Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT): Patient Characteristics, Treatment, and Outcome—A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
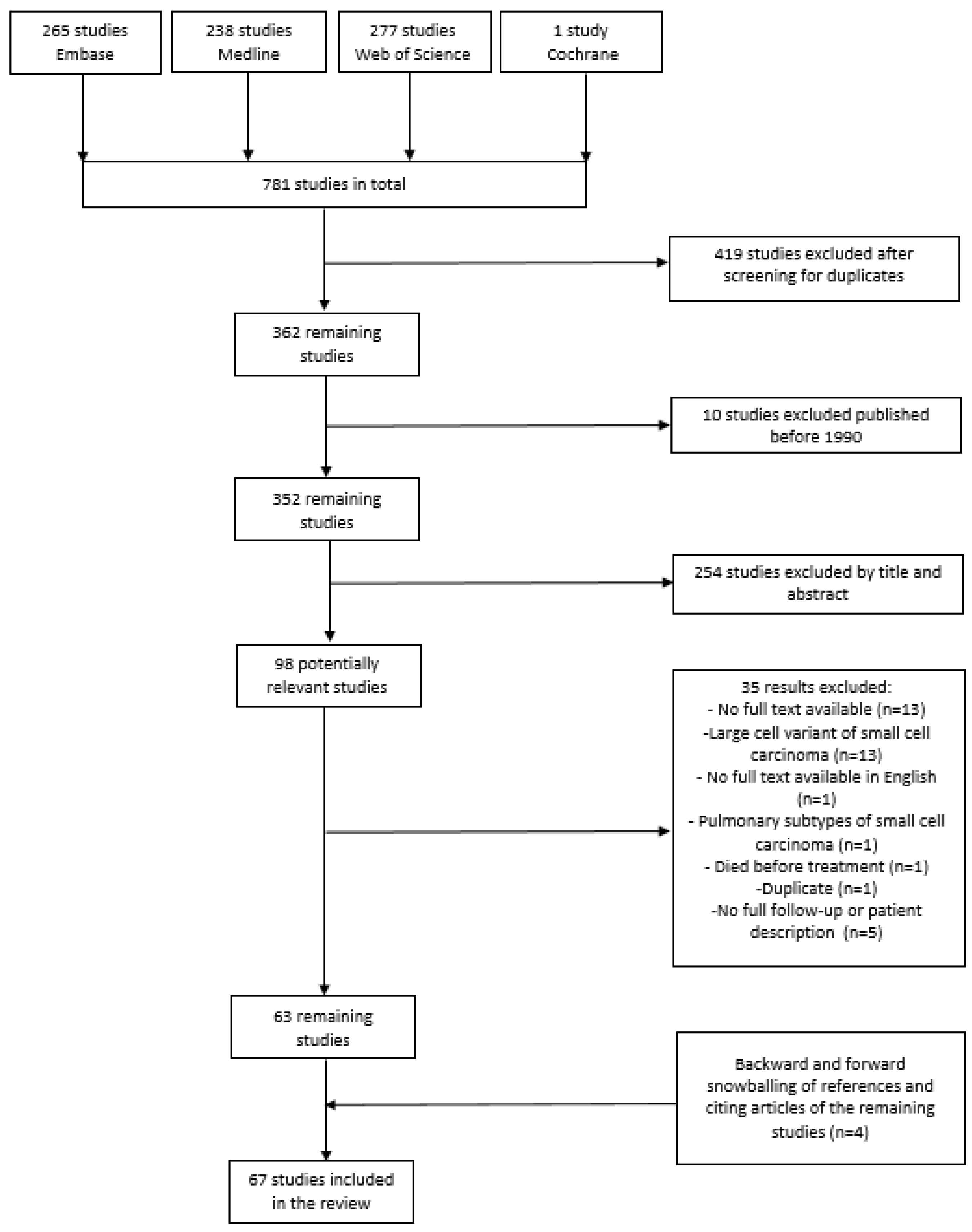
2.1. Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Quality Assessment
2.4. Data Collection and Data Items
2.5. Statistical Analyses
3. Results
3.1. Search and Selection
3.2. Quality Assessment
3.3. Patient Characteristics
3.4. Treatment
3.4.1. Surgery
3.4.2. Chemotherapy and HDC with ASCT
3.4.3. Radiotherapy
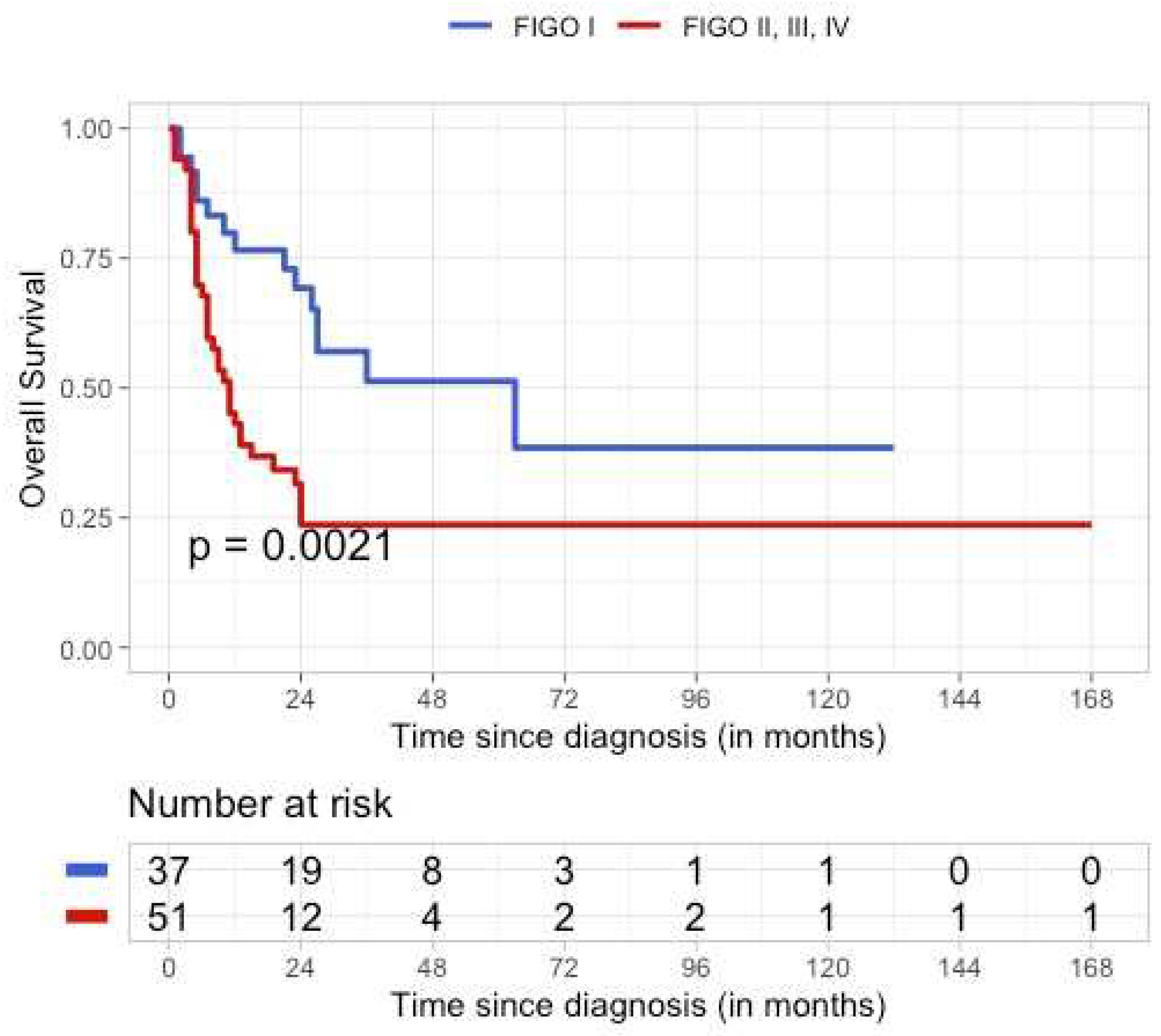
3.5. Outcome
3.6. Potential Targeted Therapies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, R.H.; Goodman, A.; Penson, R.T.; Russell, A.H.; Uppot, R.N.; Tambouret, R.H. Case records of the Massachusetts General Hospital. Case 8-2010. A 22-year-old woman with hypercalcemia and a pelvic mass. N. Engl. J. Med. 2010, 362, 1031–1040. [Google Scholar] [CrossRef] [Green Version]
- Pressey, J.G.; Dandoy, C.E.; Pater, L.E.; Sroga Rios, J.; Sisson, R.; Dasgupta, R.; Szabo, S. Small cell carcinoma of the ovary hypercalcemic type (SCCOHT): Comprehensive management of a newly diagnosed young adult. Gynecol. Oncol. 2020, 158, 538–546. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs (IARC WHO Classification of Tumours); International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Brennan, B.; Stiller, C.; Bourdeaut, F. Extracranial rhabdoid tumours: What we have learned so far and future directions. Lancet Oncol. 2013, 14, e329–e336. [Google Scholar] [CrossRef]
- Young, R.H.; Oliva, E.; Scully, R.E. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am. J. Surg. Pathol. 1994, 18, 1102–1116. [Google Scholar] [CrossRef]
- Prat, J.; Belhadj, H.; Berek, J.; Bermudez, A.; Bhatla, N.; Cain, J.; Denny, L.; Fujiwara, K.; Hacker, N.; Åvall-Lundqvist, E. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- Dykgraaf, R.H.; de Jong, D.; van Veen, M.; Ewing-Graham, P.C.; Helmerhorst, T.J.; van der Burg, M.E. Clinical management of ovarian small-cell carcinoma of the hypercalcemic type: A proposal for conservative surgery in an advanced stage of disease. Int. J. Gynecol. Cancer 2009, 19, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv1–iv18. [Google Scholar] [CrossRef]
- Longy, M.; Toulouse, C.; Mage, P.; Chauvergne, J.; Trojani, M. Familial cluster of ovarian small cell carcinoma: A new mendelian entity. J. Med. Genet. 1996, 33, 333–335. [Google Scholar] [CrossRef]
- Witkowski, L.; Carrot-Zhang, J.; Albrecht, S.; Fahiminiya, S.; Hamel, N.; Tomiak, E.; Grynspan, D.; Saloustros, E.; Nadaf, J.; Rivera, B. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat. Genet. 2014, 46, 438–443. [Google Scholar] [CrossRef]
