Postoperative Changes in Systemic Immune Tolerance Following Major Oncologic versus Minor Maxillofacial Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Collective
2.2. Sampling of Peripheral Blood
2.3. Analysis of IL6, IL10, FOXP3, and PD-L1 Expression by Quantitative Real-Time Reverse-Transcriptase Polymerase Chain Reaction (RT-qPCR)
2.4. Statistical Analysis
3. Results
3.1. Patients Collective
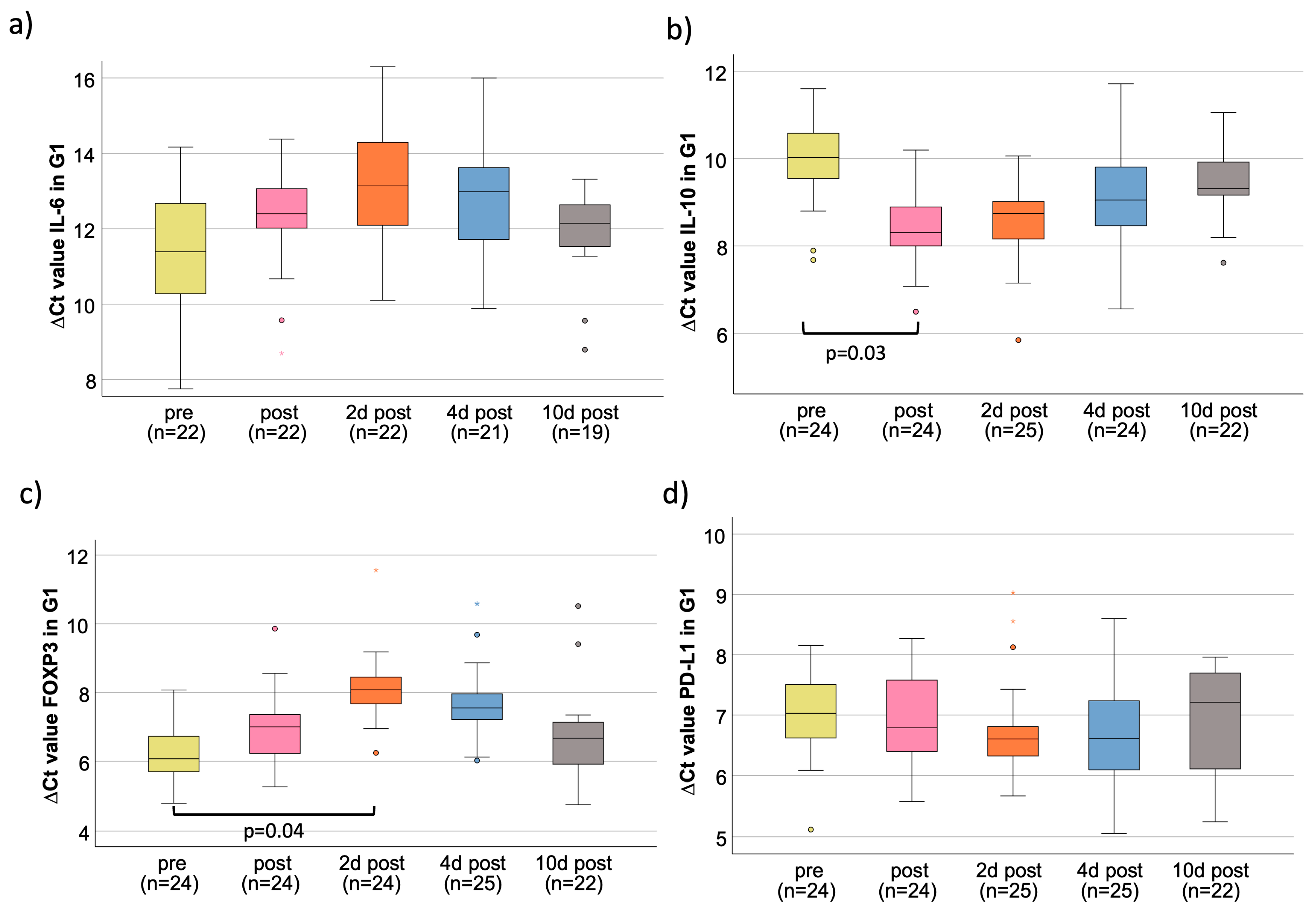
3.2. IL-6
3.3. IL-10
3.4. FOXP3
3.5. PD-L1
4. Discussion
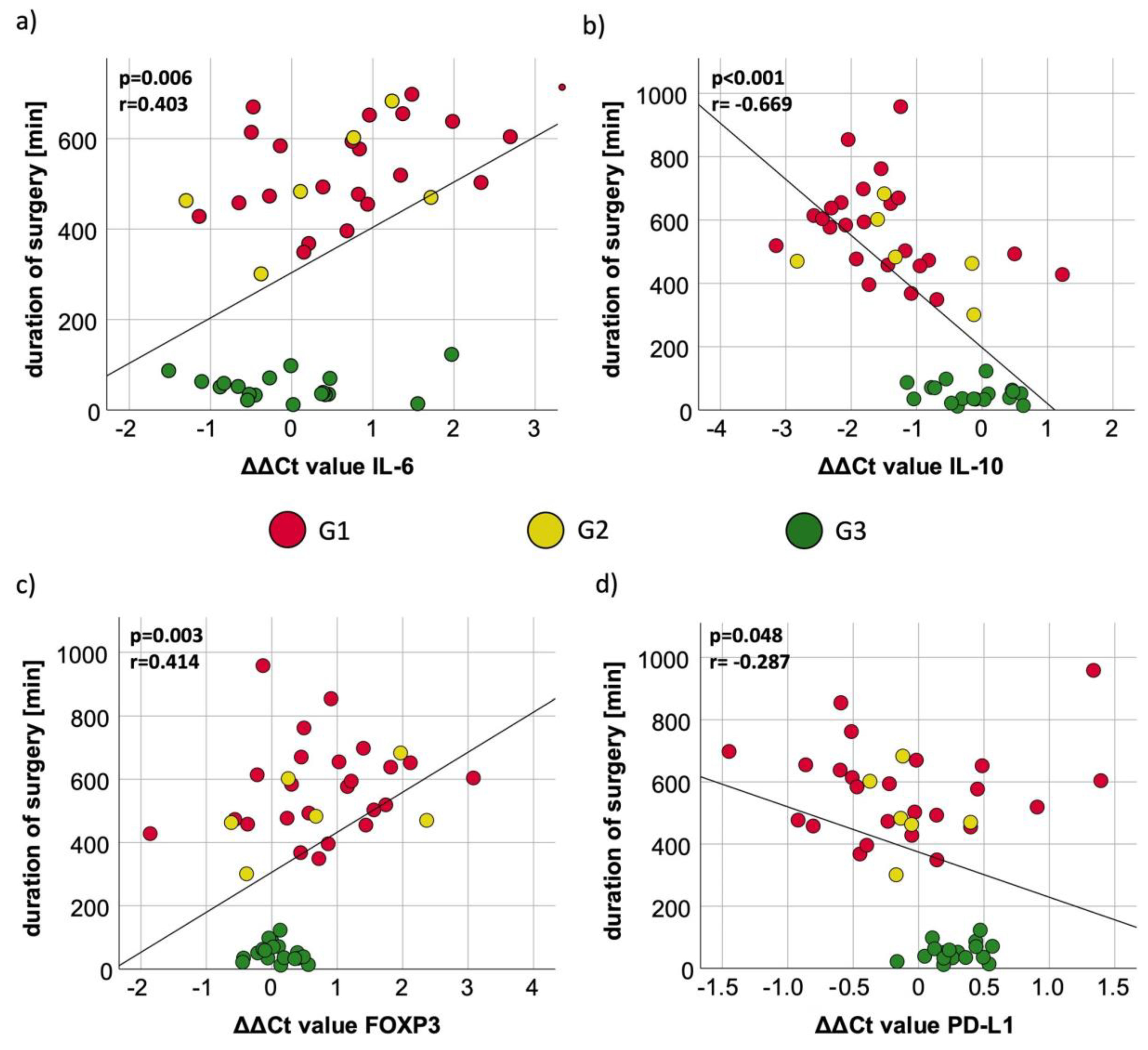
4.1. Influence of Surgical Trauma on Peripheral Immune Tolerance
4.2. Influence of Duration of Surgery on Peripheral Immune Tolerance
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torrance, H.D.T.; Longbottom, E.R.; Vivian, M.E.; Lalabekyan, B.; Abbott, T.E.F.; Ackland, G.L.; Hinds, C.J.; Pearse, R.M.; O’dwyer, M.J. Post-operative immune suppression is mediated via reversible, Interleukin-10 dependent pathways in circulating monocytes following major abdominal surgery. PLoS ONE 2018, 13, e0203795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmos, M.; Lutz, R.; Büntemeyer, T.-O.; Glajzer, J.; Nobis, C.-P.; Ries, J.; Möst, T.; Eckstein, M.; Hecht, M.; Gostian, A.-O.; et al. Case report: Patient specific combination of surgery and immunotherapy in advanced squamous cell carcinoma of the head and neck—A case series and review of literature. Front. Immunol. 2022, 13, 970823. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Della Corte, C.M.; Di Liello, R.; Viscardi, G.; Sparano, F.; Iacovino, M.L.; Paragliola, F.; Piccolo, A.; Napolitano, S.; Martini, G.; et al. Immunotherapy for head and neck cancer: Present and future. Crit. Rev. Oncol. Hematol. 2022, 174, 103679. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Park, J.J.; Mendis, S.; Rai, R.; Xu, W.; Lo, S.; Drummond, M.; Rowe, C.; Wong, A.; McArthur, G.; et al. Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br. J. Cancer 2017, 116, 1558–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Tie, Y.; Tu, C.; Wei, X. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin. Transl. Med. 2020, 10, 199–223. [Google Scholar] [CrossRef]
