Adjuvant Chemotherapy in Older Patients with Gastric Cancer: A Population-Based Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
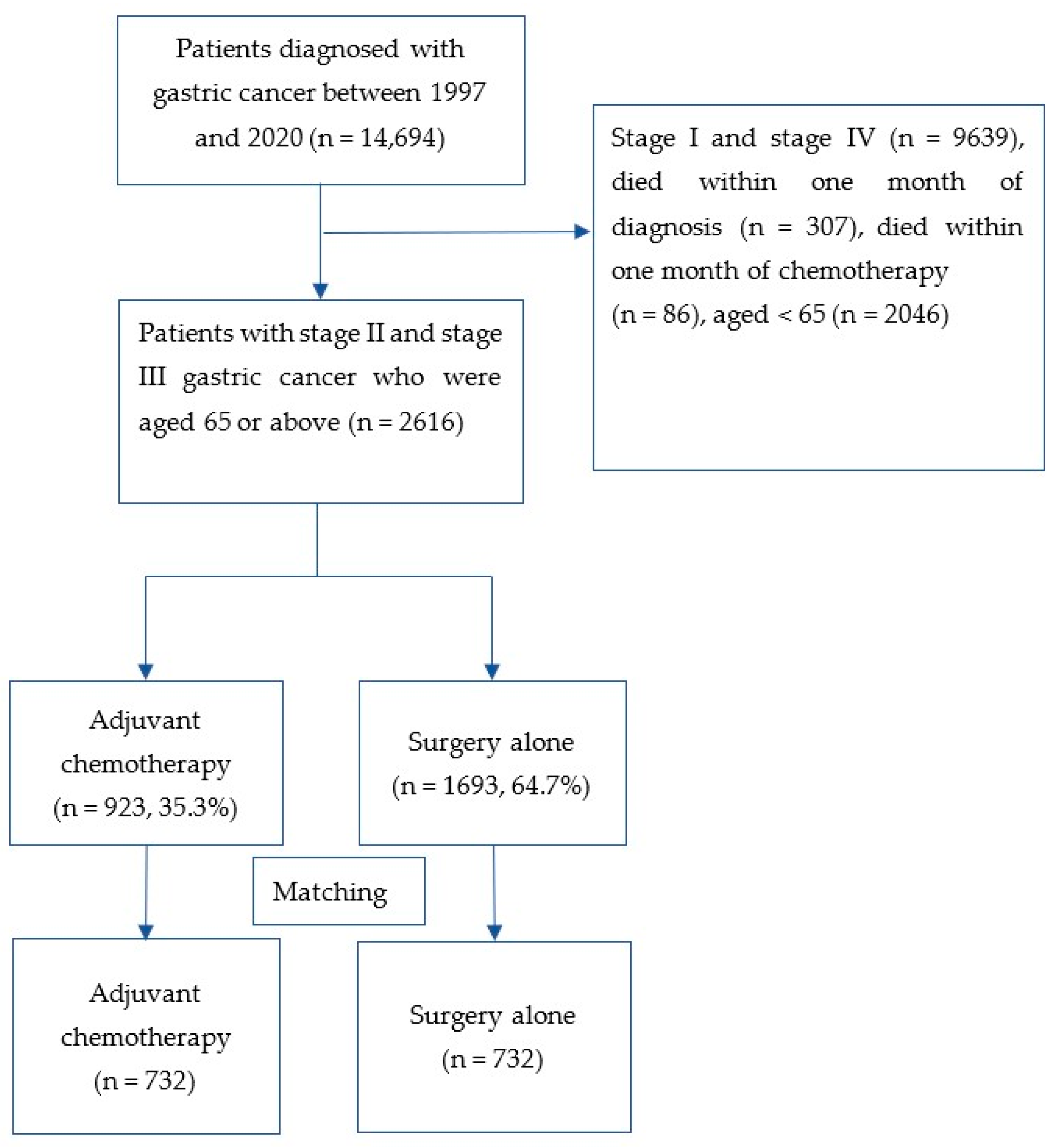
2.1. Patient Eligibility and Data Collection
2.2. Baseline Covariates
2.3. Outcomes
2.4. Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- The Surveillance, Epidemiology, and End Results (SEER) Cancer Facts: Stomach Cancer. Available online: https://seer.cancer.gov/statfacts/html/stomach.html (accessed on 4 January 2022).