- Tischkowitz, M.; Huang, S.; Banerjee, S.; Hague, J.; Hendricks, W.P.D.; Huntsman, D.G.; Lang, J.D.; Orlando, K.A.; Oza, A.M.; Pautier, P. Small-Cell Carcinoma of the Ovary, Hypercalcemic Type-Genetics, New Treatment Targets, and Current Management Guidelines. Clin. Cancer Res. 2020, 26, 3908–3917. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claes, W. Guidelines for snowballing in systematic literature studies and a replication in software engineering. ACM Int. Conf. Proceeding Ser. 2014, 38, 1–10. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taraszewski, R.; Rosman, P.M.; Knight, C.A.; Cloney, D.J. Small cell carcinoma of the ovary. Gynecol. Oncol. 1991, 41, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.L.; Jensen, M.L.; Nielsen, K.M.; Velander, G. Small cell carcinoma of the ovary diagnosed in early pregnancy. Acta Obstet. Gynecol. Scand. 1991, 70, 377–379. [Google Scholar] [CrossRef]
- Peccatori, F.; Bonazzi, C.; Lucchini, V.; Bratina, G.; Mangioni, C. Primary ovarian small cell carcinoma: Four more cases. Gynecol. Oncol. 1993, 49, 95–99. [Google Scholar] [CrossRef]
- Reed, W.C. Small cell carcinoma of the ovary with hypercalcemia: Report of a case of survival without recurrence 5 years after surgery and chemotherapy. Gynecol. Oncol. 1995, 56, 452–455. [Google Scholar] [CrossRef]
- Selvaggi, S.M. Small-cell carcinoma of the ovary in peritoneal fluid. Diagn. Cytopathol. 1994, 11, 266–270. [Google Scholar] [CrossRef]
- Tewari, K.; Brewer, C.; Cappuccini, F.; Macri, C.; Rogers, L.W.; Berman, M.L. Advanced-stage small cell carcinoma of the ovary in pregnancy: Long-term survival after surgical debulking and multiagent chemotherapy. Gynecol. Oncol. 1997, 66, 531–534. [Google Scholar] [CrossRef]
- Meganck, G.; Moerman, P.; De Schrijver, D.; Berteloot, P.; Vergrote, I. A non-diploid, small cell carcinoma of the ovary of the hypercalcemic type. Int. J. Gynecol. Cancer 1998, 8, 430–433. [Google Scholar] [CrossRef]
- Schleef, J.; Wagner, A.; Kleta, R.; Schaarschmidt, K.; Dockhorn-Dworniczak, B.; Willital, G.; Jürgens, H. Small-cell carcinoma of the ovary of the hypercalcemic type in an 8-year-old girl. Pediatr. Surg. Int. 1999, 15, 431–434. [Google Scholar] [CrossRef]
- Ferrera, P.C.; Whitman, M.C. Ovarian small cell carcinoma: A rare neoplasm in a 15-year-old female. Pediatr. Emerg. Care 2000, 16, 170–172. [Google Scholar] [CrossRef]
- Schweiger, L.M.; Hsiang, H.Y. Pathological case of the month. Acute symptomatic hypercalcemia associated with ovarian small cell carcinoma. Arch. Pediatr. Adolesc. Med. 2002, 156, 83–84. [Google Scholar] [CrossRef] [Green Version]
- Wynn, D.; Everett, G.D.; Boothby, R.A. Small cell carcinoma of the ovary with hypercalcemia causes severe pancreatitis and altered mental status. Gynecol. Oncol. 2004, 95, 716–718. [Google Scholar] [CrossRef]
- Chen, L.; Dinh, T.A.; Haque, A. Small cell carcinoma of the ovary with hypercalcemia and ectopic parathyroid hormone production. Arch. Pathol. Lab. Med. 2005, 129, 531–533. [Google Scholar] [CrossRef]
- Sholler, G.L.; Luks, F.; Mangray, S.; Meech, S.J. Advanced small cell carcinoma of the ovary in a pediatric patient with long-term survival and review of the literature. J. Pediatr. Hematol. Oncol. 2005, 27, 169–172. [Google Scholar] [CrossRef]
