Trends in Diet and Cancer Research: A Bibliometric and Visualization Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Databases and Search Strategy
2.2. PubMed Search Query
2.3. Data Analysis
2.4. Visual Network Analysis
3. Results
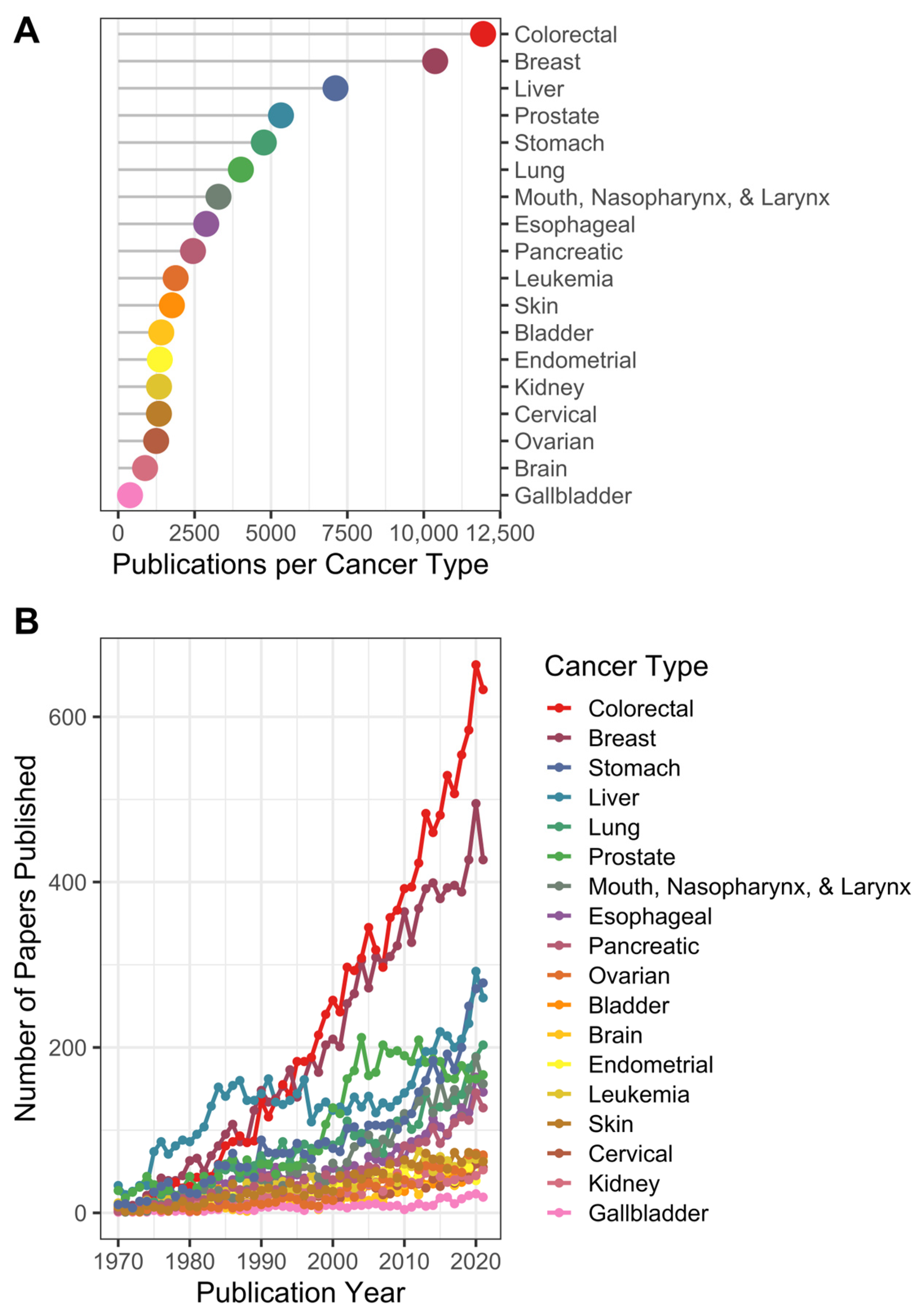
3.1. Publications and Trends over Time
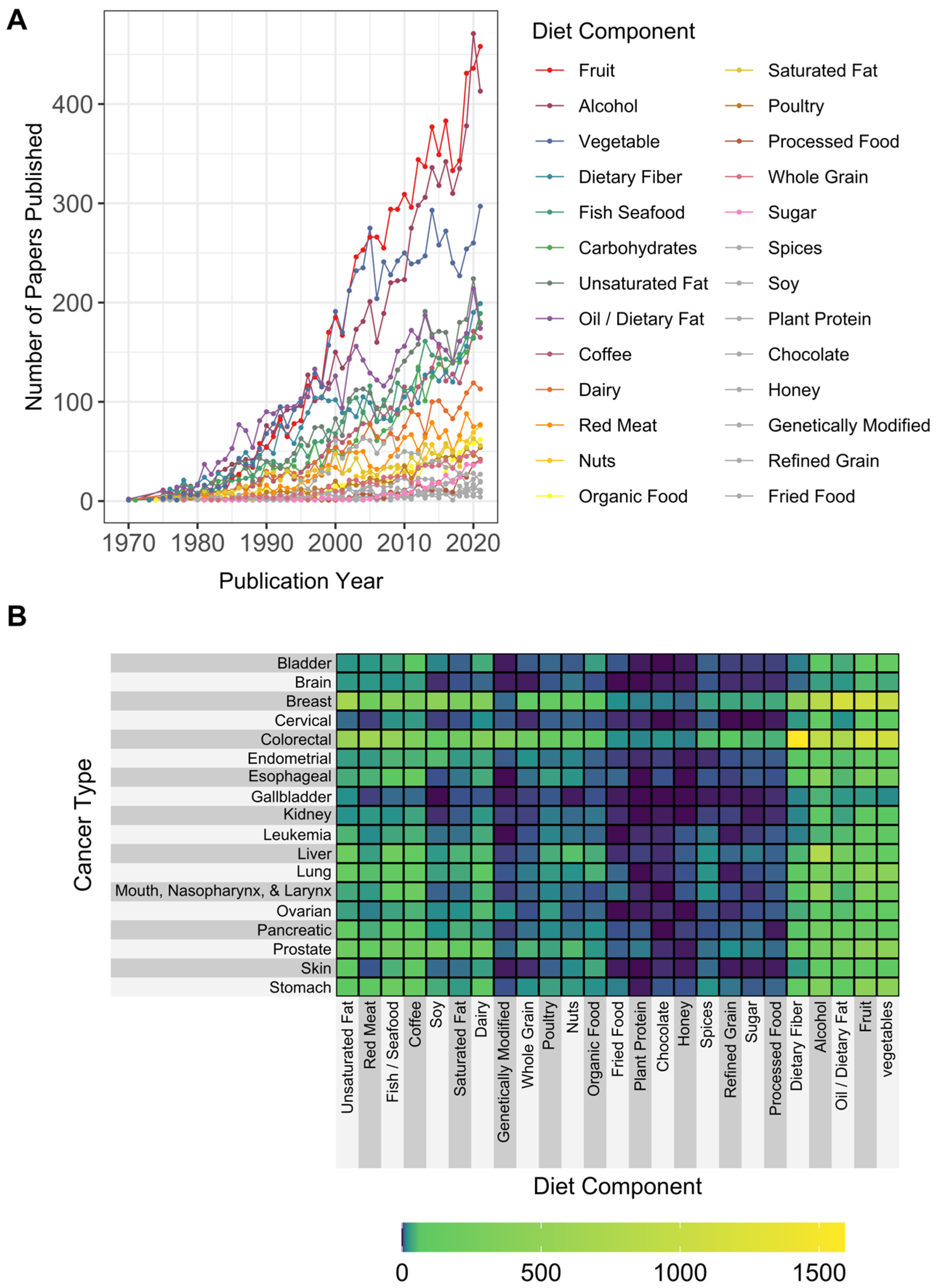
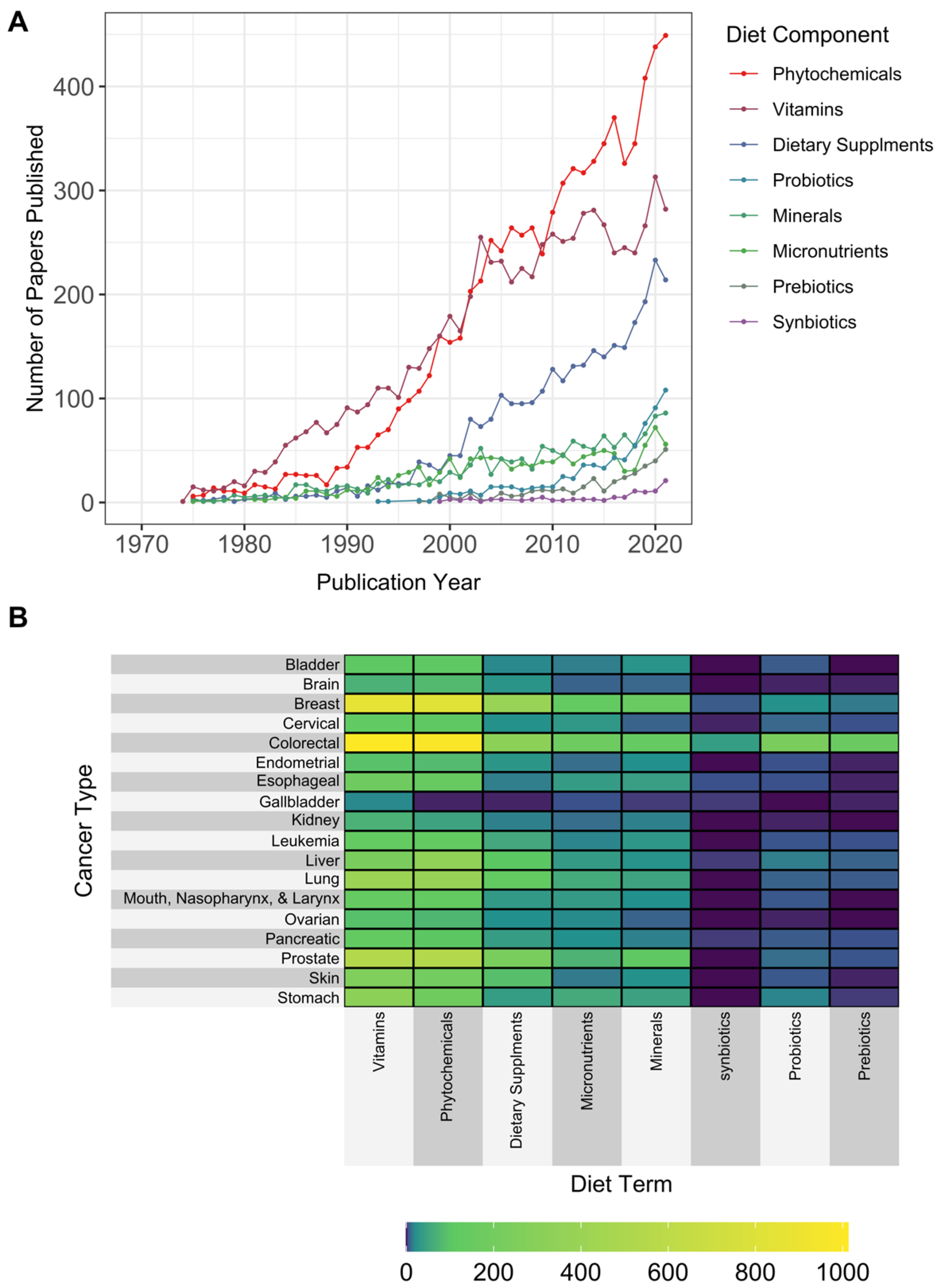
3.2. Diet Types, Components, and Bioactive Nutrient-Related Terms
3.3. Network Analysis of Keywords
4. Discussion
4.1. Principal Findings
4.1.1. Colorectal, Breast, and Liver Cancer Are Prevalent in Nutrition-Related Literature
4.1.2. Early Focus on Non-Nutritive Bioactive Compounds and Vitamins with a More Recent Transition to Focus on the Microbiome
4.1.3. WCRF/AICR Recommendations Have Remained Relatively Constant over Time
4.1.4. Interest Is Increasing in Dietary Patterns over Dietary Content of Foods
4.1.5. Nutrition and Cancer Studies Are Represented across the Scientific Spectrum
4.1.6. Comparisons to Other Bibliometric Analyses
4.1.7. Summary of Gaps in the Literature