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- Eom, S.S.; Choi, W.; Eom, B.W.; Park, S.H.; Kim, S.J.; Kim, Y.I.; Yoon, H.M.; Lee, Y.L.; Kim, C.G.; Kim, H.K.; et al. A Comprehensive and Comparative Review of Global Gastric Cancer Treatment Guidelines. J. Gastric Cancer. 2022, 22, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Guideline Committee of the Korean Gastric Cancer Association (KGCA); Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: An evidence-based, multi-disciplinary approach. J. Gastric Cancer 2019, 19, 1–48. [Google Scholar] [CrossRef]
- Wang, F.H.; Zhang, X.T.; Li, Y.F.; Tang, L.; Qu, X.J.; Ying, J.E.; Zhang, J.; Sun, L.-Y.; Lin, R.-B.; Qiu, H.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021, 41, 747–795. [Google Scholar] [CrossRef]
- Liu, D.; Lu, M.; Li, J.; Yang, Z.; Feng, Q.; Zhou, M.; Zhang, Z.; Shen, L. The patterns and timing of recurrence after curative resection for gastric cancer in China. World J. Surg. Oncol. 2016, 14, 305. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kojima, K.; Inokuchi, M.; Kato, K.; Sugita, H.; Kawano, T.; Sugihara, K. Patterns, timing and risk factors of recurrence of gastric cancer after laparoscopic gastrectomy: Reliable results following long-term follow-up. Eur. J. Surg. Oncol. 2014, 40, 1376–1382. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophagealadenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-K.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Smalley, S.R.; Benedetti, J.K.; Haller, D.G.; Hundahl, S.A.; Estes, N.C.; Ajani, J.A.; Gunderson, L.L.; Goldman, B.; Martenson, J.A.; Jessup, J.M.; et al. Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J. Clin. Oncol. 2012, 30, 2327–2333. [Google Scholar] [CrossRef]
- Park, S.H.; Sohn, T.S.; Lee, J.; Lim, D.H.; Hong, M.E.; Kim, K.M.; Sohn, I.; Jung, S.H.; Choi, M.G.; Lee, J.H.; et al. Phase III Trial to Compare Adjuvant Chemotherapy with Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J. Clin. Oncol. 2015, 33, 3130–3136. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.H.; Kim, K.M.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S.; Lee, S.J.; Kim, S.T.; et al. The Influence of Metastatic Lymph Node Ratio on the Treatment Outcomes in the Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) Trial: A Phase III Trial. J. Gastric Cancer 2016, 16, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lim, D.H.; Sohn, T.S.; Lee, J.; Zang, D.Y.; Kim, S.T.; Kang, J.H.; Oh, S.Y.; Hwang, I.G.; Ji, J.H.; et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: The ARTIST 2 trial. Ann. Oncol. 2021, 32, 368–374. [Google Scholar] [CrossRef]
- Noh, S.H.; Park, S.R.; Yang, H.K.; Chung, H.C.; Chung, I.J.; Kim, S.W.; Kim, H.H.; Choi, J.H.; Kim, H.K.; Yu, W.; et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1389–1396. [Google Scholar] [CrossRef]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef]