- Chen, F.; Koenig, C.; Heller, D.S. A 26-year-old woman with right ovarian mass. Small cell carcinoma of ovary, hypercalcemic type. Arch. Pathol. Lab. Med. 2006, 130, e56–e58. [Google Scholar] [CrossRef]
- Distelmaier, F.; Calaminus, G.; Harms, D.; Sträter, R.; Kordes, U.; Fleischhack, G.; Göbel, U.; Schneider, D.T. Ovarian small cell carcinoma of the hypercalcemic type in children and adolescents: A prognostically unfavorable but curable disease. Cancer 2006, 107, 2298–2306. [Google Scholar] [CrossRef]
- Harrison, M.L.; Hoskins, P.; du Bois, A.; Quinn, M.; Rustin, G.J.; Ledermann, J.A.; Baron-Hay, S.; Friedlander, M.L. Small cell of the ovary, hypercalcemic type—Analysis of combined experience and recommendation for management. A GCIG study. Gynecol. Oncol. 2006, 100, 233–238. [Google Scholar] [CrossRef]
- Sassi, S.H.; Bettaïeb, I.; Abbes, I.; Mrad, K.; Dhouib, R.; Driss, M. Small cell carcinoma of the ovary, hypercalcemic type in a pediatric patient: Case report. Case Rep. Clin. Pract. Rev. 2007, 8, 36–39. [Google Scholar]
- Christin, A.; Lhomme, C.; Valteau-Couanet, D.; Dubrel, M.; Hartmann, O. Successful treatment for advanced small cell carcinoma of the ovary. Pediatr. Blood Cancer 2008, 50, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Isonishi, S.; Nishii, H.; Saitou, M.; Yasuda, M.; Kiyokawa, T.; Fukunaga, M.; Ishikawa, H.; Tanaka, T. Small cell carcinoma of the ovary: Clinical and biological study. Int. J. Clin. Oncol. 2008, 13, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Kaneki, E.; Kijima, M.; Kodama, K.; Yamaguchi, S.; Yagi, H.; Yasunaga, M.; Ohgami, T.; Onoyama, I.; Okugawa, K. Two types of small cell carcinoma of the ovary: Two typical case reports. Gynecol. Oncol. Rep. 2018, 25, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Beattie, G.J.; Williams, A.R. Small cell carcinoma of the ovary: A report of three cases and review of the literature. J. Obstet. Gynaecol. 2004, 24, 169–172. [Google Scholar] [CrossRef] [PubMed]
- McCormick, T.C.; Muffly, T.; Lu, G.; Shoup, B. Aggressive small cell carcinoma of the ovary, hypercalcemic type with hypercalcemia in pregnancy, treated with conservative surgery and chemotherapy. Int. J. Gynecol. Cancer 2009, 19, 1339–1341. [Google Scholar] [CrossRef]
- Barondeau, J.; Rodgers, M.; Braun, L.; Azarow, K.; Forouhar, M.; Faucette, K. Small cell ovarian carcinoma: A rare, aggressive tumor masquerading as constipation in a teenager with a fatal outcome. J. Pediatr. Hematol. Oncol. 2010, 32, e139–e141. [Google Scholar] [CrossRef]
- Shrimali, R.K.; Correa, P.D.; Reed, N.S. Dose-dense and dose-intense chemotherapy for small cell ovarian cancer: 2 cases and review of literature. Med. Oncol. 2011, 28, 766–770. [Google Scholar] [CrossRef]
- Montalto, S.A.; Gupta, A.; Hakmi, A.; Raju, K.S. Small cell carcinoma of the ovary: Hypercalcaemic type. J. Obstet. Gynaecol. 2011, 31, 199–200. [Google Scholar] [CrossRef]