- Alieva, M.; van Rheenen, J.; Broekman, M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 2018, 35, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Rossaint, J.; Margraf, A.; Zarbock, A. Perioperative inflammation. Anaesthesist 2019, 68, 421–427. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Lu, L.; Barbi, J.; Pan, F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gong, R.; Zhao, C.; Lei, K.; Sun, X.; Ren, H. Human FOXP3 and tumour microenvironment. Immunology 2023, 168, 248–255. [Google Scholar] [CrossRef]
- Hayashi, T.; Yoshikawa, K.; Suzuki, S.; Gosho, M.; Ueda, R.; Kazaoka, Y. Tumor-infiltrating FoxP3+ T cells are associated with poor prognosis in oral squamous cell carcinoma. Clin. Exp. Dent. Res. 2022, 8, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Wang, Q.; Gu, J.; Lu, L. Clinical and Basic Research Progress on Treg-Induced Immune Tolerance in Liver Transplantation. Front. Immunol. 2021, 12, 535012. [Google Scholar] [CrossRef]
- Qiu, Y.; Ke, S.; Chen, J.; Qin, Z.; Zhang, W.; Yuan, Y.; Meng, D.; Zhao, G.; Wu, K.; Li, B.; et al. FOXP3+ regulatory T cells and the immune escape in solid tumours. Front. Immunol. 2022, 13, 982986. [Google Scholar] [CrossRef] [PubMed]
- Pesenacker, A.M.; Cook, L.; Levings, M.K. The role of FOXP3 in autoimmunity. Curr. Opin. Immunol. 2016, 43, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin. Ther. Targets 2018, 22, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.; Wehrhan, F.; Baran, C.; Agaimy, A.; Büttner-Herold, M.; Preidl, R.; Neukam, F.W.; Ries, J. PD-L1 expression in tumor tissue and peripheral blood of patients with oral squamous cell carcinoma. Oncotarget 2017, 8, 112584–112597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Irlbeck, T.; Zwissler, B.; Bauer, A. ASA classification: Transition in the course of time and depiction in the literature. Anaesthesist 2017, 66, 5–10. [Google Scholar] [CrossRef]
