; Bachelot, T.; Pierga, J.Y.; Viret, F.; Levy, C.; Salabert, L.; Le Du, F.; et al. Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY). Cancer Res. 2022, 82, PD8-02. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Zhao, F.Y.; Chen, N.; Hahn, O.M.; Nanda, R.; Olopade, O.I.; Huo, D.Z.; Howard, F.M. Clinicopathologic Characteristics and Prognosis of ERBB2-Low Breast Cancer Among Patients in the National Cancer Database. JAMA Oncol. 2023, 9, 500–510. [Google Scholar] [CrossRef]
- Andre, F.; Hee Park, Y.; Kim, S.B.; Takano, T.; Im, S.A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gavila Gregori, J.; De Laurentiis, M.; et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; Toki, T.; Yamaguchi, J.; Kitamura, M.; Kamei, R.; et al. Datopotamab Deruxtecan, a Novel TROP2-directed Antibody-drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol. Cancer Ther. 2021, 20, 2329–2340. [Google Scholar] [CrossRef]
- Krop, I.; Juric, D.; Shimizu, T.; Tolcher, A.; Spira, A.; Mukohara, T.; Lisberg, A.; Kogawa, T.; Papdopoulos, K.; Hamilton, E.; et al. Datopotamab deruxtecan in advanced/metastatic HER2-breast cancer: Results from the phase 1 TROPION-PanTumour01 study [abstract]. Cancer Res. 2022, 82, GS1-05. [Google Scholar]
- Schmid, P.; Jung, K.H.; Wysocki, P.J.; Jassem, J.; Ma, C.X.; Fernandes, R.; Huisden, R.; Stewart, R.; Vukovic, P.; Nunes, A.T.; et al. Datopotamab deruxtecan + durvalumab as first-line treatment for unresectable locally advanced/metastatic triple-negative breast cancer: Initial results from BEGONIA, a phase 1b/2 study. In Proceedings of the ESMO Breast Cancer Congress, Berlin, Germany, 3–5 May 2022. [Google Scholar]
- Krop, I.E.; Masuda, N.; Mukohara, T.; Takahashi, S.; Nakayama, T.; Inoue, K.; Iwata, H.; Toyama, T.; Yamamoto, Y.; Hansra, D.M.; et al. Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC). J. Clin. Oncol. 2022, 40, 1002. [Google Scholar] [CrossRef]
- Hamilton, E.; Dosunmu, O.; Shastry, M.; Finney, L.; Sellami, D.; Sternberg, D.W.; Wright-Browne, V.; Toppmeyer, D.; Gwin, W.; Thaddeus, T.; et al. A phase 2 study of HER3-DXd in patients (pts) with metastatic breast cancer (MBC). J. Clin. Oncol. 2023, 41, 1004. [Google Scholar] [CrossRef]
