International e-Delphi Consensus Recommendations for the Assessment and Diagnosis of Circadian rest–Activity Rhythm Disorders (CARDs) in Patients with Cancer
Abstract
Simple Summary
Abstract
1. Introduction
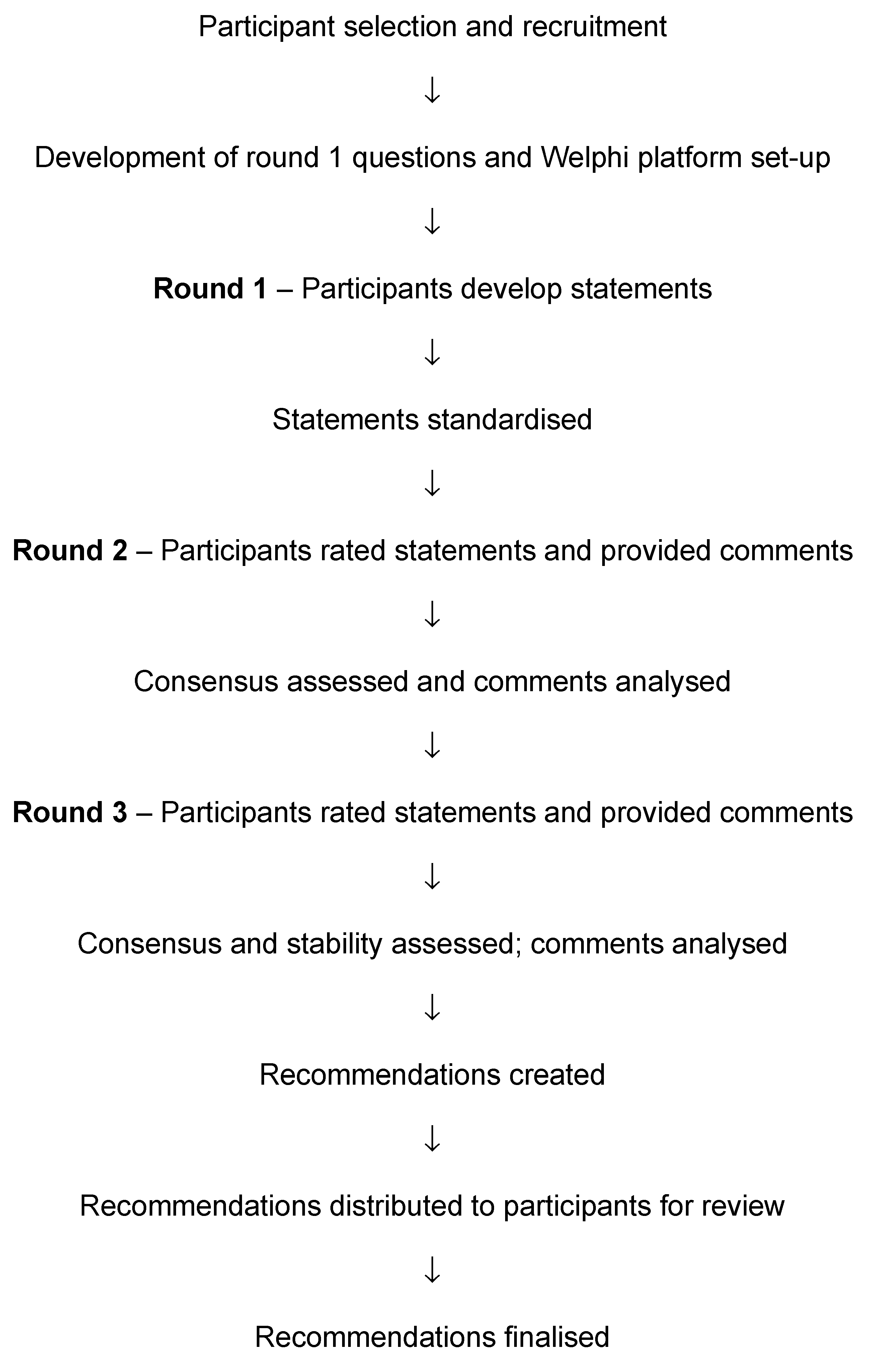
2. Methodology
Interpretation and Processing of Results
3. Results
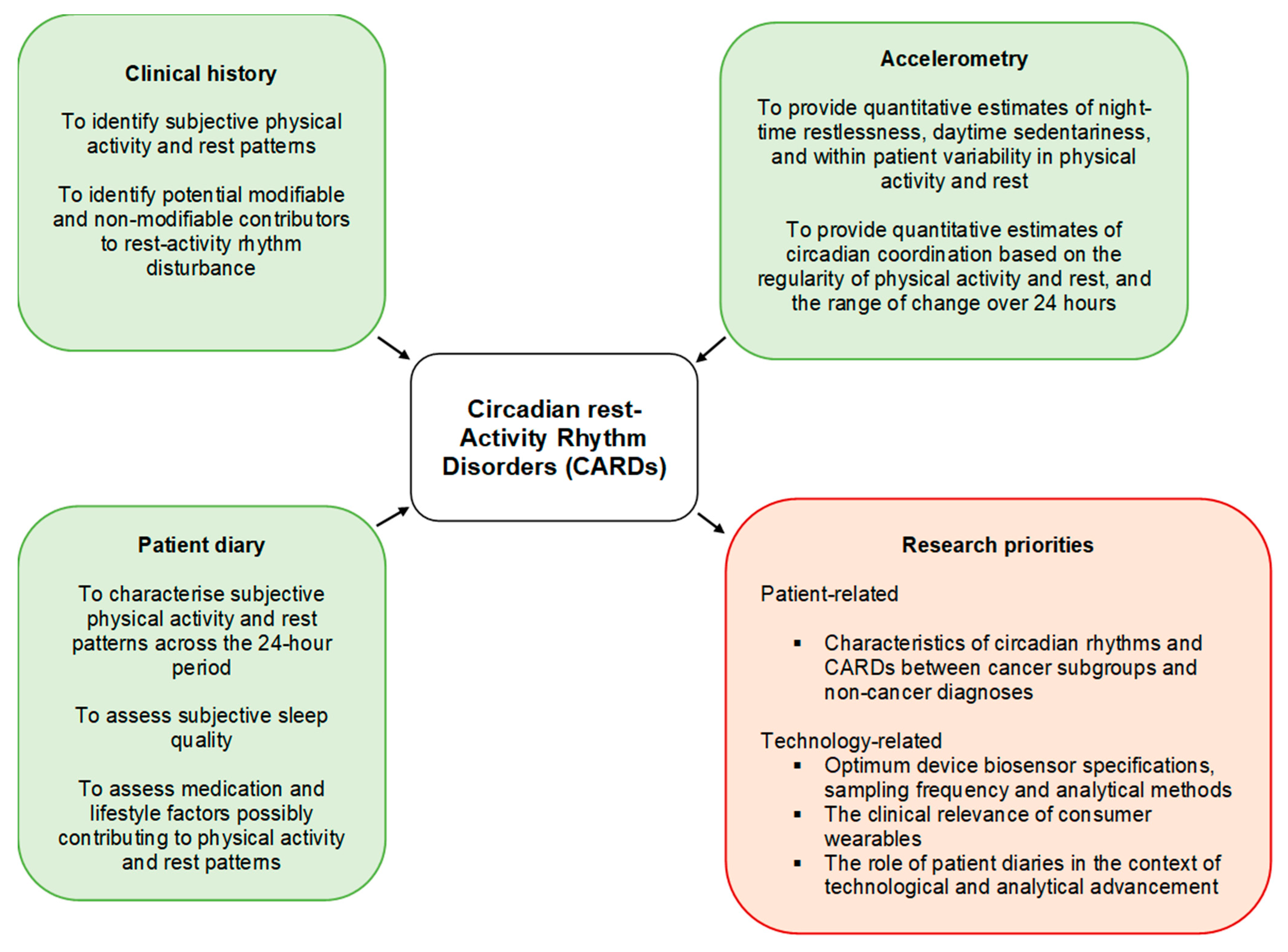
3.1. Definition of a Circadian Rest–Activity Rhythm Disorder in Patients with Cancer
3.2. Lack of Consensus and Opposing Views
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian rhythm as a therapeutic target. Nat. Rev. 2021, 20, 287–307. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Thomas, J.M.; Kern, P.A.; Bush, H.M.; McQuerry, K.J.; Black, W.S.; Clasey, J.L.; Pendergast, J.S. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. J. Clin. Investig. 2020, 5, e134270. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Touitou, Y.; Bogdan, A.; Lévi, F.; Benavides, M.; Auzéby, A. Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: Relationships with tumour marker antigens. Br. J. Cancer 1996, 74, 1248–1252. [Google Scholar] [CrossRef]
- Viviani, S.; Bidoli, P.; Spinazzé, S.; Rovelli, F.; Lissoni, P. Normalization of the light/dark rhythm of melatonin after prolonged subcutaneous administration of interleukin-2 in advanced small cell lung cancer patients. J. Pineal Res. 1992, 12, 114–117. [Google Scholar] [CrossRef]
- Innominato, P.; Komarzynski, S.; Palesh, O.; Dallmann, R.; Bjarnson, G.; Giacchetti, S.; Ulusakarya, A.; Bouchahda, M.; Haydar, M.; Ballesta, A.; et al. Circadian rest-activity rhythm as an objective marker of patient-reported outcomes in patients with advanced cancer. Cancer Med. 2018, 7, 4396–4405. [Google Scholar] [CrossRef]
- Li, J.; Somers, V.K.; Lopez-Jimenez, F.; Di, J.; Covassin, N. Demographic characteristics associated with circadian rest-activity rhythm patterns: A cross-sectional study. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 107. [Google Scholar] [CrossRef]
- Arvidsson, D.; Fridolfsson, J.; Börjesson, M. Measurement of physical activity in clinical practice using accelerometers. J. Intern. Med. 2019, 286, 137–153. [Google Scholar] [CrossRef]
- Milanti, A.; Chan, D.N.S.; Li, C.; So, W.K.W. Actigraphy-measured rest-activity circadian rhythm disruption in patients with advanced cancer: A scoping review. Support. Care Cancer 2021, 29, 7145–7169. [Google Scholar] [CrossRef]