- Wolff, K.-D.; Al-Nawas, B.; Al-Sharif, U.; Beck, J.; Bikowski, K.; Bissinger, O.; Böhme, P.; Bönte-Hieronymus, I.; Bootz, F.; Bozzato, A.; et al. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Diagnostik und Therapie des Mundhöhlenkarzinoms, Langversion 3.0, AWMF Registernummer: 007/100OL; Leitlinienprogramm Onkologie: Berlin, Germany, 2021. [Google Scholar]
- Robbins, K.T.; Clayman, G.; Levine, P.A.; Medina, J.; Sessions, R.; Shaha, A.; Som, P.; Wolf, G.T. Neck dissection classification update: Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Weber, M.; Moebius, P.; Büttner-Herold, M.; Amann, K.; Preidl, R.; Neukam, F.W.; Wehrhan, F. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas—An immunohistochemical study. Br. J. Cancer 2015, 113, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, Y.; Li, X.; Chen, G.; Liang, H.; Wu, Y.; Tong, J.; Ouyang, W. Propranolol Attenuates Surgical Stress-Induced Elevation of the Regulatory T Cell Response in Patients Undergoing Radical Mastectomy. J. Immunol. 2016, 196, 3460–3469. [Google Scholar] [CrossRef] [Green Version]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10, eaan3464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.; Wehrhan, F.; Baran, C.; Agaimy, A.; Büttner-Herold, M.; Kesting, M.; Ries, J. Prognostic significance of PD-L2 expression in patients with oral squamous cell carcinoma-A comparison to the PD-L1 expression profile. Cancer Med. 2019, 8, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Predina, J.; Eruslanov, E.; Judy, B.; Kapoor, V.; Cheng, G.; Wang, L.-C.; Sun, J.; Moon, E.K.; Fridlender, Z.G.; Albelda, S.; et al. Changes in the local tumor microenvironment in recurrent cancers may explain the failure of vaccines after surgery. Proc. Natl. Acad. Sci. USA 2013, 110, E415–E424. [Google Scholar] [CrossRef]