- Sussman, D.; Smith, L.M.; Anderson, M.E.; Duniho, S.; Hunter, J.H.; Kostner, H.; Miyamoto, J.B.; Nesterova, A.; Westendorf, L.; Van Epps, H.A.; et al. SGN-LIV1A: A Novel Antibody-Drug Conjugate Targeting LIV-1 for the Treatment of Metastatic Breast Cancer. Mol. Cancer Ther. 2014, 13, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Hadley, L.J.; Nicholson, R.I. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem. J. 2003, 375, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Pusztai, L.; Forero, A.; Mita, M.; Miller, K.D.; Weise, A.; Krop, I.; Burris, H.; Kalinsky, K.; Tsai, M.; et al. Phase 1 study of the antibody-drug conjugate SGN-LIV1A in patients with heavily pretreated triple-negative metastatic breast cancer. Cancer Res. 2018, 78, PD3-14. [Google Scholar]
- Tsai, M.; Han, H.S.; Montero, A.J.; Tkaczuk, K.H.; Assad, H.; Pusztai, L.; Hurvitz, S.A.; Wilks, S.T.; Specht, J.M.; Nanda, R.; et al. Weekly ladiratuzumab vedotin monotherapy for metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, S474–S475. [Google Scholar] [CrossRef]
- Cao, A.T.; Higgins, S.; Stevens, N.; Gardai, S.J.; Sussman, D. Additional mechanisms of action of ladiratuzumab vedotin contribute to increased immune cell activation within the tumor. Cancer Res. 2018, 78, 2742. [Google Scholar] [CrossRef]
- Pusztai, L.; Lu, H.L.; Hale, C.; Grosse-Wilde, A.; Specht, J.; Modi, S.; Han, H.; Cortes, J.; Oliveira, M.; Garfin, P.; et al. Systemic Administration of Ladiratuzumab Vedotin Alone or in Combination with Pembrolizumab Results in Significant Immune Activation in the Tumor Microenvironment in Metastatic Breast Cancer Patients. J. Immunother. Cancer 2020, 8, A198–A199. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab vedotin: A novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022, 29, 1335–1344. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, Y.J.; Zhang, Q.Y.; Feng, J.F.; Fang, J.M.; Chen, X.L.; Han, Y.Q.; Li, Q.; Zhang, P.; Yuan, P.; et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. J. Clin. Oncol. 2021, 39, 1022. [Google Scholar] [CrossRef]
- Klumper, N.; Ralser, D.J.; Ellinger, J.; Roghmann, F.; Albrecht, J.; Below, E.; Alajati, A.; Sikic, D.; Breyer, J.; Bolenz, C.; et al. Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1496–1505. [Google Scholar] [CrossRef]
