Retrospective Evaluation of a Single Surgeon’s Learning Curve of Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion via Ileal Conduit
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Elegibility
2.2. Surgeon and Surgical Technique
2.3. Study Data
2.4. Study Objectives
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Pathological Characteristics
3.3. Operation Time
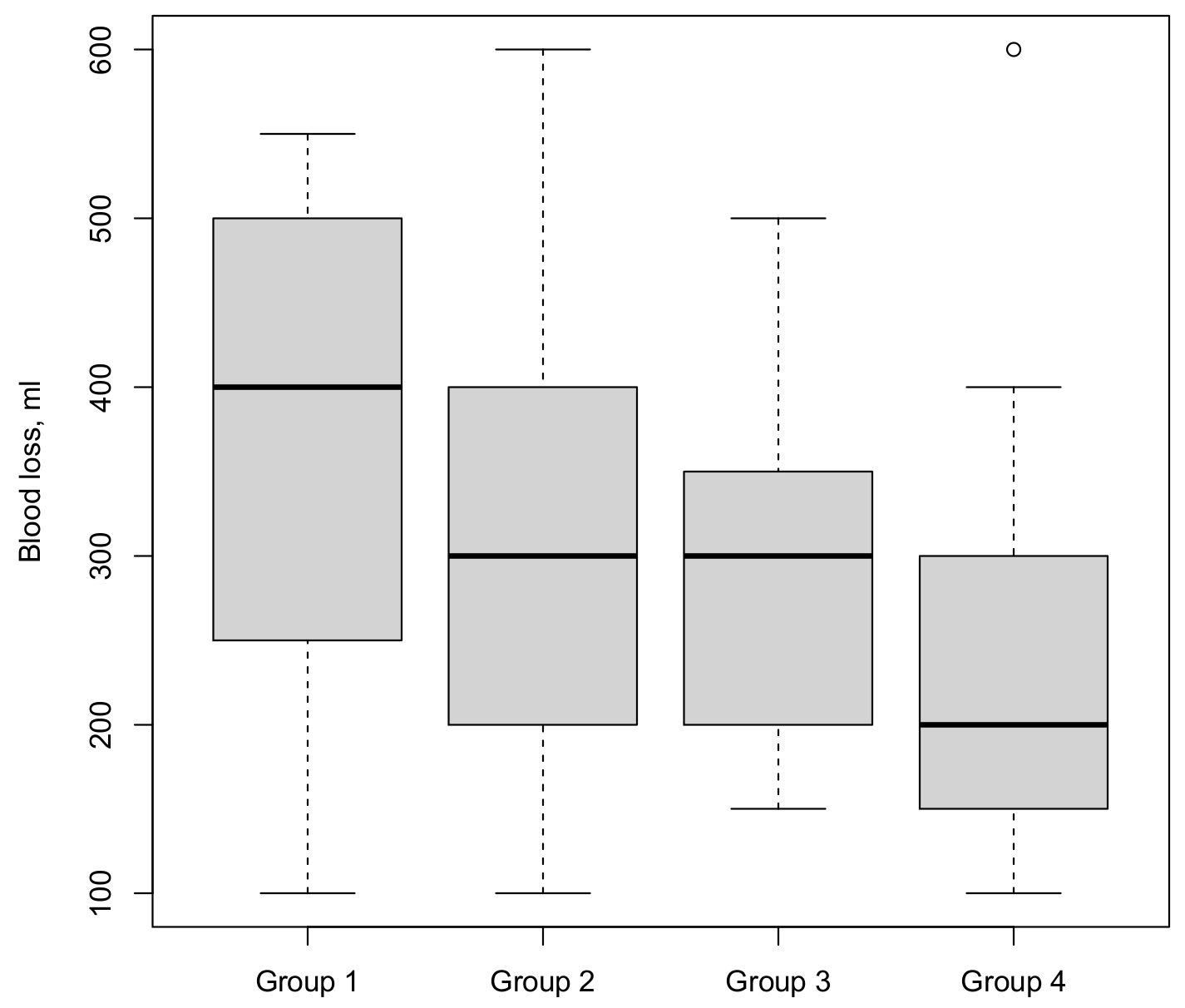
3.4. Blood Loss
3.5. Lymph Node Yield
3.6. Length of Hospital Stay
3.7. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Total | Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|
| Operation date | 01.05.2017–12.03.2019 | 02.04.2019–11.11.2019 | 12.11.2019–02.07.2020 | 30.07.2020–31.12.2021 | |
| Total Cystectomies | 95 | 31 | 17 | 19 | 28 |
| Excluded ORC | −18 | −14 | −4 | 0 | 0 |
| RARC | 77 | 17 | 13 | 19 | 28 |
| Excluded neobladder | −4 | −3 | 0 | 0 | −1 |
| Excluded ureterocutaneostomy | −3 | 0 | 0 | −3 | 0 |
| RARC, Ileum conduit | 70 | 14 | 13 | 16 | 27 |
| Excluded other surgeon | −17 | 0 | 0 | −3 | −14 |
| RARC, Ileum conduit, ASA | 53 | 14 | 13 | 13 | 13 |
| Variable | 1 vs. 2 | 2 vs. 3 | 3 vs. 4 |
|---|---|---|---|
| Median operation time (min) | 0.1 | 0.06 | <0.001 |
| Median blood loss (mL) | 0.3 | 0.8 | 0.1 |
| Median lymph node yield, n (range) | 0.049 | 0.01 | 0.1 |
| Median hospital stay, days | 0.68 | 0.02 | 0.002 |
| 90-day complication rate, n (%) | |||
| Overall | 0.7 | 0.7 | 0.04 |
| Minor | 0.3 | 1 | 0.04 |
| Major | 0.3 | 1 | 1 |
References
- Witjes, J.A.; Bruins, M.; Carrión, A.; Cathomas, R.; Compérat, E.M.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; van der Heijden, A.G.; Lorch, A.; et al. EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer 2023; Edn. presented at the EAU Annual Congress Milan 2023; European Association of Urology Guidelines Office Arnhem: Arnhem, The Netherlands, 2023. [Google Scholar]
- Beecken, W.D.; Wolfram, M.; Engl, T.; Bentas, W.; Probst, M.; Blaheta, R.; Oertl, A.; Jonas, D.; Binder, J. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur. Urol. 2003, 44, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Kobayashi, Y.; Maruyama, Y.; Kawada, T.; Sadahira, T.; Oiwa, Y.; Katayama, S.; Nishimura, S.; Takamoto, A.; Sako, T.; et al. Comparison of intracorporeal versus extracorporeal urinary diversion after robot-assisted radical cystectomy at a medium-sized facility. Int. J. Clin. Oncol. 