- Onuma, A.E.; Zhang, H.; Gil, L.; Huang, H.; Tsung, A. Surgical Stress Promotes Tumor Progression: A Focus on the Impact of the Immune Response. J. Clin. Med. 2020, 9, 4096. [Google Scholar] [CrossRef]
- Lachmann, G.; Von Haefen, C.; Kurth, J.; Yuerek, F.; Spies, C. Innate immunity recovers earlier than acquired immunity during severe postoperative immunosuppression. Int. J. Med. Sci. 2018, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakos, O.; Lawson, C.; Rouleau, S.; Tai, L.-H. Combining surgery and immunotherapy: Turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarhini, A.A.; Edington, H.; Butterfield, L.H.; Lin, Y.; Shuai, Y.; Tawbi, H.; Sander, C.; Yin, Y.; Holtzman, M.; Johnson, J.; et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE 2014, 9, e87705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, A.; Takeuchi, H.; Matsuda, S.; Ogura, M.; Miyasho, T.; Nakamura, R.; Takahashi, T.; Wada, N.; Kawakubo, H.; Saikawa, Y.; et al. Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann. Surg. Oncol. 2015, 22, 3130–3135. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Shi, X.; Xu, W.; Zuo, M. The Effect of Anesthesia on the Immune System in Colorectal Cancer Patients. Can. J. Gastroenterol. Hepatol. 2018, 2018, 7940603. [Google Scholar] [CrossRef] [Green Version]
| Real-Time qPCR | |||||
|---|---|---|---|---|---|
| Primer | Sequence (5′ to 3′) | Primer Length (bp) | Amplicon Length (bp) | Annealing Temp. (°C) | Accession Number |
| IL-6/s | TCG GTC CAG TTG CCT TCT CC | 20 | 127 | 60 | NM_000600 |
| IL-6/as | TCT GAA GAG GTC AGT GGC TGT C | 22 | 127 | 60 | |
| IL-10/s | AAG GCG CAT GTG AAC TCC C | 19 | 99 | 60 | NM_000572.3 |
| IL10/as | GGC CTT GCT CTT GTT TTC ACA G | 22 | 99 | 61 | |
| FOXP3/s | CAC TGC TGG CAA ATG GTG TC | 20 | 226 | 60 | NM_014009.4 |
| FOXP3/as | TCA TCC AGA AGA TGG TCC GC | 20 | 226 | 60 | |