- Xu, Y.; Su, S.; Li, X.; Mansuri, A.; McCall, W.V.; Wang, X. Blunted rest-activity circadian rhythm increases the risk of all-cause, cardiovascular disease and cancer mortality in US adults. Nature 2022, 12, 20665. [Google Scholar] [CrossRef]
- Miaskowski, C.P.; Lee, K.P.; Dunn, L.; Dodd, M.P.; Aouizerat, B.E.P.; West, C.M.; Paul, S.M.; Cooper, B.; Wara, W.; Swift, P. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nurs. 2011, 34, 255–268. [Google Scholar] [CrossRef]
- Innominato, P.F.; Giacchetti, S.; Bjarnson, G.A.; Focan, C.; Garufi, C.; Coudert, B.; Iacobelli, S.; Tampellini, M.; Durando, X.; Mormont, M.C.; et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int. J. Cancer 2012, 131, 2684–2692. [Google Scholar] [CrossRef]
- Cash, E.; Duck, C.; Brinkman, C.; Rebholz, W.; Albert, C.; Worthen, M.; Jusufbegovic, M.; Wilson, L.; Bumpous, J. Depressive symptoms and actigraphic-measured circadian disruption predict head and neck cancer survival. Psycho-Oncology 2018, 27, 2500–2507. [Google Scholar] [CrossRef]
- Komarzynski, S.; Huang, Q.; Lévi, F.A.; Palesh, O.G.; Ulusakarya, A.; Bouchahda, M.; Haydar, M.; Wreglesworth, N.I.; Morère, J.-F.; Adam, R.; et al. The day after: Correlates of patient-reported outcomes with actigraphy-assessed sleep in cancer patients at home (inCASA project). Sleep 2019, 42, zsz146. [Google Scholar] [CrossRef]
- Cash, E.; Sephton, S.; Chapgar, A.B.; Spiegel, D.; Rebholz, W.; Zimmaro, L.; Tillie, J.; Dhabhar, F. Circadian disruption and biomarkers of tumour progression in breast cancer patients awaiting surgery. Brain Behav. Immun. 2015, 48, 102–114. [Google Scholar] [CrossRef]
- Lévi, F.; Dugué, P.A.; Innominato, P.; Karaboué, A.; Dispersyn, G.; Parganiha, A.; Giacchetti, S.; Moreau, T.; Focan, C.; Waterhouse, J.; et al. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol. Int. 2014, 31, 891–900. [Google Scholar] [CrossRef]
- Innominato, P.F.; Focan, C.; Gorlia, T.; Moreau, T.; Garufi, C.; Waterhouse, J.; Giacchetti, S.; Coudert, B.; Iacobelli, S.; Genet, D.; et al. Circadian Rhythm in Rest and Activity: A Biological Correlate of Quality of Life and a Predictor of Survival in Patients with Metastatic Colorectal Cancer. Cancer Res. 2009, 69, 4700–4707. [Google Scholar] [CrossRef]
- Kos, M.; Brouwer, C.G.; van Laarhoven, H.W.; Hopman, M.T.; van Oijen, M.G.; Buffart, L.M. The association between wearable device metrics and clinical outcomes in oncology: A systematic review with evidence synthesis and meta-analysis. Crit. Rev. Oncol. 2023, 185, 103979. [Google Scholar] [CrossRef]