2021, 26, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Kalapara, A.; Frydenberg, M.; Lawrentschuk, N.; Weight, C.J.; Parekh, D.; Konety, B.R. Robotic Assisted Radical Cystectomy vs Open Radical Cystectomy: Systematic Review and Meta-Analysis. J. Urol. 2019, 201, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.H.; Dalbagni, G.; Sjoberg, D.D.; Silberstein, J.; Keren Paz, G.E.; Donat, S.M.; Coleman, J.A.; Mathew, S.; Vickers, A.; Schnorr, G.C.; et al. Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial. Eur. Urol. 2015, 67, 1042–1050. [Google Scholar] [CrossRef]
- Khan, M.S.; Gan, C.; Ahmed, K.; Ismail, A.F.; Watkins, J.; Summers, J.A.; Peacock, J.L.; Rimington, P.; Dasgupta, P. A Single-centre Early Phase Randomised Controlled Three-arm Trial of Open, Robotic, and Laparoscopic Radical Cystectomy (CORAL). Eur. Urol. 2016, 69, 613–621. [Google Scholar] [CrossRef]
- Nix, J.; Smith, A.; Kurpad, R.; Nielsen, M.E.; Wallen, E.M.; Pruthi, R.S. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: Perioperative and pathologic results. Eur. Urol. 2010, 57, 196–201. [Google Scholar] [CrossRef]
- Parekh, D.J.; Messer, J.; Fitzgerald, J.; Ercole, B.; Svatek, R. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. J. Urol. 2013, 189, 474–479. [Google Scholar] [CrossRef]
- Parekh, D.J.; Reis, I.M.; Castle, E.P.; Gonzalgo, M.L.; Woods, M.E.; Svatek, R.S.; Weizer, A.Z.; Konety, B.R.; Tollefson, M.; Krupski, T.L.; et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): An open-label, randomised, phase 3, non-inferiority trial. Lancet 2018, 391, 2525–2536. [Google Scholar] [CrossRef]
- Hussein, A.A.; May, P.R.; Jing, Z.; Ahmed, Y.E.; Wijburg, C.J.; Canda, A.E.; Dasgupta, P.; Shamim Khan, M.; Menon, M.; Peabody, J.O.; et al. Outcomes of Intracorporeal Urinary Diversion after Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J. Urol. 2018, 199, 1302–1311. [Google Scholar] [CrossRef]
- Jonsson, M.N.; Adding, L.C.; Hosseini, A.; Schumacher, M.C.; Volz, D.; Nilsson, A.; Carlsson, S.; Wiklund, N.P. Robot-assisted radical cystectomy with intracorporeal urinary diversion in patients with transitional cell carcinoma of the bladder. Eur. Urol. 2011, 60, 1066–1073. [Google Scholar] [CrossRef]
- Katayama, S.; Mori, K.; Pradere, B.; Mostafaei, H.; Schuettfort, V.M.; Quhal, F.; Motlagh, R.S.; Laukhtina, E.; Moschini, M.; Grossmann, N.C.; et al. Intracorporeal versus extracorporeal urinary diversion in robot-assisted radical cystectomy: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2021, 26, 1587–1599. [Google Scholar] [CrossRef]