- Rovithi, M.; Pallis, A.G.; Kalykaki, A.; Lagoudaki, E.; Giannikaki, L.; Stathopoulos, E.N.; Relakis, K.; Georgoulias, V. Small cell ovarian cancer in adolescents: Report of two cases and review of the literature. Case Rep. Med. 2011, 2011, 749516. [Google Scholar] [CrossRef] [Green Version]
- Stephens, B.; Anthony, S.P.; Han, H.; Kiefer, J.; Hostetter, G.; Barrett, M.; Von Hoff, D.D. Molecular Characterization of a Patient’s Small Cell Carcinoma of the Ovary of the Hypercalcemic Type. J. Cancer 2012, 3, 58–66. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.M.; Karabakhtsian, R.G.; Pierce, H.H.; Iocono, J.A.; Desimone, C.P.; Bayliff, S.L.; Ueland, F.R. Small cell carcinoma of the ovary of hypercalcemic type: A case report. J. Pediatr. Surg. 2012, 47, 588–592. [Google Scholar] [CrossRef]
- Wallbillich, J.J.; Nick, A.M.; Ramirez, P.T.; Watkins, J.L.; Euscher, E.D.; Schmeler, K.M. Vinblastine, cisplatin, cyclophosphamide, bleomycin, doxorubicin, and etoposide (VPCBAE) in the management of three patients with small-cell carcinoma of the ovary. Gynecol. Oncol. Case Rep. 2012, 2, 58–60. [Google Scholar] [CrossRef] [Green Version]
- Woopen, H.; Sehouli, J.; Pietzner, K.; Darb-Esfahani, S.; Braicu, E.I.; Fotopoulou, C. Clinical experience of young patients with small cell ovarian carcinoma of the hypercalcemic type (OSCCHT). Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 313–317. [Google Scholar] [CrossRef]
- Zaied, S.; Gharbi, O.; Zayène, A.; Rjiba, R.; Hadhri, R.; Hadded, A.; Hochlef, M.; Ben Fatma, L.; Ben Ahmed, S. Small cell carcinoma of the ovary of hypercalcemic type: A case report. Case Rep. Oncol. Med. 2012, 2012, 461873. [Google Scholar] [CrossRef] [Green Version]
- Zagouri, F.; Thomakos, N.; Rodolakis, A.; Bamias, A.; Chalazonitis, A.; Sotiropoulou, M.; Antsaklis, A.; Dimopoulos, M.; Papadimitriou, C.A. Quiz case: A 19-year-old woman with hypercalcemia and abdominal pain. Onkologie 2012, 35, 126–127. [Google Scholar] [CrossRef]
- Origoni, M.; Bergamini, A.; Pella, F.; Ottolina, J.; Giorgione, V.; Del Prato, D.; Almirante, G.; Candiani, M. Small Cell Carcinoma of the Ovary: Report of Three Cases of a Poor Prognosis Disease. J. Med. Cases 2013, 4, 189. [Google Scholar] [CrossRef]
- Bitton, R.C.; Mak, M.P.; Takahashi, T.K.; Aguiar, F.N.; Abdo, E.; Diz, M.D.P.E. Advanced small-cell ovarian carcinoma, hypercalcemic type: A challenging therapeutic entity. Autops. Case Rep. 2014, 4, 47–52. [Google Scholar] [CrossRef]
- Ghribi, A.; Bouden, A.; Gasmi, M.; Hamzaoui, M. Unusual malignant neoplasms of ovary in children: Two cases report. Korean J. Pediatr. 2016, 59 (Suppl. 1), S107–S111. [Google Scholar] [CrossRef] [Green Version]
- Benito Reyes, V.; Martínez Lanao, D.; Lubrano Rosales, A.; Arencibia Sánchez, O.; Falcón Vizcaíno, O. Ovarian small cell carcinoma of hypercalcaemic-type: History of the disease in a patient who refused treatment. J. Obstet. Gynaecol. 2011, 31, 354–356. [Google Scholar] [CrossRef]
- Ilić, M.B.; Jovanović, D.V.; Milosavljević, M.Z.; Stanković, V.; Djordjević, G.; Protrka, Z.; Nedović, J.; Mitrović, S.L.J. Hypercalcemic type of small cell carcinoma of the ovary. Vojnosanit. Pregl. 2015, 72, 295–298. [Google Scholar] [CrossRef]
