Diffusion-Weighted MRI for Treatment Response Assessment in Osteoblastic Metastases—A Repeatability Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patients and Imaging Protocol
2.2. Phantom Measurements
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
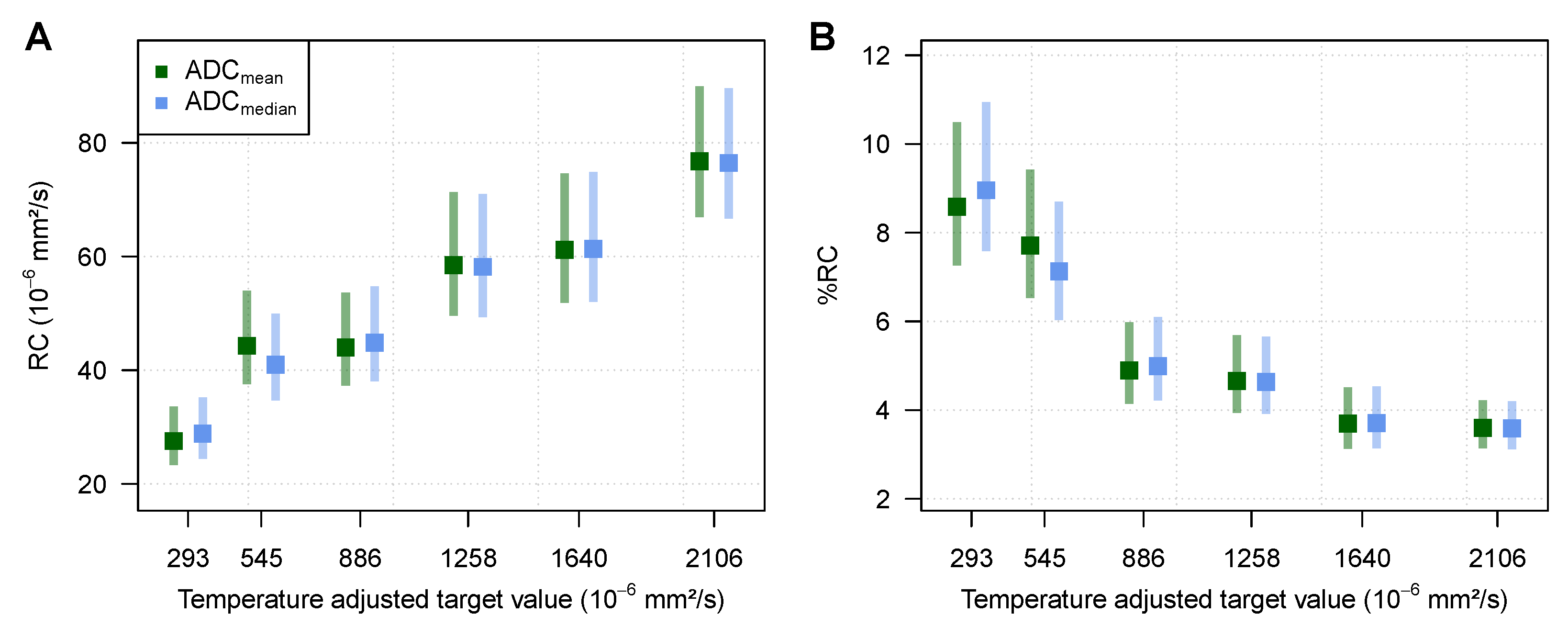
3.1. Phantom Measurements
3.2. Bone Metastases Characteristics
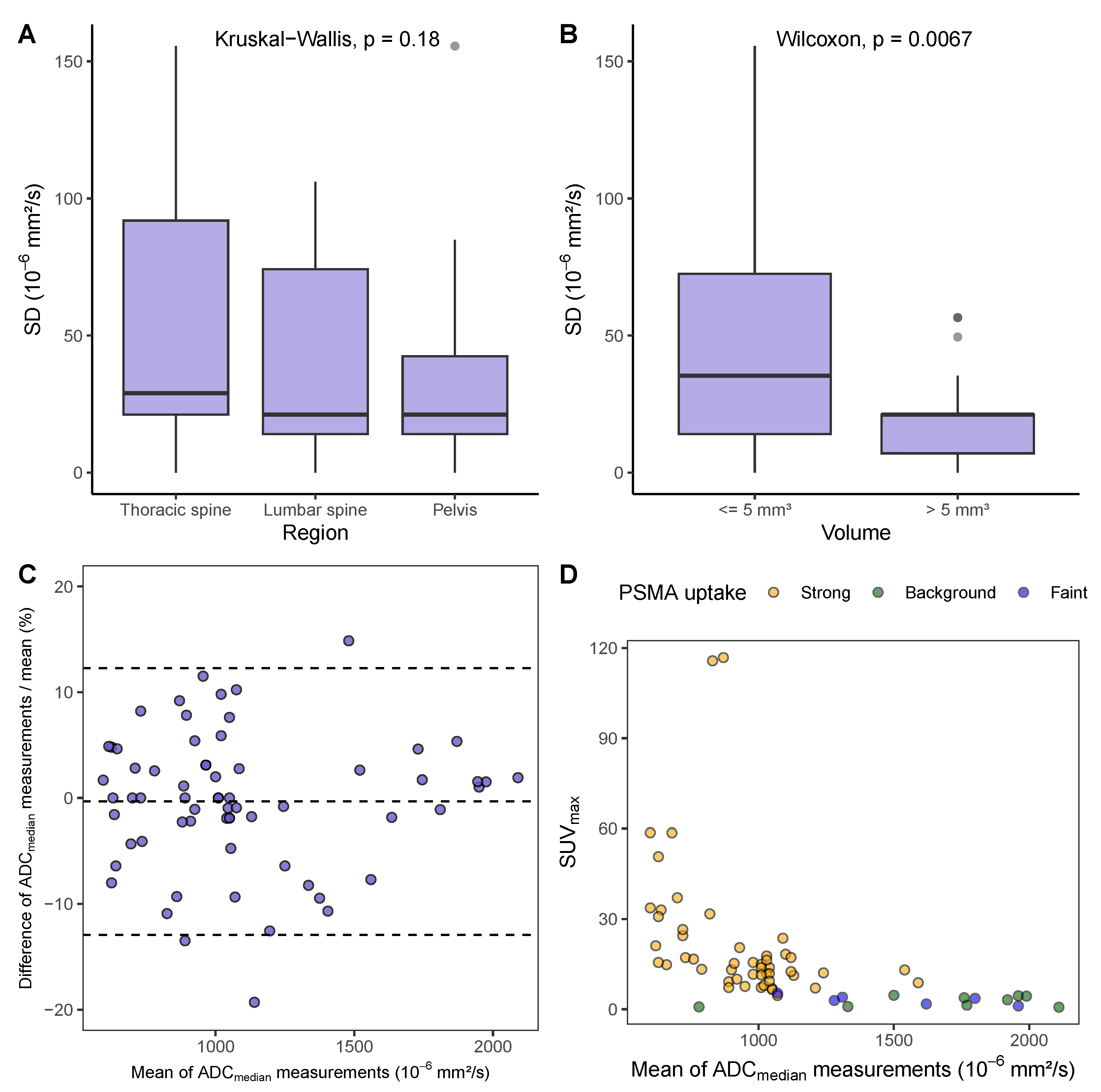
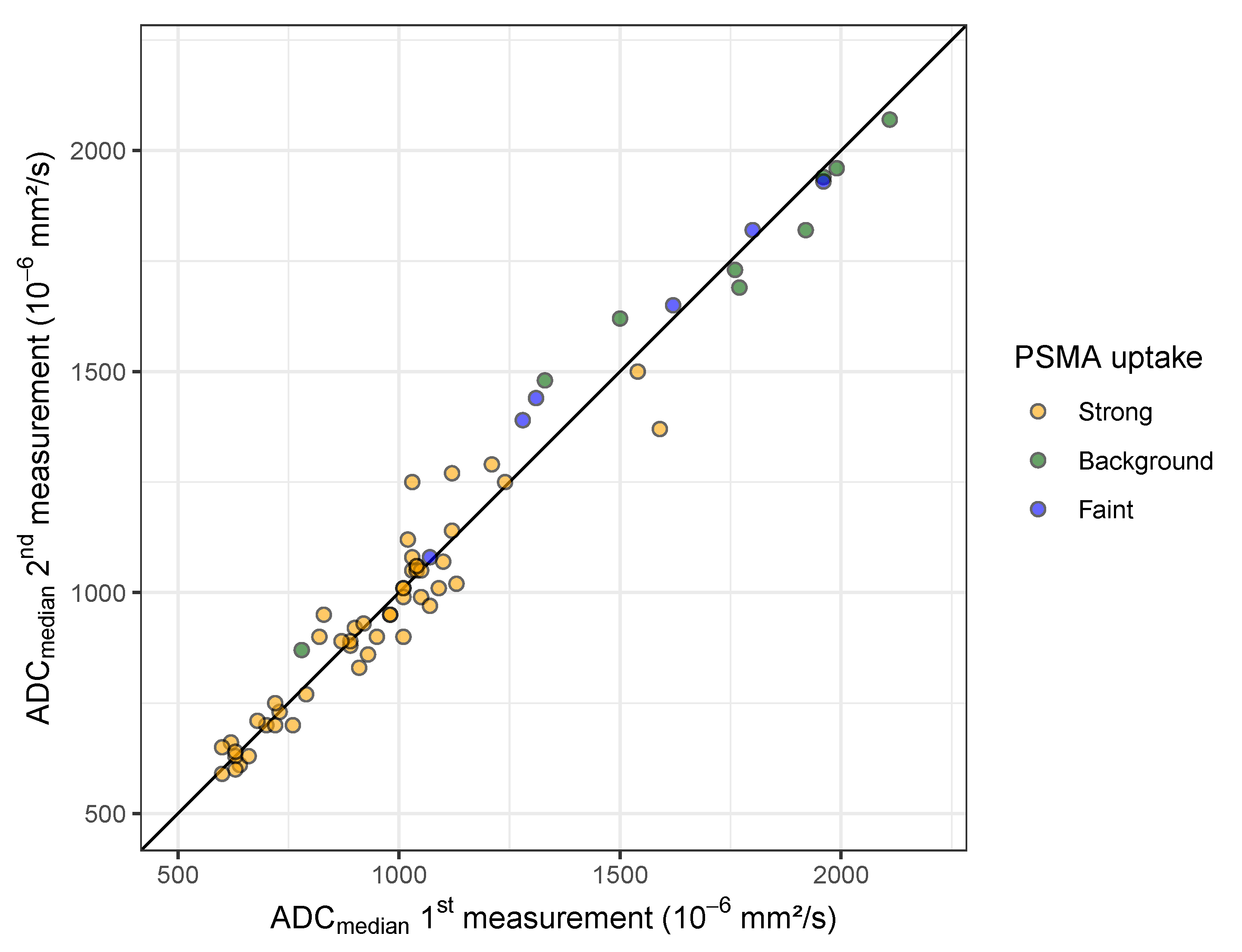
3.3. Repeatability of ADC Measurements in Bone Metastases
3.4. ADC Range in Bone Metastases and Association with PSMA-PET Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | apparent diffusion coefficient |
| CI | confidence interval |
| CT | computed tomography |
| CV | coefficient of variation |
| DWI | diffusion-weighted (magnetic resonance) imaging |
| ICC | intraclass correlation coefficient |
| IQR | interquartile range |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| PSA | prostate-specific antigen |
| PSMA | prostate-specific membrane antigen |
| PVP | polyvinylpyrrolidone |
| RC | repeatability coefficient |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| SD | standard deviation |
| SUV | standardized uptake value |
| VOI | volume of interest |
| wCV | within-subject coefficient of variation |
| wSD | within-subject standard deviation |
Appendix A
| ADCmean | ADCmedian | ||||
|---|---|---|---|---|---|
| PVP Concentration | Temperature-Adjusted Target Value | Mean Deviation from Target Value | Mean Absolute Deviation from Target Value | Mean Deviation from Target Value | Mean Absolute Deviation from Target Value |
| % | |||||
| 0 | 2106.00 | 33.81 | 36.28 (24.33) | 33.58 | 36.07 (24.20) |
| 10 | 1640.00 | 21.69 | 25.20 (17.88) | 21.90 | 25.50 (17.78) |
| 20 | 1258.00 | 3.50 | 18.54 (10.38) | 3.45 | 18.38 (10.44) |