| PD-L1/s | AGC TAT GGT GGT GCC GAC TA | 20 | 152 | 61 | NM_014143.3 NM_001314029 |
| PD-L1/as | CAG ATG ACT TCG GCC TTG GG | 20 | 152 | 61 | |
| GAPDH/s | GAC CCC TTC ATT GAC CTC AAC TA | 23 | 122 | 60 | NM_002046.5 |
| GAPDH/as | GAA TTT GCC ATG GGT GGA AT | 20 | 122 | 60 | |
| IL6 in G1 | Pre | Post | 2 d Post | 4 d Post | 10 d Post |
|---|---|---|---|---|---|
| Sample size (n) | 22 | 22 | 22 | 21 | 19 |
| Median ΔCt value | 11.39 | 12.40 | 13.14 | 12.94 | 12.15 |
| Fold change to “pre“ | 1.00 | 0.50 | 0.30 | 0.34 | 0.59 |
| Average (±standard derivation) | 11.39 (±1.57) | 12.32 (±1.41) | 13.17 (±1.70) | 12.80 (±1.69) | 11.92 (±1.15) |
| p-value to “pre“ | n.d. | 0.20 | 0.19 | 0.07 | 0.19 |
| IL6 in G2 | Pre | Post | 2 d Post | 4 d Post | 10 d Post |
| Sample size (n) | 7 | 6 | 7 | 5 | 5 |
| Median ΔCt value | 11.35 | 11.76 | 13.51 | 11.55 | 12.19 |
| Fold change to “pre“ | 1.00 | 0.75 | 0.22 | 0.87 | 0.56 |
| Average (±standard derivation) | 11.69 (±1.04) | 12.15 (±1.11) | 13.30 (±0.96) | 11.71 (±1.06) | 11.97 (±0.60) |
| p-value to “pre“ | n.d. | 0.35 | 0.12 | 0.43 | 0.49 |
| IL6 in G3 | Pre | Post | 2 d Post | 4 d Post | |
| Sample size (n) | 18 | 18 | 7 | 5 | |
| Median ΔCt value | 11.03 | 11.00 | 11.51 | 12.37 | |
| Fold change to “pre“ | 1.00 | 1.02 | 0.72 | 0.40 | |
| Average (±standard derivation) | 11.10 (±1.36) | 11.04 (±1.02) | 11.56 (±1.29) | 12.15 (±0.89) | |
| p-value to “pre“ | n.d. | 0.33 | 0.44 | 0.31 |
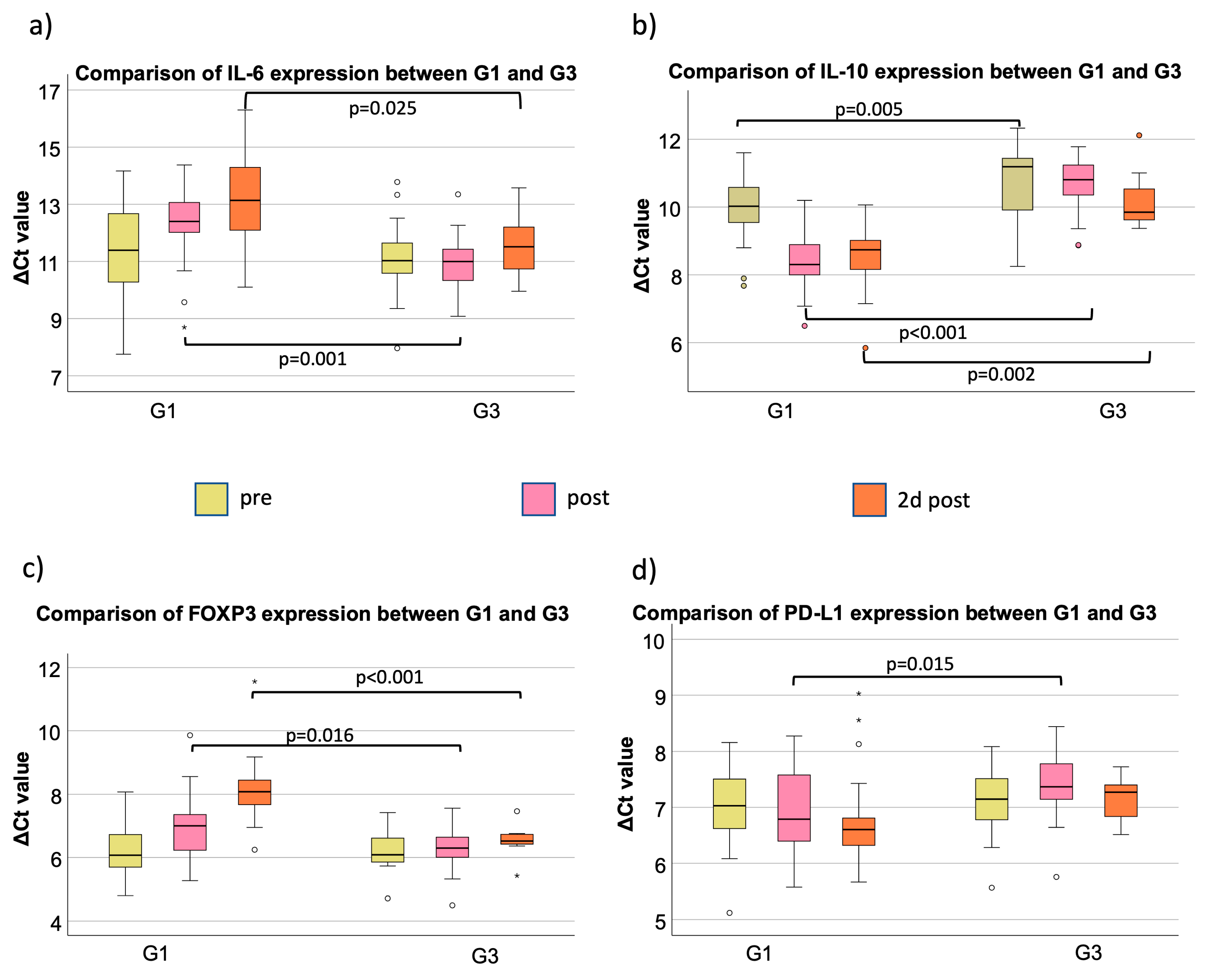
| IL6 | Pre | Post | 2 d Post | ||||||
|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | |
| Median ΔCt value | 11.39 | 11.35 | 11.03 | 12.40 | 11.76 | 11.00 | 13.14 | 13.51 | 11.51 |
| Fold change to G1 | 1.00 | 1.03 | 1.28 | 1.00 | 1.56 | 2.64 | 1.00 | 0.77 | 3.10 |
| p-value to G1 | n.d. | 0.92 | 0.45 | n.d. | 0.22 | <0.01 | n.d. | 0.80 | 0.03 |
| p-value G2/G3 | n.d. | 0.36 | n.d. | 0.02 | n.d. | 0.02 | |||
| IL10 | Pre | Post | 2 d Post | ||||||