- Hahm, B.-J.; Jo, B.; Dhabhar, F.S.; Palesh, O.; Aldridge-Gerry, A.; Bajestan, S.N.; Neri, E.; Nouriani, B.; Spiegel, D.; Zeitzer, J.M. Bedtime misalignment and progression of breast cancer. Chronobiol. Int. 2014, 31, 214–221. [Google Scholar] [CrossRef]
- Jünger, S.; Payne, S.A.; Brine, J.; Radbruch, L.; Brearley, S.G. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: Recommendations based on a methodological systematic review. Palliat. Med. 2017, 31, 684–706. [Google Scholar] [CrossRef]
- Pati, A.K.; Parganiha, A.; Kar, A.; Soni, R.; Roy, S.; Choudhary, V. Alterations of the Characteristics of the Circadian Rest-Activity Rhythm of Cancer In-Patients. Chronobiol. Int. 2007, 24, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Meyrel, M.; Scott, J.; Etain, B.; Manon, M. Chronotypes and circadian rest–activity rhythms in bipolar disorders: A meta-analysis of self- and observer rating scales. Bipolar Disord. 2021, 24, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.M.; Gonçalves, B.S.B.; Peixoto, C.A.T.; De Bruin, V.M.S.; Louzada, F.M.; DE Bruin, P.F.C. Circadian rest-activity rhythm in chronic obstructive pulmonary disease. Chronobiol. Int. 2017, 34, 1315–1319. [Google Scholar] [CrossRef]
- Musiek, E.S.; Bhimasani, M.; Zangrilli, M.A.; Morris, J.C.; Holtzman, D.M.; Ju, Y.-E.S. Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol. 2018, 75, 582–590. [Google Scholar] [CrossRef]
- Wu, J.Q.; Li, P.; Gilbert, K.S.; Hu, K.; Cronin-Golomb, A. Circadian Rest-Activity Rhythms Predict Cognitive Function in Early Parkinson’s Disease Independently of Sleep. Mov. Disord. Clin. Pr. 2018, 5, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Choudhary, V.; Parganiha, A. Worsening of rest-activity circadian rhythm and quality of life in female breast cancer patients along progression of chemotherapy cycles. Chronobiol. Int. 2017, 34, 609–623. [Google Scholar] [CrossRef]
- Rumble, M.E.; Rose, S.L.; White, K.H.; Moore, A.H.; Gehrman, P.; Benca, R.M.; Costanzo, E.S. Circadian actigraphic rest–activity rhythms following surgery for endometrial cancer: A prospective, longitudinal study. Gynecol. Oncol. 2015, 137, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Liu, L.; Rissling, M.; Natarajan, L.; Neikrug, A.B.; Palmer, B.W.; Mills, P.J.; Parker, B.A.; Sadler, G.R.; Maglione, J. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: A 1-year longitudinal study. Support. Care Cancer 2014, 22, 2535–2545. [Google Scholar] [CrossRef]