| 30 | 886.00 | 16.80 | 19.09 (12.97) | 17.08 | 19.52 (13.09) |
| 40 | 545.00 | 31.46 | 32.17 (14.45) | 32.38 | 32.68 (14.09) |
| 50 | 293.00 | 29.13 | 29.13 (9.93) | 30.27 | 30.27 (10.40) |
| PVP Concentration | Temperature-Adjusted Target Value | Number of Measurements | SD | RC | CV | %RC |
|---|---|---|---|---|---|---|
| % | % | % | ||||
| 0 | 2106.00 | 6 × 5 × 3 | 27.6 (24.1–32.3) | 76.5 (66.7–89.6) | 1.3 (1.1–1.5) | 3.6 (3.1–4.2) |
| 10 | 1640.00 | 6 × 5 × 2 | 22.1 (18.8–27.0) | 61.4 (52.0–74.8) | 1.3 (1.1–1.6) | 3.7 (3.1–4.5) |
| 20 | 1258.00 | 6 × 5 × 2 | 21.0 (17.8–25.6) | 58.2 (49.3–71.0) | 1.7 (1.4–2.0) | 4.6 (3.9–5.7) |
| 30 | 886.00 | 6 × 5 × 2 | 16.2 (13.7–19.7) | 44.9 (38.0–54.7) | 1.8 (1.5–2.2) | 5.0 (4.2–6.1) |
| 40 | 545.00 | 6 × 5 × 2 | 14.8 (12.5–18.0) | 41.0 (34.7–50.0) | 2.6 (2.2–3.1) | 7.1 (6.0–8.7) |
| 50 | 293.00 | 6 × 5 × 2 | 10.4 (8.8–12.7) | 28.8 (24.4–35.2) | 3.23 (2.7–4.0) | 9.0 (7.6–11.0) |
| PVP Concentration | Temperature-Adjusted Target Value | Number of Measurements | SD | RC | CV | %RC |
|---|---|---|---|---|---|---|
| % | % | % | ||||
| 0 | 2106.00 | 6 × 5 × 3 | 27.7 (24.2–32.5) | 76.8 (66.9–90.0) | 1.3 (1.1–1.5) | 3.6 (3.1–4.2) |
| 10 | 1640.00 | 6 × 5 × 2 | 22.1 (18.7–26.9) | 61.1 (51.9–74.6) | 1.3 (1.1–1.6) | 3.7 (3.1–4.5) |
| 20 | 1258.00 | 6 × 5 × 2 | 21.1 (17.9–25.7) | 58.5 (49.6–71.3) | 1.7 (1.4–2.1) | 4.7 (3.9–5.7) |
| 30 | 886.00 | 6 × 5 × 2 | 15.9 (13.5–19.4) | 44.0 (37.3–53.7) | 1.8 (1.5–2.2) | 4.9 (4.1–6.0) |
| 40 | 545.00 | 6 × 5 × 2 | 16.0 (13.5–19.5) | 44.3 (37.5–54.0) | 2.8 (2.4–3.4) | 7.7 (6.5–9.4) |
| 50 | 293.00 | 6 × 5 × 2 | 9.9 (8.4–12.2) | 27.5 (23.3–33.6) | 3.1 (2.6–3.8) | 8.6 (7.3–10.5) |
| Thoracic Spine | |||||||||||
| (n = 9) | |||||||||||
| T1 0 | T2 0 | T3 0 | T4 2 | T5 1 | T6 1 | T7 1 | T8 1 | T9 0 | T10 1 | T11 1 | T12 1 |