| G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | |
| Median ΔCt value | 10.02 | 9.98 | 11.19 | 8.31 | 8.90 | 10.81 | 8.74 | 8.88 | 9.85 |
| Fold change to G1 | 1.00 | 1.03 | 0.44 | 1.00 | 0.66 | 0.18 | 1.00 | 0.91 | 0.46 |
| p-value to G1 | n.d. | 0.89 | <0.01 | n.d. | 0.21 | <0.01 | n.d. | 0.19 | <0.01 |
| p-value G2/G3 | n.d. | 0.09 | n.d. | <0.01 | n.d. | <0.01 | |||
| FOXP3 | Pre | Post | 2 d Post | ||||||
| G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | |
| Median ΔCt value | 6.07 | 6.24 | 6.09 | 7.00 | 7.00 | 6.30 | 8.08 | 8.19 | 6.52 |
| Fold change to G1 | 1.00 | 0.89 | 0.99 | 1.00 | 1.00 | 1.62 | 1.00 | 0.93 | 2.95 |
| p-value to G1 | n.d. | 0.37 | 0.98 | n.d. | 0.80 | 0.02 | n.d. | 0.64 | <0.01 |
| p-value G2/G3 | n.d. | 0.40 | n.d. | 0.16 | n.d. | 0.02 | |||
| PD-L1 | Pre | Post | 2 d Post | ||||||
| G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | |
| Median ΔCt value | 7.03 | 7.45 | 7.15 | 6.79 | 7.21 | 7.37 | 6.60 | 7.19 | 7.27 |
| Fold change to G1 | 1.00 | 0.75 | 0.92 | 1.00 | 0.75 | 0.67 | 1.00 | 0.66 | 0.63 |
| p-value to G1 | n.d. | 0.32 | 0.70 | n.d. | 0.35 | 0.02 | n.d. | 0.08 | 0.05 |
| p-value G2/G3 | n.d. | 0.51 | n.d. | 0.46 | n.d. | 0.95 | |||
| IL10 in G1 | Pre | Post | 2 d Post | 4 d Post | 10 d Post |
|---|---|---|---|---|---|
| Sample size (n) | 24 | 24 | 25 | 24 | 22 |
| Median ΔCt value | 10.02 | 8.31 | 8.74 | 9.05 | 9.31 |
| Fold change to “pre“ | 1.00 | 3.27 | 2.43 | 1.96 | 1.64 |
| Average (±standard derivation) | 9.95 (±0.91) | 8.45 (±0.84) | 8.63 (±0.94) | 9.18 (±1.08) | 9.46 (±0.80) |
| p-value to “pre“ | n.d. | 0.03 | 0.06 | 0.15 | 0.08 |
| IL10 in G2 | Pre | Post | 2 d Post | 4 d Post | 10 d Post |
| Sample size (n) | 7 | 6 | 7 | 7 | 6 |
| Median ΔCt value | 9.98 | 8.90 | 8.88 | 9.21 | 9.97 |
| Fold change to “pre“ | 1.00 | 2.11 | 2.14 | 1.71 | 1.01 |
| Average (±standard derivation) | 10.11 (±0.70) | 8.88 (±0.62) | 9.03 (±0.32) | 9.36 (±0.50) | 10.20 (±0.80) |