- Rossiter, A.; Warrington, G.D.; Comyns, T.M. Effects of Long-Haul Travel on Recovery and Performance in Elite Athletes: A Systematic Review. J. Strength Cond. Res. 2022, 36, 3234–3245. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Martin, J.L.; Blackwell, T.; Buenaver, L.; Liu, L.; Meltzer, L.J.; Sadeh, A.; Spira, A.P.; Taylor, D.J. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav. Sleep Med. 2015, 13, S4–S38. [Google Scholar] [CrossRef] [PubMed]
- Fouladiun, M.; Korner, U.; Gunnebo, L.; Sixt-Ammilon, P.; Bosaeus, I.; Lundholm, K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin. Cancer Res. 2007, 13, 6379–6385. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Davies, A.; Laing, E.; Wu, H.; Mendis, J.; Dijk, D.-J. Prognostication in Advanced Cancer by Combining Actigraphy-Derived Rest-Activity and Sleep Parameters with Routine Clinical Data: An Exploratory Machine Learning Study. Cancers 2023, 15, 503. [Google Scholar] [CrossRef]
- Gresham, G.; Schrack, J.; Gresham, L.M.; Shinde, A.M.; Hendifar, A.E.; Tuli, R.; Rimel, B.; Figlin, R.; Meinert, C.L.; Piantadosi, S. Wearable activity monitors in oncology trials: Current use of an emerging technology. Contemp. Clin. Trials 2018, 64, 13–21. [Google Scholar] [CrossRef]
- Beauchamp, U.L.; Pappot, H.; Holländer-Mieritz, C. The Use of Wearables in Clinical Trials During Cancer Treatment: Systematic Review. JMIR mHealth uHealth 2020, 8, e22006. [Google Scholar] [CrossRef]
- Low, C.A. Harnessing consumer smartphone and wearable sensors for clinical cancer research. NPJ Digit. Med. 2020, 3, 140. [Google Scholar] [CrossRef]
- Komarzynski, S.; Huang, Q.; Innominato, P.F.; Maurice, M.; Arbaud, A.; Beau, J.; Bouchahda, M.; Ulusakarya, A.; Beaumatin, N.; Breda, G.; et al. Relevance of a Mobile Internet Platform for Capturing Inter- and Intrasubject Variabilities in Circadian Coordination During Daily Routine: Pilot Study. J. Med. Internet Res. 2018, 20, e204. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Mokhtar, N.; Byrne, N.M. Assessment of Physical Activity and Energy Expenditure: An Overview of Objective Measures. Front. Nutr. 2014, 1, 5. [Google Scholar] [CrossRef]
- Aunger, J.; Wagnild, J. Objective and subjective measurement of sedentary behavior in human adults: A toolkit. Am. J. Hum. Biol. 2020, 34, e23546. [Google Scholar] [CrossRef]
- Lehrer, H.M.; Yao, Z.; Krafty, R.T.; Evans, M.A.; Buysee, D.J.; Kravitz, H.M.; Matthews, K.A.; Gold, E.B.; Harlow, S.D.; Samuelsson, L.B.; et al. Comparing polysomnography, actigraphy, and sleep diary in the home environment: The Study of Women’s Health Across the Nation (SWAN) Sleep Study. Sleep Adv. 2022, 2022, zpac001. [Google Scholar] [CrossRef]