- Cai, Z.; Li, H.; Hu, J.; Qiu, D.; Yi, Z.; Chen, J.; Zu, X. Intracorporeal versus extracorporeal urinary diversion after robot-assisted radical cystectomy: A pooled analysis. Gland. Surg. 2021, 10, 706–720. [Google Scholar] [CrossRef]
- Zhang, J.H.; Ericson, K.J.; Thomas, L.J.; Knorr, J.; Khanna, A.; Crane, A.; Mittal, R.; Zampini, A.; Fascelli, M.; Murthy, P.B.; et al. Large Single Institution Comparison of Perioperative Outcomes and Complications of Open Radical Cystectomy, Intracorporeal Robot-Assisted Radical Cystectomy and Robotic Extracorporeal Approach. J. Urol. 2020, 203, 512–521. [Google Scholar] [CrossRef]
- Herrell, S.D.; Smith, J.A., Jr. Robotic-assisted laparoscopic prostatectomy: What is the learning curve? Urology 2005, 66, 105–107. [Google Scholar] [CrossRef]
- Guru, K.A.; Perlmutter, A.E.; Butt, Z.M.; Piacente, P.; Wilding, G.E.; Tan, W.; Kim, H.L.; Mohler, J.L. The learning curve for robot-assisted radical cystectomy. J. Soc. Laparoendosc. Surg. 2009, 13, 509–514. [Google Scholar] [CrossRef]
- Morozov, A.; Babaevskaya, D.; Taratkin, M.; Inoyatov, J.; Laukhtina, E.; Moschini, M.; Singla, N.; Gomez Rivas, J.; Teoh, J.Y.; Glybochko, P.; et al. Systematic Review: The Learning Curve for Robot-Assisted Radical Cystectomy-What Do We Know? J. Endourol. 2022, 36, 770–784. [Google Scholar] [CrossRef]
- Wilson, T.G.; Guru, K.; Rosen, R.C.; Wiklund, P.; Annerstedt, M.; Bochner, B.H.; Chan, K.G.; Montorsi, F.; Mottrie, A.; Murphy, D.; et al. Best practices in robot-assisted radical cystectomy and urinary reconstruction: Recommendations of the Pasadena Consensus Panel. Eur. Urol. 2015, 67, 363–375. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley: New York, NY, USA, 2022. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wijburg, C.J.; Hannink, G.; Michels, C.T.J.; Weijerman, P.C.; Issa, R.; Tay, A.; Decaestecker, K.; Wiklund, P.; Hosseini, A.; Sridhar, A.; et al. Learning Curve Analysis for Intracorporeal Robot-assisted Radical Cystectomy: Results from the EAU Robotic Urology Section Scientific Working Group. Eur. Urol. Open Sci. 2022, 39, 55–61. [Google Scholar] [CrossRef]