| p-value to “pre“ | n.d. | 0.33 | 0.28 | 0.21 | 0.48 |
| IL10 in G3 | Pre | Post | 2 d Post | 4 d Post | |
| Sample size (n) | 18 | 18 | 7 | 5 | |
| Median ΔCt value | 11.19 | 10.81 | 9.85 | 10.67 | |
| Fold change to “pre“ | 1.00 | 1.30 | 2.53 | 1.43 | |
| Average (±standard derivation) | 10.80 (±1.05) | 10.64 (±0.85) | 10.23 (±0.99) | 10.81 (±0.51) | |
| p-value to “pre“ | n.d. | 0.48 | 0.26 | 0.39 |
| FOXP3 in G1 | Pre | Post | 2 d Post | 4 d Post | 10 d Post |
|---|---|---|---|---|---|
| Sample size (n) | 24 | 24 | 24 | 25 | 22 |
| Median ΔCt value | 6.07 | 7.00 | 8.08 | 7.55 | 6.67 |
| Fold change to “pre“ | 1.00 | 0.52 | 0.25 | 0.36 | 0.66 |
| Average (±standard derivation) | 6.25 (±0.85) | 7.01 (±1.05) | 8.12 (±1.00) | 7.74 (±1.21) | 6.71 (±1.29) |
| p-value to “pre“ | n.d. | 0.27 | 0.04 | 0.06 | 0.27 |
| FOXP3 in G2 | Pre | Post | 2 d Post | 4 d Post | 10 d Post |
| Sample size (n) | 7 | 6 | 7 | 7 | 6 |
| Median ΔCt value | 6.24 | 7.00 | 8.19 | 7.66 | 6.79 |
| Fold change to “pre“ | 1.00 | 0.59 | 0.26 | 0.37 | 0.68 |
| Average (±standard derivation) | 6.43 (±0.98) | 7.17 (±1.33) | 8.15 (±1.01) | 7.69 (±1.29) | 6.70 (±0.71) |
| p-value to “pre“ | n.d. | 0.33 | 0.18 | 0.25 | 0.39 |
| FOXP3 in G3 | Pre | Post | 2 d Post | 4 d Post | |
| Sample size (n) | 18 | 18 | 7 | 5 | |
| Median ΔCt value | 6.09 | 6.30 | 6.52 | 5.96 | |
| Fold change to “pre“ | 1.00 | 0.86 | 0.74 | 1.09 | |
| Average (±standard derivation) | 6.18 (±0.61) | 6.25 (±0.67) | 6.53 (±0.61) | 5.96 (±0.69) | |
| p-value to “pre“ | n.d. | 0.39 | 0.46 | 0.30 |
| PD-L1 in G1 | Pre | Post | 2 d Post | 4 d Post | 10 d Post |
|---|---|---|---|---|---|
| Sample size (n) | 24 | 24 | 25 | 25 | 22 |
| Median ΔCt value | 7.03 | 6.79 | 6.60 | 6.61 | 7.21 |
| Fold change to “pre“ | 1.00 | 1.18 | 1.35 | 1.34 | 0.88 |
| Average (±standard derivation) | 7.00 (±0.68) | 6.88 (±0.72) | 6.76 (±0.80) | 6.65 (±0.82) | 6.87 (±0.96) |