- Nasa, P.; Jain, R.; Juneja, D. Delphi methodology in healthcare research: How to decide its appropriateness. World J. Methodol. 2021, 11, 116–129. [Google Scholar] [CrossRef]
- Niederberger, M.; Spranger, J. Delphi Technique in Health Sciences: A Map. Front. Public Health 2020, 8, 457. [Google Scholar] [CrossRef]
- Gargon, E.; Crew, R.; Burnside, G.; Williamson, P.R. Higher number of items associated with significantly lower response rates in COS Delphi surveys. J. Clin. Epidemiol. 2019, 108, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Holey, E.A.; Feeley, J.L.; Dixon, J.; Whittaker, V.J. An exploration of the use of simple statistics to measure consensus and stability in Delphi studies. BMC Med. Res. Methodol. 2007, 7, 52. [Google Scholar] [CrossRef]
- Bernatchez, M.S.; Savard, J.; Ivers, H. Disruptions in sleep–wake cycles in community-dwelling cancer patients receiving palliative care and their correlates. Chronobiol. Int. 2018, 35, 49–62. [Google Scholar] [CrossRef]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014, 11, 16. [Google Scholar] [CrossRef]
- Ortiz-Tudela, E.; Innominato, P.F.; Rol, M.A.; Lévi, F.; Madrid, J.A. Relevance of internal time and circadian robustness for cancer patients. BMC Cancer 2016, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Du-Quiton, J.; Wood, P.A.; Burch, J.B.; Grutsch, J.F.; Gupta, D.; Tyer, K.; Lis, C.G.; Levin, R.D.; Quiton, D.F.T.; Reynolds, J.L.; et al. Actigraphic assessment of daily sleep-activity pattern abnormalities reflects self-assessed depression and anxiety in outpatients with advanced non-small cell lung cancer. Psycho-Oncology 2010, 19, 180–189. [Google Scholar] [CrossRef]
- Chen, H.-M.; Tsai, C.-M.; Wu, Y.-C.; Lin, K.-C.; Lin, C.-C. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: A randomised controlled trial. Br. J. Cancer 2016, 115, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.; Innominato, P.F.; Boreggiani, M.; Tonetti, L.; Filardi, M.; Parganiha, A.; Fabbri, M.; Martoni, M.; Lévi, F. The difference between in bed and out of bed activity as a behavioral marker of cancer patients: A comparative actigraphic study. Chronobiol. Int. 2015, 32, 925–933. [Google Scholar] [CrossRef]
- Jakobsen, G.; Engstrøm, M.; Thronæs, M.; Løhre, E.T.; Kaasa, S.; Fayers, P.; Hjermstad, M.J.; Klepstad, P. Sleep quality in hospitalized patients with advanced cancer: An observational study using self-reports of sleep and actigraphy. Support. Care Cancer 2020, 28, 2015–2023. [Google Scholar] [CrossRef]