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 2019, 30, 1183–1200. [Google Scholar] [CrossRef] [Green Version]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- Mayne, S.T.; Playdon, M.C.; Rock, C.L. Diet, nutrition, and cancer: Past, present and future. Nat. Rev. Clin. Oncol. 2016, 13, 504–515. [Google Scholar] [CrossRef]
- Key, T.J.; Schatzkin, A.; Willett, W.C.; Allen, N.E.; Spencer, E.A.; Travis, R.C. Diet, nutrition and the prevention of cancer. Public Health Nutr. 2004, 7, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Manoj Kumar, L.; George, R.J.; Anisha, P.S. Bibliometric Analysis for Medical Research. Indian J. Psychol. Med. 2022, 45, 277–282. [Google Scholar] [CrossRef]
- Thompson, D.F.; Walker, C.K. A Descriptive and Historical Review of Bibliometrics with Applications to Medical Sciences. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 551–559. [Google Scholar] [CrossRef]
- Zyoud, S.e.H.; Al-Jabi, S.W.; Amer, R.; Shakhshir, M.; Shahwan, M.; Jairoun, A.A.; Akkawi, M.; Abu Taha, A. Global research trends on the links between the gut microbiome and cancer: A visualization analysis. J. Transl. Med. 2022, 20, 83. [Google Scholar] [CrossRef]
- Li, R.; Huang, Q.; Ye, C.; Wu, C.; Luo, N.; Lu, Y.; Fang, J.; Wang, Y. Bibliometric and visual analysis in the field of ketogenic diet on cancer from 2012 to 2021. Front. Nutr. 2022, 9, 1060436. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J. A bibliometric analysis of Mediterranean diet on cancer from 2012 to 2021. Front. Nutr. 2023, 10, 1128432. [Google Scholar] [CrossRef]
- Kotepui, M.; Wannaiampikul, S.; Chupeerach, C.; Duangmano, S. A bibliometric analysis of diets and breast cancer research. Asian Pac. J. Cancer Prev. 2014, 15, 7625–7628. [Google Scholar] [CrossRef] [Green Version]
- Youn, B.-Y.; Lee, S.-Y.; Cho, W.; Bae, K.-R.; Ko, S.-G.; Cheon, C. Global Trends of Nutrition in Cancer Research: A Bibliometric and Visualized Analysis Study over the Past 10 Years. Int. J. Environ. Res. Public Health 2022, 19, 4165. [Google Scholar] [CrossRef]
- PubMed. About PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/about/ (accessed on 20 May 2023).
- Pubmed. Welcome to Medical Subject Headings. Available online: https://www.nlm.nih.gov/mesh/meshhome.html (accessed on 4 October 2022).
- Sargaço, B.; Oliveira, P.A.; Antunes, M.L.; Moreira, A.C. Effects of the Ketogenic Diet in the Treatment of Gliomas: A Systematic Review. Nutrients 2022, 14, 1007. [Google Scholar] [CrossRef]