- M-Rabet, M.; Cabaud, O.; Josselin, E.; Finetti, P.; Castellano, R.; Farina, A.; Agavnian-Couquiaud, E.; Saviane, G.; Collette, Y.; Viens, P.; et al. Nectin-4: A new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann. Oncol. 2017, 28, 769–776. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Stefansson, I.M.; Chappuis, P.O.; Begin, L.R.; Goffin, J.R.; Wong, N.; Trudel, M.; Akslen, L.A. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J. Natl. Cancer Inst. 2003, 95, 1482–1485. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Bose, R.; Kavuri, S.M.; Searleman, A.C.; Shen, W.; Shen, D.; Koboldt, D.C.; Monsey, J.; Goel, N.; Aronson, A.B.; Li, S.; et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013, 3, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.; Won, H.H.; Andre, F.; Arnedos, M.; Meric-Bernstam, F.; Bedard, P.L.; Shaw, K.R.; Horlings, H.; Micheel, C.; Park, B.H.; et al. Landscape of somatic ERBB2 Mutations: Findings from AACR GENIE and comparison to ongoing ERBB2 mutant basket study. Cancer Res. 2017, 77, LB-103. [Google Scholar] [CrossRef]
- Zuo, W.J.; Jiang, Y.Z.; Wang, Y.J.; Xu, X.E.; Hu, X.; Liu, G.Y.; Wu, J.; Di, G.H.; Yu, K.D.; Shao, Z.M. Dual Characteristics of Novel HER2 Kinase Domain Mutations in Response to HER2-Targeted Therapies in Human Breast Cancer. Clin. Cancer Res. 2016, 22, 4859–4869. [Google Scholar] [CrossRef]
- Turner, N.C.; Kingston, B.; Kilburn, L.S.; Kernaghan, S.; Wardley, A.M.; Macpherson, I.R.; Baird, R.D.; Roylance, R.; Stephens, P.; Oikonomidou, O.; et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020, 21, 1296–1308. [Google Scholar] [CrossRef]
- Millis, S.Z.; Gatalica, Z.; Winkler, J.; Vranic, S.; Kimbrough, J.; Reddy, S.; O’Shaughnessy, J.A. Predictive Biomarker Profiling of > 6000 Breast Cancer Patients Shows Heterogeneity in TNBC, With Treatment Implications. Clin. Breast Cancer 2015, 15, 473–481.e473. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Sheehan, C.E.; Boguniewicz, A.B.; Otto, G.; Downing, S.R.; Sun, J.; He, J.; Curran, J.A.; Ali, S.; et al. Relapsed classic E-cadherin (CDH1)-mutated invasive lobular breast cancer shows a high frequency of HER2 (ERBB2) gene mutations. Clin. Cancer Res. 2013, 19, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef] [PubMed]