- Gibbins, J.; McCoubrie, R.; Kendrick, A.H.; Senior-Smith, G.; Davies, A.N.; Hanks, G.W. Sleep-wake disturbances in patients with advanced cancer and their family carers. J. Pain Symptom Manag. 2009, 38, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Grutsch, J.F.; Ferrans, C.; Wood, P.A.; Du-Quiton, J.; Quiton, D.F.T.; Reynolds, J.L.; Ansell, C.M.; Oh, E.Y.; Daehler, M.A.; Levin, R.D.; et al. The association of quality of life with potentially remediable disruptions of circadian sleep/activity rhythms in patients with advanced lung cancer. BMC Cancer 2011, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.S.T.; Takemura, N.; Lam, T.C.; Ho, J.C.M.; Deng, W.; Smith, R.; Yan, Y.; Lee, A.W.M.; Lin, C.C. Feasibility of Aerobic Exercise and Tai-Chi Interventions in Advanced Lung Cancer Patients: A Randomized Controlled Trial. Integr. Cancer Ther. 2021, 20, 15347354211033352. [Google Scholar] [CrossRef] [PubMed]
| Job title | Professor (n = 7) |
| Associate Professor (n = 4) | |
| Medical Consultant/Attending Physician (n = 3) | |
| Senior Lecturer (n = 1) | |
| No response (n = 1) | |
| Specialty | Oncology (n = 3) |
| Biosciences (n = 2) | |
| Psychiatry (n = 2) | |
| Psychology (n = 2) | |
| Sleep and Physiology (n = 2) | |
| Anaesthesiology (n = 1) | |
| Nursing (n = 1) | |
| Oncology, Palliative Medicine and Sleep (n = 1) | |
| Palliative Medicine (n = 1) | |
| No response (n = 1) | |
| Location | United States (n = 5) |
| UK (n = 3) | |
| India (n = 2) | |
| Norway (n = 2) | |
| Canada (n = 1) | |
| France (n = 1) | |
| Ireland (n = 1) | |
| No response (n = 1) | |
| Time spent researching activity rhythms in patients with cancer | Median 15 years (range 5–35 years) |
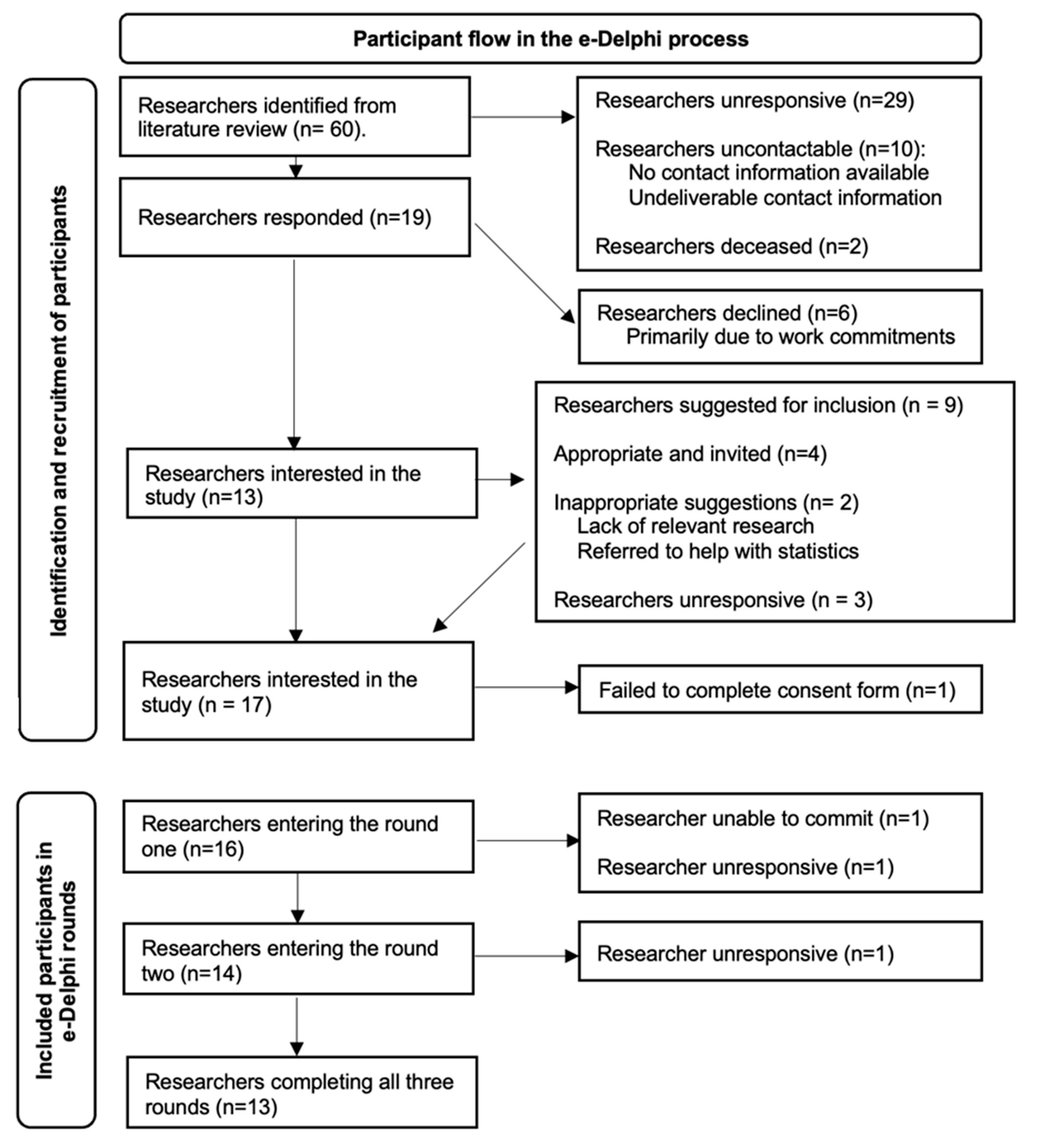
| Retention Rates | |||
|---|---|---|---|
| Round 1 | Round 2 | Round 3 | |
| Of recruited participants | 14/16 | 13/16 | 13/16 |
| 87.5% | 81.3% | 81.3% | |
| Of participants entering the round | 14/16 | 13/14 | 13/13 |
| 87.5% | 92.9% | 100% | |
| Attrition reason | Unable to commit (1), no response (1) | IT problems / no response (1) | n/a |
| The Patient Must Demonstrate an Altered Circadian Rest–Activity Rhythm Evidenced by One of the Following | Level of Agreement |
|---|---|
| Relatively less daytime and more night-time physical activity | 92% |
| Rest and physical activity spread across the 24-h period, rather than in distinct rest and active periods | 77% |