- Bailey, S.; Murray, M.J.; Witkowski, L.; Hook, E.; Hasselblatt, M.; Crawford, R.; Foulkes, W.D.; Tischkowitz, M.; Nicholson, J.C. Biallelic somatic SMARCA4 mutations in small cell carcinoma of the ovary, hypercalcemic type (SCCOHT). Pediatr. Blood Cancer 2015, 62, 728–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agaimy, A.; Thiel, F.; Hartmann, A.; Fukunaga, M. SMARCA4-deficient undifferentiated carcinoma of the ovary (small cell carcinoma, hypercalcemic type): Clinicopathologic and immunohistochemical study of 3 cases. Ann. Diagn. Pathol. 2015, 19, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, A.; Ayaz, A.; Hamid, T.; Farooq, M.U.; Islam, N. Small cell carcinoma of the ovary hypercalcemic type (SCCOHT): A rare case after in vitro fertilization (IVF). Pak. J. Med. Sci. 2017, 33, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Garg, R.; Garcia, R.; Swisher, E. Small cell ovarian carcinoma: Long term survival in juvenile case with poor prognostic features. Gynecol. Oncol. Rep. 2016, 18, 45–48. [Google Scholar] [CrossRef]
- Kascak, P.; Zamecnik, M.; Bystricky, B. Small Cell Carcinoma of the Ovary (Hypercalcemic Type): Malignant Rhabdoid Tumor. Case Rep. Oncol. 2016, 9, 305–311. [Google Scholar] [CrossRef]
- Lavrut, P.M.; Le Loarer, F.; Normand, C.; Grosos, C.; Dubois, R.; Buenerd, A.; Conter, C.; Dijoud, F.; Blay, J.; Collardeau-Frachon, S. Small Cell Carcinoma of the Ovary, Hypercalcemic Type: Report of a Bilateral Case in a Teenager Associated with SMARCA4 Germline Mutation. Pediatr. Dev. Pathol. 2016, 19, 56–60. [Google Scholar] [CrossRef]
- Agarwal, S.; Pandey, P.; Ralli, M.; Singh, S. Ovarian Small Cell Carcinoma: A Rare Case Report and Review of Literature. Iran. J. Pathol. 2018, 13, 99–102. [Google Scholar]
- Najib, F.S.; Momtahan, M.; Mokhtari, M.; Alamdarloo, S.M.; Poordast, T. Small Cell Carcinoma of the Ovary: Report of a Case with Unusual and Aggressive Presentation. Middle East J. Cancer 2017, 8, 161–165. [Google Scholar]
- Qin, Q.; Ajewole, V.B.; Sheu, T.G.; Donohue, R.; Singh, M. Successful treatment of a stage IIIC small-cell carcinoma of the ovary hypercalcemic subtype using multi-modality therapeutic approach. Ecancermedicalscience 2018, 12, 832. [Google Scholar] [CrossRef] [Green Version]
- David, M.P.; Venkatramani, R.; Lopez-Terrada, D.H.; Roy, A.; Patil, N.; Fisher, K.E. Multimodal molecular analysis of an atypical small cell carcinoma of the ovary, hypercalcemic type. Cold Spring Harb Mol. Case Stud. 2018, 4, a002956. [Google Scholar] [CrossRef] [Green Version]
- Kalogeropoulos, S.; Chronopoulou, E.; Kourea, E.; Siampalis, A.; Kaponis, A.; Decavalas, G.O. Small cell ovarian carcinoma hypercalcemic type and endometrial adenocarcinoma in a 49-year-old patient: A very rare case. Eur. J. Gynaecol. Oncol. 2019, 40, 1064–1066. [Google Scholar]