- LoRusso, P.M. Inhibition of the PI3K/AKT/mTOR Pathway in Solid Tumors. J. Clin. Oncol. 2016, 34, 3803–3815. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Altomare, D.A.; Testa, J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005, 24, 7455–7464. [Google Scholar] [CrossRef]
- Perez-Tenorio, G.; Alkhori, L.; Olsson, B.; Waltersson, M.A.; Nordenskjold, B.; Rutqvist, L.E.; Skoog, L.; Stal, O. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin. Cancer Res. 2007, 13, 3577–3584. [Google Scholar] [CrossRef]
- Li, J.; Davies, B.R.; Han, S.; Zhou, M.; Bai, Y.; Zhang, J.; Xu, Y.; Tang, L.; Wang, H.; Liu, Y.J.; et al. The AKT inhibitor AZD5363 is selectively active in PI3KCA mutant gastric cancer, and sensitizes a patient-derived gastric cancer xenograft model with PTEN loss to Taxotere. J. Transl. Med. 2013, 11, 241. [Google Scholar] [CrossRef]
- Kim, S.B.; Dent, R.; Im, S.A.; Espie, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef]
- Perez-Garcia, J.; Munoz-Couselo, E.; Soberino, J.; Racca, F.; Cortes, J. Targeting FGFR pathway in breast cancer. Breast 2018, 37, 126–133. [Google Scholar] [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Thike, A.A.; Tan, S.Y.; Chua, P.J.; Bay, B.H.; Tan, P.H. Expression of FGFR1 is an independent prognostic factor in triple-negative breast cancer. Breast Cancer Res. Treat. 2015, 151, 99–111. [Google Scholar] [CrossRef]
- Turner, N.; Pearson, A.; Sharpe, R.; Lambros, M.; Geyer, F.; Lopez-Garcia, M.A.; Natrajan, R.; Marchio, C.; Iorns, E.; Mackay, A.; et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010, 70, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Turczyk, L.; Kitowska, K.; Mieszkowska, M.; Mieczkowski, K.; Czaplinska, D.; Piasecka, D.; Kordek, R.; Skladanowski, A.C.; Potemski, P.; Romanska, H.M.; et al. FGFR2-Driven Signaling Counteracts Tamoxifen Effect on ERalpha-Positive Breast Cancer Cells. Neoplasia 2017, 19, 791–804. [Google Scholar] [CrossRef]
- Shaver, T.M.; Lehmann, B.D.; Beeler, J.S.; Li, C.I.; Li, Z.; Jin, H.; Stricker, T.P.; Shyr, Y.; Pietenpol, J.A. Diverse, Biologically Relevant, and Targetable Gene Rearrangements in Triple-Negative Breast Cancer and Other Malignancies. Cancer Res. 2016, 76, 4850–4860. [Google Scholar] [CrossRef]