- Lenardis, M.; Harper, B.; Satkunasivam, R.; Klaassen, Z.; Wallis, C.J.D. The association between patient body mass index and perioperative outcomes following radical cystectomy: An analysis using the American College of Surgeons National Surgical Quality Improvement Program database. Can. Urol. Assoc. J. 2020, 14, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Ray-Zack, M.D.; Shan, Y.; Mehta, H.B.; Yu, X.; Kamat, A.M.; Williams, S.B. Hospital length of stay following radical cystectomy for muscle-invasive bladder cancer: Development and validation of a population-based prediction model. Urol. Oncol. 2019, 37, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, D.; Artibani, W.; Graefen, M.; Remzi, M.; Rouprêt, M.; Truss, M. EAU Guidelines on Reporting and Grading of Complications after Urologic Surgical Procedures. Eur. Urol. 2012, 61, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Peyton, C.C.; Reich, R.R.; Tang, D.; Alford, B.; Azizi, M.; Li, R.; Sexton, W.J.; Poch, M.; Gilbert, S.M. Identifying and Codifying Complications after Radical Cystectomy: Comparison of Administrative Diagnostic and Procedure Codes, and Clinical Chart Review. J. Urol. 2019, 202, 913–919. [Google Scholar] [CrossRef]
- Dell’Oglio, P.; Mazzone, E.; Lambert, E.; Vollemaere, J.; Goossens, M.; Larcher, A.; Van Der Jeugt, J.; Devos, G.; Poelaert, F.; Uvin, P.; et al. The Effect of Surgical Experience on Perioperative and Oncological Outcomes After Robot-assisted Radical Cystectomy with Intracorporeal Urinary Diversion: Evidence from a Referral Centre with Extensive Experience in Robotic Surgery. Eur. Urol. Focus 2021, 7, 352–358. [Google Scholar] [CrossRef]
- Porreca, A.; Mineo Bianchi, F.; Romagnoli, D.; D’Agostino, D.; Corsi, P.; Giampaoli, M.; Salvaggio, A.; Bianchi, L.; Schiavina, R.; Brunocilla, E.; et al. Robot-assisted radical cystectomy with totally intracorporeal urinary diversion: Surgical and early functional outcomes through the learning curve in a single high-volume center. J. Robot. Surg. 2020, 14, 261–269. [Google Scholar] [CrossRef]
- Novara, G.; Catto, J.W.; Wilson, T.; Annerstedt, M.; Chan, K.; Murphy, D.G.; Motttrie, A.; Peabody, J.O.; Skinner, E.C.; Wiklund, P.N.; et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur. Urol. 2015, 67, 376–401. [Google Scholar] [CrossRef]




| Variable | Overall n = 53 | First Group n = 14 | Second Group n = 13 | Third Group n = 13 | Fourth Group n = 13 | p Value |
|---|---|---|---|---|---|---|
| Sex female (%) | 9 (17) | 3 (21) | 2 (15) | 4 (31) | 0 (0) | 0.2 |
| Median age, years (IQR) | 72 (66–78) | 69 (63–80) | 71 (66–72) | 73 (66–76) | 74 (69–80) | 0.8 |
| Median BMI, kg/m2 (interquartile range) | 25.7 (23.7–29.1) | 27.8 (25.2–30.3) | 25.4 (23.4–27.5) | 25.0 (24.1–28.3) | 25.2 (22.9–29.0) | 0.3 |