| Lumbar Spine | |||||||||||
| (n = 15) | |||||||||||
| L1 3 | L2 2 | L3 6 | L4 2 | L5 2 | |||||||
| Pelvis | |||||||||||
| (n = 41) | |||||||||||
| right iliac bone 13 | left iliac bone 7 | sacral bone 12 | right pubic bone 1 | left pubic bone 2 | right ischium 2 | left ischium 2 | right femur 1 | left femur 1 | |||

References
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Fournier, L.; de Geus-Oei, L.F.; Regge, D.; Oprea-Lager, D.E.; D’Anastasi, M.; Bidaut, L.; Bäuerle, T.; Lopci, E.; Cappello, G.; Lecouvet, F.; et al. Twenty years on: RECIST as a biomarker of response in solid tumours an EORTC imaging group–ESOI joint paper. Front. Oncol. 2022, 11, 800547. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 052–060. [Google Scholar] [CrossRef]
- Roudier, M.P.; Morrissey, C.; True, L.D.; Higano, C.S.; Vessella, R.L.; Ott, S.M. Histopathological assessment of prostate cancer bone osteoblastic metastases. J. Urol. 2008, 180, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Oprea-Lager, D.E.; Cysouw, M.C.; Boellaard, R.; Deroose, C.M.; de Geus-Oei, L.F.; Lopci, E.; Bidaut, L.; Herrmann, K.; Fournier, L.S.; Bäuerle, T.; et al. Bone metastases are measurable: The role of whole-body MRI and positron emission tomography. Front. Oncol. 2021, 11, 772530. [Google Scholar] [CrossRef]
- Messiou, C.; Cook, G.; Reid, A.H.; Attard, G.; Dearnaley, D.; de Bono, J.S.; de Souza, N.M. The CT flare response of metastatic bone disease in prostate cancer. Acta Radiol. 2011, 52, 557–561. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1148. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar]
- Perez-Lopez, R.; Rodrigues, D.N.; Figueiredo, I.; Mateo, J.; Collins, D.J.; Koh, D.M.; de Bono, J.S.; Tunariu, N. Multiparametric magnetic resonance imaging of prostate cancer bone disease: Correlation with bone biopsy histological and molecular features. Investig. Radiol. 2018, 53, 96. [Google Scholar] [CrossRef]