- Roidl, A.; Foo, P.; Wong, W.; Mann, C.; Bechtold, S.; Berger, H.J.; Streit, S.; Ruhe, J.E.; Hart, S.; Ullrich, A.; et al. The FGFR4 Y367C mutant is a dominant oncogene in MDA-MB453 breast cancer cells. Oncogene 2010, 29, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Hong, F.; Vaklavas, C.; Cheng, H.H.; Hammerman, P.; Mitchell, E.P.; Zwiebel, J.A.; Ivy, S.P.; Gray, R.J.; Li, S.; et al. Phase II Study of AZD4547 in Patients With Tumors Harboring Aberrations in the FGFR Pathway: Results From the NCI-MATCH Trial (EAY131) Subprotocol W. J. Clin. Oncol. 2020, 38, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.; Smyth, E.; Babina, I.S.; Herrera-Abreu, M.T.; Tarazona, N.; Peckitt, C.; Kilgour, E.; Smith, N.R.; Geh, C.; Rooney, C.; et al. High-Level Clonal FGFR Amplification and Response to FGFR Inhibition in a Translational Clinical Trial. Cancer Discov. 2016, 6, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.J.; Leon-Ferre, R.A.; Sinnwell, J.P.; Zahrieh, D.M.; Suman, V.J.; Metzger, F.O.; Asad, S.; Stover, D.G.; Carey, L.; Sikov, W.M.; et al. Luminal androgen receptor breast cancer subtype and investigation of the microenvironment and neoadjuvant chemotherapy response. NAR Cancer 2022, 4, zcac018. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Murillas, I.; Chopra, N.; Comino-Mendez, I.; Beaney, M.; Tovey, H.; Cutts, R.J.; Swift, C.; Kriplani, D.; Afentakis, M.; Hrebien, S.; et al. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer. JAMA Oncol. 2019, 5, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Cutts, R.J.; Huang, X.; Hrebien, S.; Liu, Y.; Andre, F.; Loibl, S.; Loi, S.; Garcia-Murillas, I.; Cristofanilli, M.; et al. Circulating Tumor DNA Markers for Early Progression on Fulvestrant With or Without Palbociclib in ER+ Advanced Breast Cancer. J. Natl. Cancer Inst. 2021, 113, 309–317. [Google Scholar] [CrossRef]
- Berger, F.; Marce, M.; Delaloge, S.; Hardy-Bessard, A.C.; Bachelot, T.; Bieche, I.; Pradines, A.; De La Motte Rouge, T.; Canon, J.L.; Andre, F.; et al. Randomised, open-label, multicentric phase III trial to evaluate the safety and efficacy of palbociclib in combination with endocrine therapy, guided by ESR1 mutation monitoring in oestrogen receptor-positive, HER2-negative metastatic breast cancer patients: Study design of PADA-1. BMJ Open 2022, 12, e055821. [Google Scholar] [CrossRef]