- Minoia, C.; Gerardi, C.; Allocati, E.; Daniele, A.; De Sanctis, V.; Bari, A.; Guarini, A. The Impact of Healthy Lifestyles on Late Sequelae in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors. A Systematic Review by the Fondazione Italiana Linfomi. Cancers 2021, 13, 3135. [Google Scholar] [CrossRef]
- Noorlag, L.; De Vos, F.Y.; Kok, A.; Broekman, M.L.D.; Seute, T.; Robe, P.A.; Snijders, T.J. Treatment of malignant gliomas with ketogenic or caloric restricted diets: A systematic review of preclinical and early clinical studies. Clin. Nutr. 2019, 38, 1986–1994. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Waltman, L.; van Eck, N.J.; Noyons, E.C.M. A unified approach to mapping and clustering of bibliometric networks. J. Informetr. 2010, 4, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Stewart, S.L.; King, J.B.; Thompson, T.D.; Friedman, C.; Wingo, P.A. Cancer Mortality Surveillance—United States, 1990–2000. MMWR Surveill. Summ. 2004, 53, 1–108. [Google Scholar]
- Giovannucci, E.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Ascherio, A.; Willett, W.C. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994, 54, 2390–2397. [Google Scholar]
- The Breast Cancer Research Foundation; a cure in our lifetime. Neoplasia 2002, 4, 275–277. [CrossRef]
- The Susan G. Komen Breast Cancer Foundation. Neoplasia 1999, 1, 379–380.