| American Society of Anesthesiology Class (%) | ||||||
| II | 26 (49) | 8 (57) | 5 (38) | 6 (46) | 7 (54) | 0.8 |
| III | 27 (51) | 6 (43) | 8 (62) | 7 (54) | 6 (46) | |
| Charlson comorbidity index (%) | ||||||
| 0 | 36 (68) | 8 (57) | 10 (77) | 9 (69) | 9 (69) | 0.8 |
| 1 | 8 (15) | 1 (7) | 2 (15) | 2 (15) | 3 (23) | |
| 2 | 7 (13) | 4 (29) | 1 (8) | 1 (8) | 1 (8) | |
| 3 | 2 (4) | 1 (7) | 0 (0) | 1 (8) | 0 (0) | |
| Previous treatment (%) | ||||||
| BCG instillations | 6 (11) | 1 (7) | 1 (8) | 1 (8) | 3 (23) | 0.7 |
| Chemotherapy instillation | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (15) | 0.2 |
| Neoadjuvant chemotherapy | 6 (11) | 3 (21) | 3 (23) | 0 (0) | 0 (0) | 0.07 |
| Thereof, CP-based | 3 (6) | 1 (7) | 2 (15) | 0 (0) | 0 (0) | 0.5 |
| Variable | Overall | First Group | Second Group | Third Group | Fourth Group | p Value |
|---|---|---|---|---|---|---|
| Pathological tumor stage (%) | ||||||
| <T2 | 12 (23) | 0 (0) | 3 (23) | 4 (31) | 5 (39) | 0.04 |
| T2 | 18 (34) | 9 (64) | 2 (15) | 3 (23) | 4 (31) | |
| T3 | 17 (32) | 3 (21) | 7 (54) | 3 (23) | 4 (31) | |
| T4 | 6 (11) | 2 (14) | 1 (8) | 3 (23) | 0 (0) | |
| Pathological lymph node status (%) | ||||||
| pN0, Nx | 41 (77) | 10 (71) | 12 (92) | 10 (77) | 9 (69) | 0.6 |
| pN1, N2 | 12 (23) | 4 (29) | 1 (8) | 3 (23) | 4 (31) | |
| Positive surgical margins (%) | 5 (9) | 0 (0) | 1 (8) | 4 (31) | 0 (0) | 0.02 |
| Overall | First Group | Second Group | Third Group | Fourth Group | p Value Groups | p Value Continuous | |
|---|---|---|---|---|---|---|---|
| Median operation time, min (IQR) | 396 (365–432) | 415 (397–432) | 390 (371–415) | 441 (421–445) | 361 a (283–372) | 0.02 2 | 0.01 3 |
| Median blood loss, mL(IQR) | 300 (200–400) | 400 (263–500) | 300 (200–400) | 300 (200–350) | 200 (150–300) | 0.01 2 | 0.01 3 |
| Median lymph node yield, n (IQR) | 20 (16–31) | 19 (14–28) | 29 a (20–35) | 19 a (15–20) | 23 (17–32) | 0.8 2 | 0.8 3 |
| Median hospital stay, days (IQR) | 16 (15–22) | 16 (13–26) | 16 (15–18) | 22 (17–26) | 16 a (15–16) | 0.8 2 | 0.8 3 |
| 90-day complication rate, n (%) | |||||||
| Overall | 36 (68) | 11 (79) | 9 (69) | 11 (85) | 5 a (38) | 0.07 1 | 0.1 4 |
| Minor | 26 (49) | 10 (71) | 6 (46) | 8 (62) | 2 a (15) | 0.02 1 | 0.03 4 |
| Major | 10 (19) | 1 (7) | 3 (23) | 3 (23) | 3 (23) | 0.7 1 | 0.3 4 |
| Intraoperative complication rate, n (%) | 1 (2) | 0 (0) | 0 (0) | 1 | 1 (8) | 0.17 1 | 0.3 4 |