- Bidard, F.C.; Hardy-Bessard, A.C.; Dalenc, F.; Bachelot, T.; Pierga, J.Y.; de la Motte Rouge, T.; Sabatier, R.; Dubot, C.; Frenel, J.S.; Ferrero, J.M.; et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hancock, B.A.; Solzak, J.P.; Brinza, D.; Scafe, C.; Miller, K.D.; Radovich, M. Next-generation sequencing of circulating tumor DNA to predict recurrence in triple-negative breast cancer patients with residual disease after neoadjuvant chemotherapy. NPJ Breast Cancer 2017, 3, 24. [Google Scholar] [CrossRef]
- Turner, N.C.; Swift, C.; Jenkins, B.; Kilburn, L.; Coakley, M.; Beaney, M.; Fox, L.; Goddard, K.; Garcia-Murillas, I.; Proszek, P.; et al. Results of the c-TRAK TN trial: A clinical trial utilising ctDNA mutation tracking to detect molecular residual disease and trigger intervention in patients with moderate- and high-risk early-stage triple-negative breast cancer. Ann. Oncol. 2023, 34, 200–211. [Google Scholar] [CrossRef]
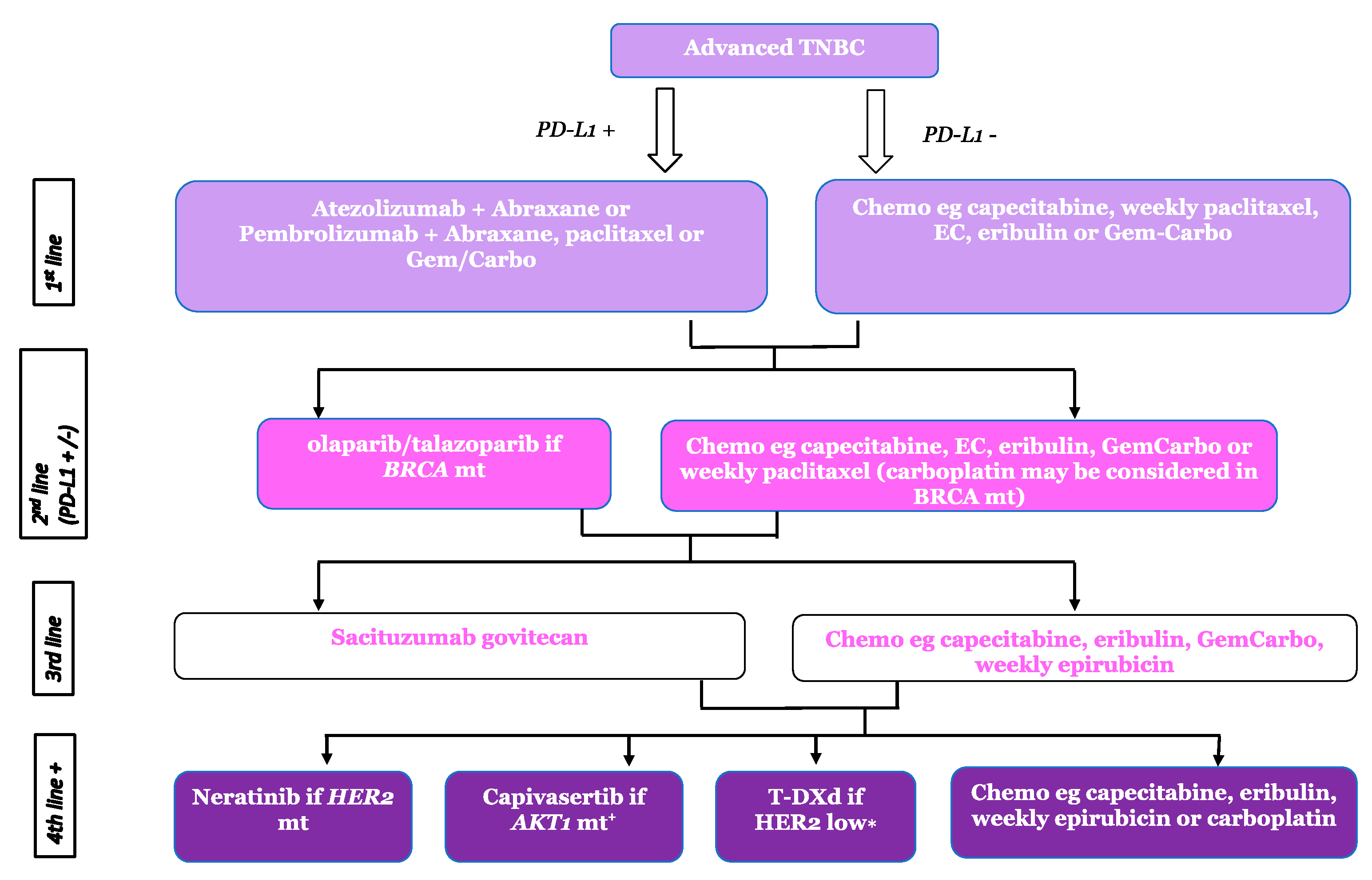
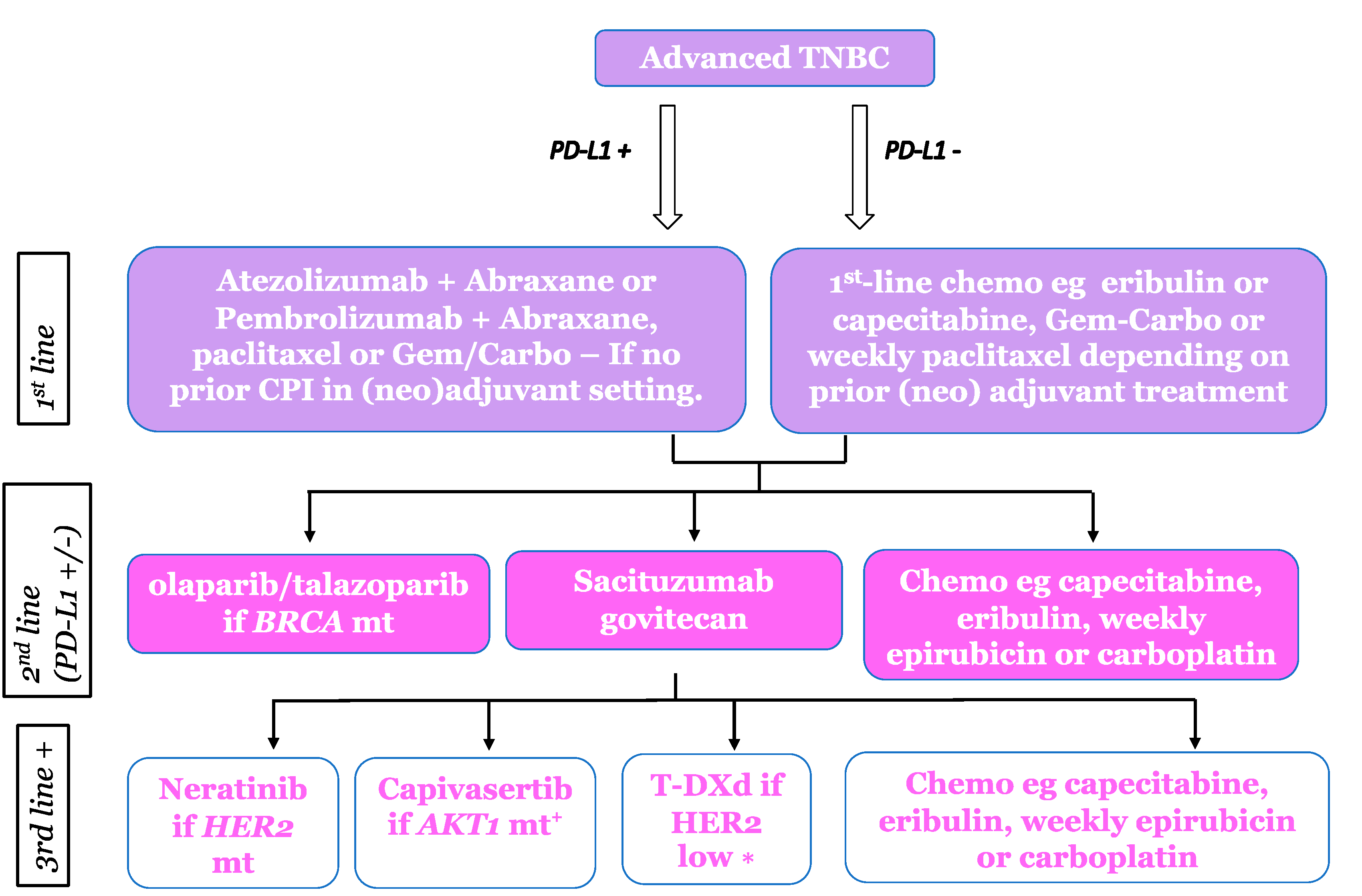
| Trial Name and Sample Size | Treatment Arms | Response Rate (%) | Median PFS/Months | Median OS/Months (ITT) |
|---|---|---|---|---|
| ADCs | ||||
| ASCENT [35] n = 468 | Chemotherapy (Eribulin, vinorelbine, capecitabine or gemcitabine) | 5 | 1.7 | 6.7 |
| Sacituzumab govitecan | 35 | 5.6 | 12.1 | |
| DESTINY BREAST-04 [36] n = 63 with TNBC (ITT 557 HER2 Low) | Chemotherapy (capecitabine, eribulin, gemcitabine, paclitaxel or nab-paclitaxel) | 16.3 | 5.4 | 8.3 (16.8) |
| Trastuzumab deruxtecan | 52.6 | 10.1 | 18.2 (23.4) | |
| PARP Inhibitors | ||||
| OlympiAD [37] n = 150 TNBC (ITT = 302 BRCA mt) | Chemotherapy (capecitabine, eribulin or vinorelbine) | 28.8 | 4.2 | 19.3 |
| Olaparib | 59.9 | 7.0 | 19.6 | |
| EMBRACA [38] n = 190 TNBC (ITT = 431 BRCA mt) | Chemotherapy (capecitabine, eribulin, gemcitabine or vinorelbine) | 27.2 | 5.6 | 19.5 |
| Talazoparib | 67.6 | 8.6 | 22.3 | |
| AKT pathway targeted agents | ||||
| PAKT [39] n = 28 AKT pathway mutations (ITT n = 140) All TNBC | Chemotherapy (paclitaxel) + placebo | 18.2 (28.8) | 3.6 (3.6) | 10.4 (12.6) |
| Chemotherapy (paclitaxel) + Capivasertib | 35.3 (34.8) | 9.3 (5.5) | NR (19.1) | |
| LOTUS [40] n= 42 AKT pathway mutations (ITT n = 124) All TNBC | Chemotherapy (paclitaxel) + placebo | 26 (32) | 4.9 (4.9) | 22.1 (16.9) |