- Sedrak, M.S.; Freedman, R.A.; Cohen, H.J.; Muss, H.B.; Jatoi, A.; Klepin, H.D.; Klepin, H.D.; Wildes, T.M.; Le-Rademacher, J.G.; Kimmick, G.G.; et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J. Clin. 2021, 71, 78–92. [Google Scholar] [CrossRef]
- Chang, S.H.; Kim, S.N.; Choi, H.J.; Park, M.; Kim, R.B.; Go, S.I.; Lee, W.S. Adjuvant Chemotherapy for Advanced Gastric Cancer in Elderly and Non-elderly Patients: Meta-Analysis of Randomized Controlled Trials. Cancer Res. Treat. 2017, 49, 263–273. [Google Scholar] [CrossRef]
- Jeong, J.W.; Kwon, I.G.; Son, Y.G.; Ryu, S.W. Could Adjuvant Chemotherapy after Surgery Benefit Elderly Patients with Advanced Gastric Cancer? J. Gastric Cancer 2016, 16, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qiu, M.Z.; Wang, D.S.; Zhang, D.S.; Ren, C.; Bai, L.; Luo, H.-Y.; Wang, Z.-Q.; Wang, F.H.; Li, Y.-H.; et al. Adjuvant chemotherapy for elderly patients with gastric cancer after D2 gastrectomy. PLoS ONE 2013, 8, e53149. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.C.; Baek, J.H.; Koh, S.J.; Kim, H.; Min, Y.J.; Lee, B.U.; Kim, B.G.; Jeong, I.D.; Cho, H.R.; Kim, G.Y. Adjuvant chemotherapy for elderly patients (aged 70 or older) with gastric cancer after a gastrectomy with D2 dissection: A single center experience in Korea. Asia Pac. J. Clin. Oncol. 2015, 11, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, L.; Chen, H.; Lin, T.; Chen, T.; Zhao, M.; Hu, Y.; Yu, J.; Liu, H.; Li, G. Survival analysis of elderly patients over 65 years old with stage II/III gastric cancer treated with adjuvant chemotherapy after laparoscopic D2 gastrectomy: A retrospective cohort study. BMC Cancer 2021, 21, 196. [Google Scholar]
- Liang, Y.X.; Deng, J.Y.; Guo, H.H.; Ding, X.W.; Wang, X.N.; Wang, B.G.; Zhang, L.; Liang, H. Characteristics and prognosis of gastric cancer in patients aged ≥ 70 years. World J. Gastroenterol. 2013, 19, 6568–6578. [Google Scholar] [CrossRef]
- Extermann, M.; Boler, I.; Reich, R.R.; Lyman, G.H.; Brown, R.H.; DeFelice, J.; Levine, R.M.; Lubiner, E.T.; Reyes, P.; Schreiber, F.J., 3rd; et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012, 118, 3377–3386. [Google Scholar] [CrossRef]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Prediciting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef]


| Total (n = 1464) | Non-Chemotherapy (n = 732) | Chemotherapy (n = 732) | |
|---|---|---|---|
| General information | |||
| Age (year), Mean ± SE | 73.7 ± 0.2 | 74.4 ± 0.2 | 73.1 ± 0.2 |
| Age group, % | |||
| ≤80 | 88.3 | 88.7 | 87.8 |
| >80 | 11.8 | 11.3 | 12.2 |
| Sex, n (%) | |||
| Male | 68.9 | 68.4 | 69.5 |
| Treatment | |||
| Radiotherapy, % | 9.3 | 8.9 | 9.7 |
| Chemotherapy drugs | |||