- Lee, K.C.; Sud, S.; Meyer, C.R.; Moffat, B.A.; Chenevert, T.L.; Rehemtulla, A.; Pienta, K.J.; Ross, B.D. An imaging biomarker of early treatment response in prostate cancer that has metastasized to the bone. Cancer Res. 2007, 67, 3524–3528. [Google Scholar] [CrossRef][Green Version]
- Graham, T.J.; Box, G.; Tunariu, N.; Crespo, M.; Spinks, T.J.; Miranda, S.; Attard, G.; de Bono, J.; Eccles, S.A.; Davies, F.E.; et al. Preclinical evaluation of imaging biomarkers for prostate cancer bone metastasis and response to cabozantinib. J. Natl. Cancer Inst. 2014, 106, dju033. [Google Scholar] [CrossRef] [PubMed]
- Rozel, S.; Galbán, C.J.; Nicolay, K.; Lee, K.C.; Sud, S.; Neeley, C.; Snyder, L.A.; Chenevert, T.L.; Rehemtulla, A.; Ross, B.D.; et al. Synergy between anti-CCL2 and docetaxel as determined by DW-MRI in a metastatic bone cancer model. J. Cell. Biochem. 2009, 107, 58–64. [Google Scholar] [CrossRef]
- Reischauer, C.; Froehlich, J.M.; Koh, D.M.; Graf, N.; Padevit, C.; John, H.; Binkert, C.A.; Boesiger, P.; Gutzeit, A. Bone metastases from prostate cancer: Assessing treatment response by using diffusion-weighted imaging and functional diffusion maps—initial observations. Radiology 2010, 257, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Blackledge, M.D.; Collins, D.J.; Tunariu, N.; Orton, M.R.; Padhani, A.R.; Leach, M.O.; Koh, D.M. Assessment of treatment response by total tumor volume and global apparent diffusion coefficient using diffusion-weighted MRI in patients with metastatic bone disease: A feasibility study. PLoS ONE 2014, 9, e91779. [Google Scholar] [CrossRef] [PubMed]