- Khosla, D.; Gupta, N.; Koshy, A.; Dalal, A.; Pandey, A.K.; Dimri, K. Ovarian Small Cell Carcinoma of Hypercalcemic Type in an Adolescent Girl. J. Obstet. Gynaecol. India 2019, 69 (Suppl. 1), 60–62. [Google Scholar] [CrossRef]
- Connor, Y.D.; Miao, D.; Lin, D.I.; Hayne, C.; Howitt, B.E.; Dalrymple, J.L.; DeLeonardis, K.R.; Hacker, M.R.; Esselen, K.M.; Shea, M. Germline mutations of SMARCA4 in small cell carcinoma of the ovary, hypercalcemic type and in SMARCA4-deficient undifferentiated uterine sarcoma: Clinical features of a single family and comparison of large cohorts. Gynecol. Oncol. 2020, 157, 106–114. [Google Scholar] [CrossRef]
- Mathey, M.P.; de Jolinière, J.B.; Major, A.; Conrad, B.; Khomsi, F.; Betticher, D.; Devouassoux, M.; Feki, A.L. Rare case of remission of a patient with small cell carcinoma of the ovary, hypercalcaemic type (SCCOHT) stage IV: Case report. Int. J. Surg. Case Rep. 2020, 66, 398–403. [Google Scholar] [CrossRef]
- Feng, M.; Yang, K.; Xu, L.; Zhang, Y.; Zou, J. Primary ovarian small cell carcinoma of hypercalcemic type in a pregnant woman: A case report. Medicine 2020, 99, e20387. [Google Scholar] [CrossRef]
- Gupta, P.; Kapatia, G.; Gupta, N.; Dey, P.; Rohilla, M.; Gupta, A.; Rai, B.; Suri, V.; Rajwanshi, A.; Srinivasan, R. Small Cell Carcinoma of the Ovary: Clinicopathologic and Immunohistochemical Analysis of 7 New Cases of a Rare Malignancy. Int. J. Surg. Pathol. 2021, 29, 236–245. [Google Scholar] [CrossRef]
- Blanc-Durand, F.; Lefeuvre-Plesse, C.; Ray-Coquard, I.; Chaltiel, D.; Floquet, A.; Meriaux, É.; Berton, D.; Bello-Roufai, D.; Guillemet, C.; Dupre, P. Dose-intensive regimen treatment for small-cell carcinoma of the ovary of hypercalcemic type (SCCOHT). Gynecol. Oncol. 2020, 159, 129–135. [Google Scholar] [CrossRef]
- Sahay, A.; Kante, K.; Menon, S.; Ghosh, J.; Kerkar, R.A.; Deodhar, K.K. Small Cell Carcinoma of Ovary, Hypercalcemic Type (Malignant Rhabdoid Tumor of Ovary) with Loss of SMARCA4 (BRG1) Expression: Report of Two Cases. Turk. Patoloji Derg. 2020, 1, 261–267. [Google Scholar] [CrossRef]
- Montebello, A.; Gruppetta, M. Hypercalcaemia due to ovarian small cell carcinoma of the hypercalcaemic type. BMJ Case Rep. 2021, 14, e243571. [Google Scholar] [CrossRef]
- Reyes-Tobar, P.; Altamirano-Assad, R.; Osses-Donoso, L.; Nazzal-Nazal, O.; Suárez-Pacheco, E. Small cell ovarian carcinoma of the hypercalcemic subtype: A case report. Ginecol. Obstet. Mex. 2021, 89, 84–89. [Google Scholar]
- Aggarwal, D.; Gupta, P.; Chhabra, P.; Peters, N.J.; Bansal, D.; Srinivasan, R.; Kakkar, N. Small Cell Carcinoma of Ovary, Hypercalcemic Type: Cytologic, Histopathologic, and Immunohistochemical Landscapes of a Rare Case. J. Pediatr. Adolesc. Gynecol. 2021, 34, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Atwi, D.; Quinton, M.R.; Kiser, R.M.; Pokala, H.R.; Rooms, L.M.; Yu, Z. Small Cell Carcinoma of the Ovary, Hypercalcemic Type, in a 12-Month-Old Girl. Pediatr. Dev. Pathol. 2021, 24, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Vivod, G.; Merlo, S.; Kovačević, N. Hypercalcemia and Unilateral Ovarian Mass in a Young Adult: A Case Report of Small Cell Ovarian Carcinoma. Am. J. Case Rep. 2021, 22, e928959. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.F.E.; da Costa, A.A.B.A.; Silva, T.N.; Fernandes, L.; Bovolim, G.; Torrezan GTCarraro, D.M.; Baiocchi, G.; Menezes, A.N.O.; Dos Santos, E.S. Case Report of Small Cell Carcinoma of the Ovary, Hypercalcemic Type (Ovarian Rhabdoid Tumor) with SMARCB1 Mutation: A Literature Review of a Rare and Aggressive Condition. Curr. Oncol. 2022, 29, 411–422. [Google Scholar] [CrossRef]
- Li, G.; Jiang, Y. Case Report: A Durable Response to Camrelizumab and Apatinib Combination Therapy in a Heavily Treated Small Cell Carcinoma of the Ovary, Hypercalcemic Type. Front. Oncol. 2022, 12, 916790. [Google Scholar] [CrossRef]
- Sanders, B.E.; Wolsky, R.; Doughty, E.S.; Wells, K.L.; Ghosh, D.; Ku, L.; Pressey, J.G.; Bitler, B.B.; Brubaker, L.W. Small cell carcinoma of the ovary hypercalcemic type (SCCOHT): A review and novel case with dual germline SMARCA4 and BRCA2 mutations. Gynecol. Oncol. Rep. 2022, 44, 101077. [Google Scholar] [CrossRef]
- Han, Q.; Guo, H. Multi-modality Therapies for Advanced Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT): Three Case Reports and Literature Review. Eur. J. Gynaecol. Oncol. 2022, 43, 4301002. [Google Scholar]
- Estel, R.; Hackethal, A.; Kalder, M.; Münstedt, K. Small cell carcinoma of the ovary of the hypercalcaemic type: An analysis of clinical and prognostic aspects of a rare disease on the basis of cases published in the literature. Arch. Gynecol. Obstet. 2011, 284, 1277–1282. [Google Scholar] [CrossRef]
- Mulita, F.; Tavlas, P.; Maroulis, I. A giant ovarian mass in a 68-year-old female with persistent abdominal pain and elevated serum CA-125 level. Prz. Menopauzalny 2020, 19, 108–110. [Google Scholar] [CrossRef]
- Callegaro-Filho, D.; Gershenson, D.M.; Nick, A.M.; Munsell, M.F.; Ramirez, P.T.; Eifel, P.J.; Euscher, E.D.; Marques, R.M.; Nicolau, S.M.; Schmeler, K.M. Small cell carcinoma of the ovary-hypercalcemic type (SCCOHT): A review of 47 cases. Gynecol. Oncol. 2016, 140, 53–57. [Google Scholar] [CrossRef]
| Number of Patients | |
| N | 306 |
| Age at diagnosis (years) | |
| Median Range | 25 1–60 |
| Symptoms (N) | |
| Abdominal pain Abdominal swelling General/other symptoms Asymptomatic | 172 87 173 3 |
| Palpable mass at physical examination (N) | |
| Palpable pelvic mass No palpable pelvic mass | 54 252 |
| Imaging (N) | |
| USS Unspecified Abdominal Transvaginal Mediastinum CT Unspecified Abdominal Pelvic Chest Cerebrum Total body PET MRI Unspecified Abdominal Pelvic Total body X-ray Abdominal Chest Skeletal Scintigraphy Unknown | 15 13 8 1 19 24 14 9 2 1 1 1 6 3 1 3 4 6 244 |
| Tumor laterality (N) | |
| Right Left Bilateral Unknown | 112 96 5 93 |
| Hypercalcemia (N) | |
| Yes No Unknown | 98 94 114 |
| FIGO Stage (N) | |
| I II III IV Unknown | 140 19 123 23 1 |
| Epithelial marker CA-125 (N) | |
| Elevated Normal Unknown | 43 11 252 |
| Mutation status (N) | |
| Reported SMARCA4 loss | 24 |