| Surgical Experience | Age | Body Mass Index | Tumor Stage (T4) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Operation parameters | ||||||||
| Operation time | −1.1 (−2.1 to −0.1) | 0.04 | −1.2 (−2.8 to 0.5) | 0.2 | 2.9 (−1.0 to 6.9) | 0.14 | 62 (14 to 110) | 0.01 |
| Blood loss | −2.2 (−4.7 to 0.2) | 0.08 | −1.3 (−5.3 to 2.7) | 0.5 | 10.9 (1.4 to 20.3) | 0.03 | 45 (−70 to 159) | 0.4 |
| Hospital stay | −0.06 (−0.2 to 0.1) | 0.4 | 0.3 (0.02 to 0.5) | 0.04 | −0.5 (−1.1 to 0.08) | 0.09 | 6.0 (−0.9 to 13) | 0.09 |
| Lymph node yield | −0.04 (−0.3 to 0.2) | 0.7 | 0.1 (−0.2 to 0.5) | 0.4 | −0.2 (−1.0 to 0.6) | 0.6 | 2.3 (−7.2 to 12) | 0.6 |
| Positive surgical margins | 1.1 (1.0 to 1.4) | 0.2 | 1.0 (0.8 to 1.4) | 0.8 | 1.1 (0.7 to 1.6) | 0.63 | 754 (18 to 2′227′969) | 0.01 |
| Complication rate | ||||||||
| Overall | 1.0 (1.0 to 1.1) | 0.2 | 1.1 (1.0 to 1.2) | 0.1 | 1.1 (0.9 to 1.3) | 0.5 | 0.6 (0.03 to 4.7) | 0.7 |
| Minor | 1.0 (0.9 to 1.0) | 0.08 | 0.9 (0.8 to 0.98) | 0.02 | 1.1 (0.9 to 1.3) | 0.4 | 0.8 (0.1 to 5.1) | 0.8 |
| Major | 1.0 (1.0 to 1.1) | 0.7 | 1.1 (1.0 to 1.2) | 0.2 | 0.8 (0.6 to 1.0) | 0.1 | 2.6 (0.3 to 21) | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achermann, C.; Sauer, A.; Cattaneo, M.; Walz, J.; Wyler, S.F.; Kwiatkowski, M.; Prause, L.W. Retrospective Evaluation of a Single Surgeon’s Learning Curve of Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion via Ileal Conduit. Cancers 2023, 15, 3799. https://doi.org/10.3390/cancers15153799
Achermann C, Sauer A, Cattaneo M, Walz J, Wyler SF, Kwiatkowski M, Prause LW. Retrospective Evaluation of a Single Surgeon’s Learning Curve of Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion via Ileal Conduit. Cancers. 2023; 15(15):3799. https://doi.org/10.3390/cancers15153799
Chicago/Turabian StyleAchermann, Christof, Andreas Sauer, Marco Cattaneo, Jochen Walz, Stephen F. Wyler, Maciej Kwiatkowski, and Lukas W. Prause. 2023. "Retrospective Evaluation of a Single Surgeon’s Learning Curve of Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion via Ileal Conduit" Cancers 15, no. 15: 3799. https://doi.org/10.3390/cancers15153799
APA StyleAchermann, C., Sauer, A., Cattaneo, M., Walz, J., Wyler, S. F., Kwiatkowski, M., & Prause, L. W. (2023). Retrospective Evaluation of a Single Surgeon’s Learning Curve of Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion via Ileal Conduit. Cancers, 15(15), 3799. https://doi.org/10.3390/cancers15153799