- Emi, M.; Fujiwara, Y.; Nakajima, T.; Tsuchiya, E.; Tsuda, H.; Hirohashi, S.; Maeda, Y.; Tsuruta, K.; Miyaki, M.; Nakamura, Y. Frequent loss of heterozygosity for loci on chromosome 8p in hepatocellular carcinoma, colorectal cancer, and lung cancer. Cancer Res. 1992, 52, 5368–5372. [Google Scholar]
- Reischauer, C.; Patzwahl, R.; Koh, D.M.; Froehlich, J.M.; Gutzeit, A. Texture analysis of apparent diffusion coefficient maps for treatment response assessment in prostate cancer bone metastases—A pilot study. Eur. J. Radiol. 2018, 101, 184–190. [Google Scholar] [CrossRef]
- Perez-Lopez, R.; Mateo, J.; Mossop, H.; Blackledge, M.D.; Collins, D.J.; Rata, M.; Morgan, V.A.; Macdonald, A.; Sandhu, S.; Lorente, D.; et al. Diffusion-weighted imaging as a treatment response biomarker for evaluating bone metastases in prostate cancer: A pilot study. Radiology 2017, 283, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Messiou, C.; Collins, D.J.; Giles, S.; De Bono, J.; Bianchini, D.; de Souza, N. Assessing response in bone metastases in prostate cancer with diffusion weighted MRI. Eur. Radiol. 2011, 21, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Calderoni, L.; Maietti, E.; Farolfi, A.; Mei, R.; Louie, K.S.; Groaning, M.; Fanti, S. Prostate-Specific Membrane Antigen Expression on PET/CT in Patients with Metastatic Castration-Resistant Prostate Cancer: A Retrospective Observational Study. J. Nucl. Med. 2023, 64, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.i.; Lee, J.L.; Wibmer, A.G.; Schoder, H.; Ryu, J.S.; Vargas, H.A. Concordance between response assessment using prostate-specific membrane antigen PET and serum prostate-specific antigen levels after systemic treatment in patients with metastatic castration resistant prostate cancer: A systematic review and meta-analysis. Diagnostics 2021, 11, 663. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Cordes, M.; Goetz, T.I.; Prante, O.; Kuwert, T.; Ritt, P.; Uder, M.; Wullich, B.; Goebell, P.; Bäuerle, T. 68Ga-PSMA-11 PET/CT derived quantitative volumetric tumor parameters for classification and evaluation of therapeutic response of bone metastases in prostate cancer patients. Ann. Nucl. Med. 2019, 33, 766–775. [Google Scholar] [CrossRef]
- Shagera, Q.A.; Artigas, C.; Karfis, I.; Critchi, G.; Chanza, N.M.; Sideris, S.; Peltier, A.; Paesmans, M.; Gil, T.; Flamen, P. 68Ga-PSMA PET/CT for response assessment and outcome prediction in metastatic prostate cancer patients treated with taxane-based chemotherapy. J. Nucl. Med. 2022, 63, 1191–1198. [Google Scholar] [CrossRef]
- ElGendy, K.; Barwick, T.D.; Auner, H.W.; Chaidos, A.; Wallitt, K.; Sergot, A.; Rockall, A. Repeatability and test–retest reproducibility of mean apparent diffusion coefficient measurements of focal and diffuse disease in relapsed multiple myeloma at 3T whole body diffusion-weighted MRI (WB-DW-MRI). Br. J. Radiol. 2022, 95, 20220418. [Google Scholar] [CrossRef]
- Messiou, C.; Collins, D.; Morgan, V.; Desouza, N. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur. Radiol. 2011, 21, 1713–1718. [Google Scholar] [CrossRef]
- Lehmann, E.L.; Romano, J.P. Testing Statistical Hypotheses, 4th ed.; Springer Texts in Statistics; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistics Notes: Measurement error. BMJ 1996, 313, 744. [Google Scholar] [CrossRef]
- Bartlett, J.; Frost, C. Reliability, repeatability and reproducibility: Analysis of measurement errors in continuous variables. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 31, 466–475. [Google Scholar] [CrossRef]