| A lack of regularity in rest and active periods between days. | 77% |
| The circadian rest–activity rhythm alteration must have all the following | |
| Have been present for at least 1 month. | 70% |
| Have a clinical impact on the patient. | 84% |
| Be demonstrable by objective measures. | 92% |
| Be demonstrable by subjective measures | 69% |
| Not primarily be due to another cause. | NA |
| A clinical history (“needed”; 92%), accelerometry (“essential”; 92%) and patient diary (“suggested”; 70%) are recommended to assess a CARD in people with cancer. | |
| A clinical history should consider | Level of agreement |
| An oncological history (cancer site, stage, site of metastases, and cancer treatments) | 92% |
| The presence and timing of symptoms (e.g., fatigue, daytime sleepiness, pain) | 100% |
| Medical, surgical, and psychiatric comorbidities | 100% |
| Daily routine, type, and duration of physical activity | 100% |
| If daytime sedentariness and/or night-time restlessness is present, the duration should be considered | 100% |
| Sleep history, assessment of chronotype, and peak alertness | 85–100% |
| Medication history (e.g., melatonin, beta blockers, steroids, stimulants, and sedatives) | 100% |
| The use of tobacco, alcohol, caffeine, and illicit drugs | 100% |
| Environmental factors (e.g., family, newborns, occupation, shift work, jet lag, noise and light exposure) | 100% |
| Assessment using accelerometry | |
| Accelerometry should take place for at least 72 consecutive hours | LC |
| The location of an accelerometer device should be documented | NA |
| When using wrist actigraphy, the non-dominant wrist should be used unless contraindicated | 100% |
| Removal of the device should be documented | 93% |
| Relevant accelerometry parameters | |
| Evidence of night-time restlessness (e.g., sleep efficiency, number and duration of night-time awakenings) | 77–84% |
| Evidence of daytime sedentariness (e.g., daytime sedentariness, number and duration of daytime naps) | 85–92% |
| Evidence of daytime sedentariness and night-time restlessness (e.g., dichotomy index, physical activity relative amplitude, intra-daily variability) | 85–92% |
| Evidence of a lack of regularity in rest and active periods between days (e.g., the 24-h autocorrelation coefficient, interdaily stability) | 77–100% |
| Phase markers (e.g., most active 10 h (M10), activity acrophase | 69–70% |
| A patient sleep and activity diary | |