- Prentice, R.L.; Pepe, M.; Self, S.G. Dietary fat and breast cancer: A quantitative assessment of the epidemiological literature and a discussion of methodological issues. Cancer Res. 1989, 49, 3147–3156. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- World Cancer Research Fund International. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.A.; Fetterman, B.; Cheung, L.C.; Wentzensen, N.; Gage, J.C.; Katki, H.A.; Befano, B.; Demarco, M.; Schussler, J.; Kinney, W.K.; et al. Epidemiologic Evidence That Excess Body Weight Increases Risk of Cervical Cancer by Decreased Detection of Precancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1184–1191. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Harvey, C.E.; Milne, R.L.; Pottinger, C.A.; Vachon, C.M.; Wilkens, L.R.; Gapstur, S.M.; Johansson, M.; Weiderpass, E.; Winn, D.M. The National Cancer Institute Cohort Consortium: An International Pooling Collaboration of 58 Cohorts from 20 Countries. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1307–1319. [Google Scholar] [CrossRef] [Green Version]
- Peto, R.; Doll, R.; Buckley, J.D.; Sporn, M.B. Can dietary beta-carotene materially reduce human cancer rates? Nature 1981, 290, 201–208. [Google Scholar] [CrossRef]
- Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef] [Green Version]
- Gaziano, J.M.; Glynn, R.J.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Sesso, H.D.; Buring, J.E. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2009, 301, 52–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Cook, N.R.; Albert, C.; Zaharris, E.; Gaziano, J.M.; Van Denburgh, M.; Buring, J.E.; Manson, J.E. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J. Natl. Cancer Inst. 2009, 101, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, B.F.; Baron, J.A.; Sandler, R.S.; Haile, R.W.; Ahnen, D.J.; Bresalier, R.S.; McKeown-Eyssen, G.; Summers, R.W.; Rothstein, R.I.; Burke, C.A.; et al. Folic acid for the prevention of colorectal adenomas: A randomized clinical trial. JAMA 2007, 297, 2351–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebbing, M.; Bønaa, K.H.; Nygård, O.; Arnesen, E.; Ueland, P.M.; Nordrehaug, J.E.; Rasmussen, K.; Njølstad, I.; Refsum, H.; Nilsen, D.W.; et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009, 302, 2119–2126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, J.; Na, X.; Zhao, A. Association between β-carotene supplementation and risk of cancer: A meta-analysis of randomized controlled trials. Nutr. Rev. 2023, nuac110. [Google Scholar] [CrossRef]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar]
- Schuz, J.; Espina, C.; Villain, P.; Herrero, R.; Leon, M.E.; Minozzi, S.; Romieu, I.; Segnan, N.; Wardle, J.; Wiseman, M.; et al. European Code Against Cancer 4th Edition—12 Ways to Reduce Your Cancer Risk. Cancer Epidemiol. 2015, 39 (Suppl. 1), S1–S10. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Barroso, S.; Trius-Soler, M.; Lamuela-Raventos, R.M.; Zamora-Ros, R. Vegetable and Fruit Consumption and Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Adv. Nutr. 2020, 11, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W. Diet and Cancer Prevention Research: From Mechanism to Implementation. J. Cancer Prev. 2020, 25, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Glenny, E.M.; Coleman, M.F.; Giles, E.D.; Wellberg, E.A.; Hursting, S.D. Designing Relevant Preclinical Rodent Models for Studying Links between Nutrition, Obesity, Metabolism, and Cancer. Annu. Rev. Nutr. 2021, 41, 253–282. [Google Scholar] [CrossRef]
- Giles, E.D.; Jackman, M.R.; MacLean, P.S. Modeling Diet-Induced Obesity with Obesity-Prone Rats: Implications for Studies in Females. Front. Nutr. 2016, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Giles, E.D.; Wellberg, E.A. Preclinical Models to Study Obesity and Breast Cancer in Females: Considerations, Caveats, and Tools. J. Mammary Gland. Biol. Neoplasia 2020, 25, 237–253. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Continuing Survey of Food Intakes by Individuals (1988–1996); United States Department of Agriculture: Washington, DC, USA, 1997. [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey (1999–2018); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. [Google Scholar]