| Chemotherapy (paclitaxel) + ipatasertib | 48 (40) | 9.0 (6.2) | 25.8 (25.8) | |
| HER2 mutation | ||||
| SUMMIT [41] (TNBC) n = 18 (ITT n= 18) | Neratinib + Fulvestrant + Trastuzumab | 33.3 (46.2) | 6.2 | NR |
| AR targeted trials | ||||
| MDV3100-11 [42] n = 83 TNBC (ITT n = 118) | Enzalutamide 160 mg/day | 6 | 2.9 | 12.7 |
| NCT00468715 [43] n = 26 | Bicalutamide 150 mg/day | 0 | 12 weeks | NR |
| UCBG 12-1 [44] n = 34 | Abiraterone 1000 mg/day | 6.7 | 2.8 | NR |
| Clinical Trials.gov Identifier; Trial Name | Patient Population | ADC | Target | Combination or Monotherapy | Phase | Planned (n) |
|---|---|---|---|---|---|---|
| Combination studies of approved ADCs: | ||||||
| NCT04039230 | Metastatic TNBC | Sacituzumab govitecan | TROP-2 | Talozaparib | 1/2 | 75 |
| NCT04468061 | Metastatic TNBC | Sacituzumab govitecan | TROP-2 | Pembrolizumab | Randomised phase 2 | 110 |
| NCT03424005 (MORPHEUS-TNBC) | Metastatic TNBC | Sacituzumab govitecan | TROP-2 | Atezolizumab | 1/2 | 242 |
| NCT05374512 (TROPION-BREAST02) | Metastatic TNBC | Dato-DXd | TROP-2 | Monotherapy | 3 | 600 |
| NCT04556773 (DESTINY-BREAST08) | HER2 low MBC | Trastuzumab deruxtecan | HER2 | Chemotherapy/immunotherapy/hormone therapy | 1b | 139 |
| NCT05382299 (ASCENT-03) | Metastatic TNBC | Sacituzumab govitecan | TROP-2 | Monotherapy | 3 | 540 |
| NCT05382286 (ASCENT-04) | Metastatic TNBC | Sacituzumab govitecan | TROP-2 | Pembrolizumab/chemotherapy | 3 | 440 |
| Novel ADCs in development in breast cancer | ||||||
| NCT04742153 | HER2-low MBC | MRG002 | HER2 | Monotherapy | 2 | 66 |
| NCT04152499 | TNBC (and other solid tumours) | SKB264 | TROP-2 | Monotherapy | 1/2 | 78 |
| NCT04064359 | CD205+ HER2-negative MBC (plus other solid tumours) | OBT076 | CD205 | Monotherapy | 1 | 70 |
| NCT03504488 | TNBC (or NSCLC, or STS) | CAB-ROR2-ADC | ROR2 | Monotherapy | 1/2 | 120 |
| NCT04300556 | TNBC and other selected solid tumours | MORAb-202 | Folate receptor alpha | Monotherapy | 1/2 | 196 |
| NCT03401385 TROPION-PANTUMOUR01) | TNBC and ER+ breast cancer (and NSCLC) | Datopotamab Detuxtecan, | TROP2 | Monotherapy | 1 | 770 |
| NCT03742102 | TNBC | Datopotamab Detuxtecan, | TROP2 | Combination with Durvalumab | 1/2 | 57 |