- Quan, H.; Shih, W.J. Assessing reproducibility by the within-subject coefficient of variation with random effects models. Biometrics 1996, 52, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Shukla-Dave, A.; Obuchowski, N.A.; Chenevert, T.L.; Jambawalikar, S.; Schwartz, L.H.; Malyarenko, D.; Huang, W.; Noworolski, S.M.; Young, R.J.; Shiroishi, M.S.; et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J. Magn. Reson. Imaging 2019, 49, e101–e121. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. J. R. Stat. Soc. Ser. D 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420. [Google Scholar] [CrossRef]
- Brown, H.; Prescott, R. Applied Mixed Models in Medicine, 3rd ed.; John Wiley and Sons Ltd.: Chichester, UK, 2015. [Google Scholar]
- Danzer, M.F.; Eveslage, M.; Görlich, D.; Noto, B. A statistical framework for planning and analysing test-retest studies for repeatability of quantitative biomarker measurements. arXiv 2023, arXiv:2301.11690. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Reischauer, C.; Patzwahl, R.; Koh, D.M.; Froehlich, J.M.; Gutzeit, A. Non-mono-exponential analysis of diffusion-weighted imaging for treatment monitoring in prostate cancer bone metastases. Sci. Rep. 2017, 7, 5809. [Google Scholar] [CrossRef]
- Donners, R.; Figueiredo, I.; Tunariu, N.; Blackledge, M.; Koh, D.M.; de la Maza, M.d.l.D.F.; Chandran, K.; De Bono, J.S.; Fotiadis, N. Multiparametric bone MRI can improve CT-guided bone biopsy target selection in cancer patients and increase diagnostic yield and feasibility of next-generation tumour sequencing. Eur. Radiol. 2022, 32, 4647–4656. [Google Scholar] [CrossRef]
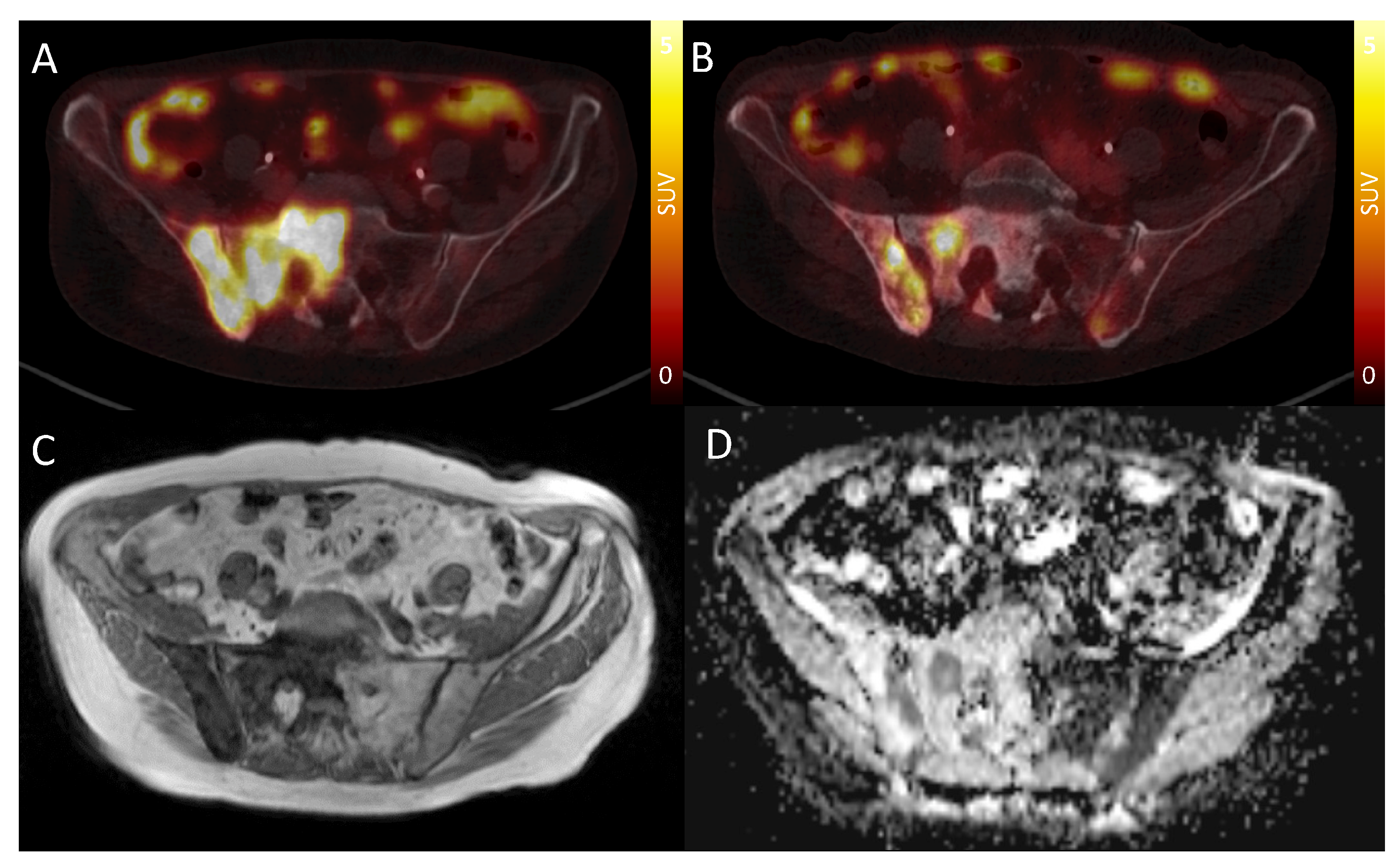
| ID | Age | PSA 1 | Pretreatments | Area Covered by DWI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | ng/mL | Px | RTx | LHRH | Abi | Enza | PSMA | RTxB | CTX | Apa | Thorax | Lumbar | Pelvis | |
| 1 | 77 | 0.34 | x | x | x | x | x | x | x | x | x | x | x | |