| S-1 | NA | NA | 17.6 |
| CAPOX | NA | NA | 27.9 |
| CAPECITABINE | NA | NA | 45.6 |
| FOLFOX4 | NA | NA | 4.5 |
| OTHERS | NA | NA | 4.4 |
| Chemotherapy regimen, % | |||
| Monotherapy | NA | NA | 65.6 |
| Doublet | NA | NA | 34.4 |
| Comorbidity, % | |||
| Coronary heart disease | 4.2 | 3.7 | 4.8 |
| Heart failure | 1.0 | 1.0 | 1.1 |
| Stroke | 3.4 | 3.1 | 3.6 |
| Atrial fibrillation | 1.4 | 1.4 | 1.5 |
| Diabetes mellitus | 18.5 | 20.1 | 16.9 |
| Hypertension | 24.4 | 24.3 | 24.5 |
| Liver cirrhosis | 1.8 | 1.5 | 2.1 |
| CCI, Mean ± SE | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.1 ± 0.1 |
| CCI, n (%) | |||
| ≤7 | 90.4 | 91.5 | 89.3 |
| Eight or above | 9.6 | 8.5 | 10.7 |
| Biological parameters, Mean ± SD | |||
| WBC, 109/L | 8.1 ± 0.1 | 8.1 ± 0.1 | 8.1 ± 0.1 |
| RBC, 1012/L | 3.8 ± 0.1 | 3.8 ± 0.1 | 3.9 ± 0.1 |
| Platelet, 109/L | 277.4 ± 2.7 | 275.8 ± 3.7 | 279.7 ± 3.9 |
| Neutrophil, 109/L | 6.1 ± 0.1 | 6.3 ± 0.1 | 6.0 ± 0.2 |
| Lymphocyte, 109/L | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.3 |
| ALP, U/L | 80.9 ± 1.7 | 84.6 ± 2.8 | 77.2 ± 1.9 |
| Total bilirubin, μmol/L | 10.4 ± 0.2 | 10.7 ± 0.1 | 10.0 ± 0.2 |
| eGFR, mL/min/1.73 m2 | 103.0 ± 0.8 | 103.9 ± 1.3 | 102.1 ± 1.1 |
| Albumin, g/L | 33.9 ± 0.2 | 34.0 ± 0.2 | 33.8 ± 0.2 |
| Grade 3/4 Hematological Toxicities | Percentage |
|---|---|
| Anemia | 30.9% |
| Neutropenia | 12.8% |
| Thrombocytopenia | 8.6% |
| Febrile neutropenia | 7.4% |
| Pneumonia | 7.5% |
| Urinary tract infection | 2.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, W.-L.; Liu, X.; Wong, C.K.-H.; Wong, M.S.-N.; Wong, I.Y.-H.; Lam, K.-O.; Yun, B.H.-K.; Cheung, E.E.; Tse, R.P.-Y.; Chan, F.; et al. Adjuvant Chemotherapy in Older Patients with Gastric Cancer: A Population-Based Cohort Study. Cancers 2023, 15, 3768. https://doi.org/10.3390/cancers15153768
Chan W-L, Liu X, Wong CK-H, Wong MS-N, Wong IY-H, Lam K-O, Yun BH-K, Cheung EE, Tse RP-Y, Chan F, et al. Adjuvant Chemotherapy in Older Patients with Gastric Cancer: A Population-Based Cohort Study. Cancers. 2023; 15(15):3768. https://doi.org/10.3390/cancers15153768
Chicago/Turabian StyleChan, Wing-Lok, Xiaodong Liu, Carlos King-Ho Wong, Michael Siu-Nam Wong, Ian Yu-Hong Wong, Ka-On Lam, Bryan Ho-Kwan Yun, Emina Edith Cheung, Rosa Pui-Ying Tse, Fion Chan, and et al. 2023. "Adjuvant Chemotherapy in Older Patients with Gastric Cancer: A Population-Based Cohort Study" Cancers 15, no. 15: 3768. https://doi.org/10.3390/cancers15153768
APA StyleChan, W.-L., Liu, X., Wong, C. K.-H., Wong, M. S.-N., Wong, I. Y.-H., Lam, K.-O., Yun, B. H.-K., Cheung, E. E., Tse, R. P.-Y., Chan, F., Law, S., & Kwong, D. (2023). Adjuvant Chemotherapy in Older Patients with Gastric Cancer: A Population-Based Cohort Study. Cancers, 15(15), 3768. https://doi.org/10.3390/cancers15153768