| NCT04441099 | TNBC and other solid tumours + sarcoma | NBE-002 | ROR1 | Monotherapy | 1/2 | 100 |
| NCT05498597 | TNBC and other solid tumours | AMT-151 | Folate receptor alpha | Monotherapy | 1 | 30 |
| NCT04699630 | TNBC and other solid tumours | US-1402 | HER3 | Monotherapy | 2 | 120 |
| NCT05579366 | TNBC and other solid tumours | PRO1184-001 | Folate receptor alpha | Monotherapy | 1/2 | 134 |
| NCT03310957 | Metastatic TNBC | Ladiratuzumab vedotin | LIV-1 | Pembrolizumab | 1/2 | 211 |
| NCT05866432 (TUXEDO-2) | Metastatic TNBC with brain metastases | Datopotamab deruxtecan | TROP-2 | Monotherapy | 2 | 20 |
| NCT05377996 | TNBC and other solid tumours | XMT-1660 | B7-H4 | MONOTHERAPY | 1 | 166 |
| NCT04925284 (JEWEL-101) | TNBC and other solid tumours | XB002 | Tissue factor | Monotherapy/with nivolumab/bevacizumab | 1 | 561 |
| NCT05208762 | TNBC and other solid tumours | SGN-PD-L1V | PDL1 | Monotherapy | 1 | 315 |
| NCT05194072 | TNBC and other solid tumours | SGN-B7H4V | B7-H4 | Monotherapy | 1 | 400 |
| NCT04225117 | TNBC and other solid tumours | Enfortumab vedotin | Nectin-4 | Monotherapy | 2 | 288 |
| NCT 02980341 | HER3 Positive BC | Patritumab deruxtecan | HER3 | Monotherapy | 1/2 | 184 |
| NCT04699630 | TNBC and other breast cancer subtypes | Patritumab deruxtecan | HER3 | Monotherapy | 2 | 120 |
| NCT05831878 | Advanced HER2 low breast cancer | Disitimab vedotin | HER2 | Monotherapy | N/a | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrison, L.; Okines, A. Systemic Therapy for Metastatic Triple Negative Breast Cancer: Current Treatments and Future Directions. Cancers 2023, 15, 3801. https://doi.org/10.3390/cancers15153801
Morrison L, Okines A. Systemic Therapy for Metastatic Triple Negative Breast Cancer: Current Treatments and Future Directions. Cancers. 2023; 15(15):3801. https://doi.org/10.3390/cancers15153801
Chicago/Turabian StyleMorrison, Laura, and Alicia Okines. 2023. "Systemic Therapy for Metastatic Triple Negative Breast Cancer: Current Treatments and Future Directions" Cancers 15, no. 15: 3801. https://doi.org/10.3390/cancers15153801
APA StyleMorrison, L., & Okines, A. (2023). Systemic Therapy for Metastatic Triple Negative Breast Cancer: Current Treatments and Future Directions. Cancers, 15(15), 3801. https://doi.org/10.3390/cancers15153801