- DeClercq, V.; Nearing, J.T.; Sweeney, E. Plant-Based Diets and Cancer Risk: What is the Evidence? Curr. Nutr. Rep. 2022, 11, 354–369. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Salamanca-Fernández, E.; Garcia-Villanova, B.; Sánchez, M.J. The Impact of Plant-Based Dietary Patterns on Cancer-Related Outcomes: A Rapid Review and Meta-Analysis. Nutrients 2020, 12, 2010. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lin, X.; Guan, Y.; Huang, J. Bibliometric and visual analysis of time-restricted eating. Front. Nutr. 2022, 9, 979702. [Google Scholar] [CrossRef]
- Chen, S.; Han, R.; Liu, H. A Bibliometric and Visualization Analysis of Intermittent Fasting. Front. Public Health 2022, 10, 946795. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Clifton, K.K.; Ma, C.X.; Fontana, L.; Peterson, L.L. Intermittent fasting in the prevention and treatment of cancer. CA Cancer J. Clin. 2021, 71, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Huang, J.; Wang, J.; Chan, P.S.F.; Lok, V.; Chen, X.; Leung, C.; Wang, H.H.X.; Lao, X.Q.; Zheng, Z.J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 2020, 35, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Delahanty, L.M.; Wadden, T.A.; Goodwin, P.J.; Alfano, C.M.; Thomson, C.A.; Irwin, M.L.; Neuhouser, M.L.; Crane, T.E.; Frank, E.; Spears, P.A.; et al. The Breast Cancer Weight Loss trial (Alliance A011401): A description and evidence for the lifestyle intervention. Obesity 2022, 30, 28–38. [Google Scholar] [CrossRef]
- Parsons, J.K.; Pierce, J.P.; Mohler, J.; Paskett, E.; Jung, S.H.; Morris, M.J.; Small, E.; Hahn, O.; Humphrey, P.; Taylor, J.; et al. Men’s Eating and Living (MEAL) study (CALGB 70807 [Alliance]): Recruitment feasibility and baseline demographics of a randomized trial of diet in men on active surveillance for prostate cancer. BJU Int. 2018, 121, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Thomson, C.A.; Crane, T.E.; Miller, A.; Gold, M.A.; Powell, M.; Bixel, K.; Van Le, L.; DiSilvestro, P.; Ratner, E.; Lele, S.; et al. Lifestyle intervention in ovarian cancer enhanced survival (LIVES) study (NRG/GOG0225): Recruitment, retention and baseline characteristics of a randomized trial of diet and physical activity in ovarian cancer survivors. Gynecol. Oncol. 2023, 170, 11–18. [Google Scholar] [CrossRef]
- Parsons, J.K.; Zahrieh, D.; Mohler, J.L.; Paskett, E.; Hansel, D.E.; Kibel, A.S.; Liu, H.; Seisler, D.K.; Natarajan, L.; White, M.; et al. Effect of a Behavioral Intervention to Increase Vegetable Consumption on Cancer Progression among Men with Early-Stage Prostate Cancer: The MEAL Randomized Clinical Trial. JAMA 2020, 323, 140–148. [Google Scholar] [CrossRef]
- Prado, C.M.; Anker, S.D.; Coats, A.J.S.; Laviano, A.; von Haehling, S. Nutrition in the spotlight in cachexia, sarcopenia and muscle: Avoiding the wildfire. J. Cachexia Sarcopenia Muscle 2021, 12, 3–8. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giles, E.D.; Purcell, S.A.; Olson, J.; Vrieling, A.; Hirko, K.A.; Woodruff, K.; Playdon, M.C.; Thomas, G.A.; Gilmore, L.A.; Moberly, H.K.; et al. Trends in Diet and Cancer Research: A Bibliometric and Visualization Analysis. Cancers 2023, 15, 3761. https://doi.org/10.3390/cancers15153761
Giles ED, Purcell SA, Olson J, Vrieling A, Hirko KA, Woodruff K, Playdon MC, Thomas GA, Gilmore LA, Moberly HK, et al. Trends in Diet and Cancer Research: A Bibliometric and Visualization Analysis. Cancers. 2023; 15(15):3761. https://doi.org/10.3390/cancers15153761
Chicago/Turabian StyleGiles, Erin D., Sarah A. Purcell, Jessica Olson, Alina Vrieling, Kelly A. Hirko, Kary Woodruff, Mary C. Playdon, Gwendolyn A. Thomas, L. Anne Gilmore, Heather K. Moberly, and et al. 2023. "Trends in Diet and Cancer Research: A Bibliometric and Visualization Analysis" Cancers 15, no. 15: 3761. https://doi.org/10.3390/cancers15153761
APA StyleGiles, E. D., Purcell, S. A., Olson, J., Vrieling, A., Hirko, K. A., Woodruff, K., Playdon, M. C., Thomas, G. A., Gilmore, L. A., Moberly, H. K., & Newell-Fugate, A. E. (2023). Trends in Diet and Cancer Research: A Bibliometric and Visualization Analysis. Cancers, 15(15), 3761. https://doi.org/10.3390/cancers15153761