| p-value to “pre“ | n.d. | 0.44 | 0.21 | 0.26 | 0.50 |
| PD-L1 in G2 | Pre | Post | 2 d Post | 4 d Post | 10 d Post |
| Sample size (n) | 7 | 6 | 7 | 7 | 6 |
| Median ΔCt value | 7.45 | 7.21 | 7.19 | 7.30 | 7.11 |
| Fold change to “pre“ | 1.00 | 1.18 | 1.20 | 1.11 | 1.27 |
| Average (±standard derivation) | 7.23 (±0.63) | 7.12 (±0.70) | 7.14 (±0.56) | 7.14 (±0.66) | 6.69 (±1.93) |
| p-value to “pre“ | n.d. | 0.38 | 0.44 | 0.44 | 0.48 |
| PD-L1 in G3 | Pre | Post | 2 d Post | 4 d Post | |
| Sample size (n) | 18 | 18 | 7 | 5 | |
| Median ΔCt value | 7.15 | 7.37 | 7.27 | 6.98 | |
| Fold change to “pre“ | 1.00 | 0.86 | 0.92 | 1.13 | |
| Average (±standard derivation) | 7.12 (±0.63) | 7.40 (±0.62) | 7.14 (±0.44) | 7.01 (±0.25) | |
| p-value to “pre“ | n.d. | 0.45 | 0.41 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trumet, L.; Ries, J.; Sobl, P.; Ivenz, N.; Wehrhan, F.; Lutz, R.; Kesting, M.; Weber, M. Postoperative Changes in Systemic Immune Tolerance Following Major Oncologic versus Minor Maxillofacial Surgery. Cancers 2023, 15, 3755. https://doi.org/10.3390/cancers15153755
Trumet L, Ries J, Sobl P, Ivenz N, Wehrhan F, Lutz R, Kesting M, Weber M. Postoperative Changes in Systemic Immune Tolerance Following Major Oncologic versus Minor Maxillofacial Surgery. Cancers. 2023; 15(15):3755. https://doi.org/10.3390/cancers15153755
Chicago/Turabian StyleTrumet, Leah, Jutta Ries, Philip Sobl, Niclas Ivenz, Falk Wehrhan, Rainer Lutz, Marco Kesting, and Manuel Weber. 2023. "Postoperative Changes in Systemic Immune Tolerance Following Major Oncologic versus Minor Maxillofacial Surgery" Cancers 15, no. 15: 3755. https://doi.org/10.3390/cancers15153755
APA StyleTrumet, L., Ries, J., Sobl, P., Ivenz, N., Wehrhan, F., Lutz, R., Kesting, M., & Weber, M. (2023). Postoperative Changes in Systemic Immune Tolerance Following Major Oncologic versus Minor Maxillofacial Surgery. Cancers, 15(15), 3755. https://doi.org/10.3390/cancers15153755