| 2 | 79 | 110.00 | x | x | x | x | x | x | x | x | x | x | ||
| 3 | 83 | 323.00 | x | x | x | x | x | x | ||||||
| 4 | 73 | 3.87 | x | x | x | x | x | x | ||||||
| 5 * | 53 | 3.98 | x | x | x | x | x | |||||||
| 6 | 65 | 110.00 | x | x | x | x | x | x | x | |||||
| 7 | 78 | 407.00 | x | x | x | x | x | x | x | x | ||||
| 8 | 67 | 3.02 | x | x | x | x | x | |||||||
| 9 | 74 | 1.49 | x | x | x | |||||||||
| ADCmean | ADCmedian | |
|---|---|---|
| wSD () | 59.95 (51.18–72.37) | 50.71 (43.29–61.22) |
| RC () | 166.18 (141.87–200.61) | 140.56 (120.00–169.68) |
| wCV (%) | 5.51 (4.57–6.69) | 4.65 (3.85–5.66) |
| %RC (%) | 15.27 (12.66–18.55) | 12.90 (10.68–15.70) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eveslage, M.; Rassek, P.; Riegel, A.; Maksoud, Z.; Bauer, J.; Görlich, D.; Noto, B. Diffusion-Weighted MRI for Treatment Response Assessment in Osteoblastic Metastases—A Repeatability Study. Cancers 2023, 15, 3757. https://doi.org/10.3390/cancers15153757
Eveslage M, Rassek P, Riegel A, Maksoud Z, Bauer J, Görlich D, Noto B. Diffusion-Weighted MRI for Treatment Response Assessment in Osteoblastic Metastases—A Repeatability Study. Cancers. 2023; 15(15):3757. https://doi.org/10.3390/cancers15153757
Chicago/Turabian StyleEveslage, Maria, Philipp Rassek, Arne Riegel, Ziad Maksoud, Jochen Bauer, Dennis Görlich, and Benjamin Noto. 2023. "Diffusion-Weighted MRI for Treatment Response Assessment in Osteoblastic Metastases—A Repeatability Study" Cancers 15, no. 15: 3757. https://doi.org/10.3390/cancers15153757
APA StyleEveslage, M., Rassek, P., Riegel, A., Maksoud, Z., Bauer, J., Görlich, D., & Noto, B. (2023). Diffusion-Weighted MRI for Treatment Response Assessment in Osteoblastic Metastases—A Repeatability Study. Cancers, 15(15), 3757. https://doi.org/10.3390/cancers15153757