| Surgery | 11 |
| Surgery + Chemotherapy | 225 * |
| Surgery + Radiotherapy | Unclear * |
| Chemotherapy only (palliative treatment) | 1 |
| Chemotherapy + Radiotherapy | 1 |
| Surgery + Chemotherapy + Radiotherapy | 21 * |
| Surgery + Chemotherapy + HDC ASCT | 23 |
| Surgery + Chemotherapy + Radiotherapy + HDC ASCT | 23 |
| No treatment | 1 |
| Chemotherapy Regimens |
|---|
| Platinum-Paclitaxel-based regimens |
| PTax, PETax, CarboTax, CarboTaxE, PE+CarboTax, CarboIfoCtax, CarboE, TaxA |
| Platinum-Bleomycin-based regimens PCBAE, BEP, VPCBAE, BEP MTX AP, VPB, |
| Platinum-Ifosfamide-based regimens PIfoA, CarboEIfoCVCRAct, VCRAC IfoE, VCRACIfoE, CarboIfo, EIfoP, IfoTopo |
| Platinum-Doxorubicin-based regimens PAEC, PAVE, VCR VACPE, PCA |
| High-dose chemotherapy CarboEMel, CarboEC, BuMelThio, CarboE |
| Miscellaneous Doc, Gem, Topo, IriA, Pem, CapP |
| Medicine | Mechanism |
|---|---|
| EZH2 inhibitor | Inactivation of SMARCA4 leads to overexpression of the oncogenic activities of EZH2 through transcriptional repression caused by aberrant H3K27me3. |
| PD-1 inhibitor | SCCOHT tumors express PD-L1 with associated T-cell infiltration, PD-1 and PD-L1 inhibitors may offset adaptive immune evasion of the SCCOHT tumor cells |
| CDK-4/6 inhibitor | SMARCA4 loss has been shown to lead to downregulation of cyclin D1, limiting the activity of CDK-4/6 and promoting sensitivity to CDK-4/6 inhibitors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wens, F.S.P.L.; Hulsker, C.C.C.; Fiocco, M.; Zsiros, J.; Smetsers, S.E.; de Krijger, R.R.; van der Steeg, A.F.W.; Zweemer, R.P.; Baas, I.O.; Roes, E.M.; et al. Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT): Patient Characteristics, Treatment, and Outcome—A Systematic Review. Cancers 2023, 15, 3794. https://doi.org/10.3390/cancers15153794
Wens FSPL, Hulsker CCC, Fiocco M, Zsiros J, Smetsers SE, de Krijger RR, van der Steeg AFW, Zweemer RP, Baas IO, Roes EM, et al. Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT): Patient Characteristics, Treatment, and Outcome—A Systematic Review. Cancers. 2023; 15(15):3794. https://doi.org/10.3390/cancers15153794
Chicago/Turabian StyleWens, Francis S. P. L., Caroline C. C. Hulsker, Marta Fiocco, József Zsiros, Stephanie E. Smetsers, Ronald R. de Krijger, Alida F. W. van der Steeg, Ronald P. Zweemer, Inge O. Baas, Eva Maria Roes, and et al. 2023. "Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT): Patient Characteristics, Treatment, and Outcome—A Systematic Review" Cancers 15, no. 15: 3794. https://doi.org/10.3390/cancers15153794
APA StyleWens, F. S. P. L., Hulsker, C. C. C., Fiocco, M., Zsiros, J., Smetsers, S. E., de Krijger, R. R., van der Steeg, A. F. W., Zweemer, R. P., Baas, I. O., Roes, E. M., Looijenga, L. H. J., Gerestein, C. G., & Mavinkurve-Groothuis, A. M. C. (2023). Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT): Patient Characteristics, Treatment, and Outcome—A Systematic Review. Cancers, 15(15), 3794. https://doi.org/10.3390/cancers15153794