| A sleep and activity diary supports accelerometry and the diagnosis of circadian rest–activity rhythm disorders | 85–93% |
| A diary should consider day and night-time events and be used for the duration of accelerometry monitoring | NA |
| Relevant information to document in a sleep and activity diary include | |
| Time and duration of daytime naps | 93% |
| Subjective daytime sleepiness | 85% |
| Time, description, duration, and perceived level of exertion of physical activity | 70–92% |
| Presence of symptoms during physical activity (e.g., pain or fatigue) | 69% |
| Medication use | 84% |
| Alcohol, smoking, caffeine, and substance use | 85% |
| Bedtime | 84% |
| Time to lights out | 84% |
| Sleep onset | 76% |
| Time and duration of night-time awakenings | 85% |
| Wake-up time | 92% |
| Get-out-of-bed time | 100% |
| Subjective sleep quality | 77% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouldthorpe, C.; Ancoli-Israel, S.; Cash, E.; Innominato, P.; Jakobsen, G.; Lévi, F.; Miaskowski, C.; Parganiha, A.; Pati, A.K.; Pereira, D.; et al. International e-Delphi Consensus Recommendations for the Assessment and Diagnosis of Circadian rest–Activity Rhythm Disorders (CARDs) in Patients with Cancer. Cancers 2023, 15, 3784. https://doi.org/10.3390/cancers15153784
Gouldthorpe C, Ancoli-Israel S, Cash E, Innominato P, Jakobsen G, Lévi F, Miaskowski C, Parganiha A, Pati AK, Pereira D, et al. International e-Delphi Consensus Recommendations for the Assessment and Diagnosis of Circadian rest–Activity Rhythm Disorders (CARDs) in Patients with Cancer. Cancers. 2023; 15(15):3784. https://doi.org/10.3390/cancers15153784
Chicago/Turabian StyleGouldthorpe, Craig, Sonia Ancoli-Israel, Elizabeth Cash, Pasquale Innominato, Gunnhild Jakobsen, Francis Lévi, Christine Miaskowski, Arti Parganiha, Atanu Kumar Pati, Deidre Pereira, and et al. 2023. "International e-Delphi Consensus Recommendations for the Assessment and Diagnosis of Circadian rest–Activity Rhythm Disorders (CARDs) in Patients with Cancer" Cancers 15, no. 15: 3784. https://doi.org/10.3390/cancers15153784
APA StyleGouldthorpe, C., Ancoli-Israel, S., Cash, E., Innominato, P., Jakobsen, G., Lévi, F., Miaskowski, C., Parganiha, A., Pati, A. K., Pereira, D., Revell, V., Zeitzer, J. M., & Davies, A. (2023). International e-Delphi Consensus Recommendations for the Assessment and Diagnosis of Circadian rest–Activity Rhythm Disorders (CARDs) in Patients with Cancer. Cancers, 15(15), 3784. https://doi.org/10.3390/cancers15153784










