Efficacy and Safety of Rechallenge with BRAF/MEK Inhibitors in Advanced Melanoma Patients: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
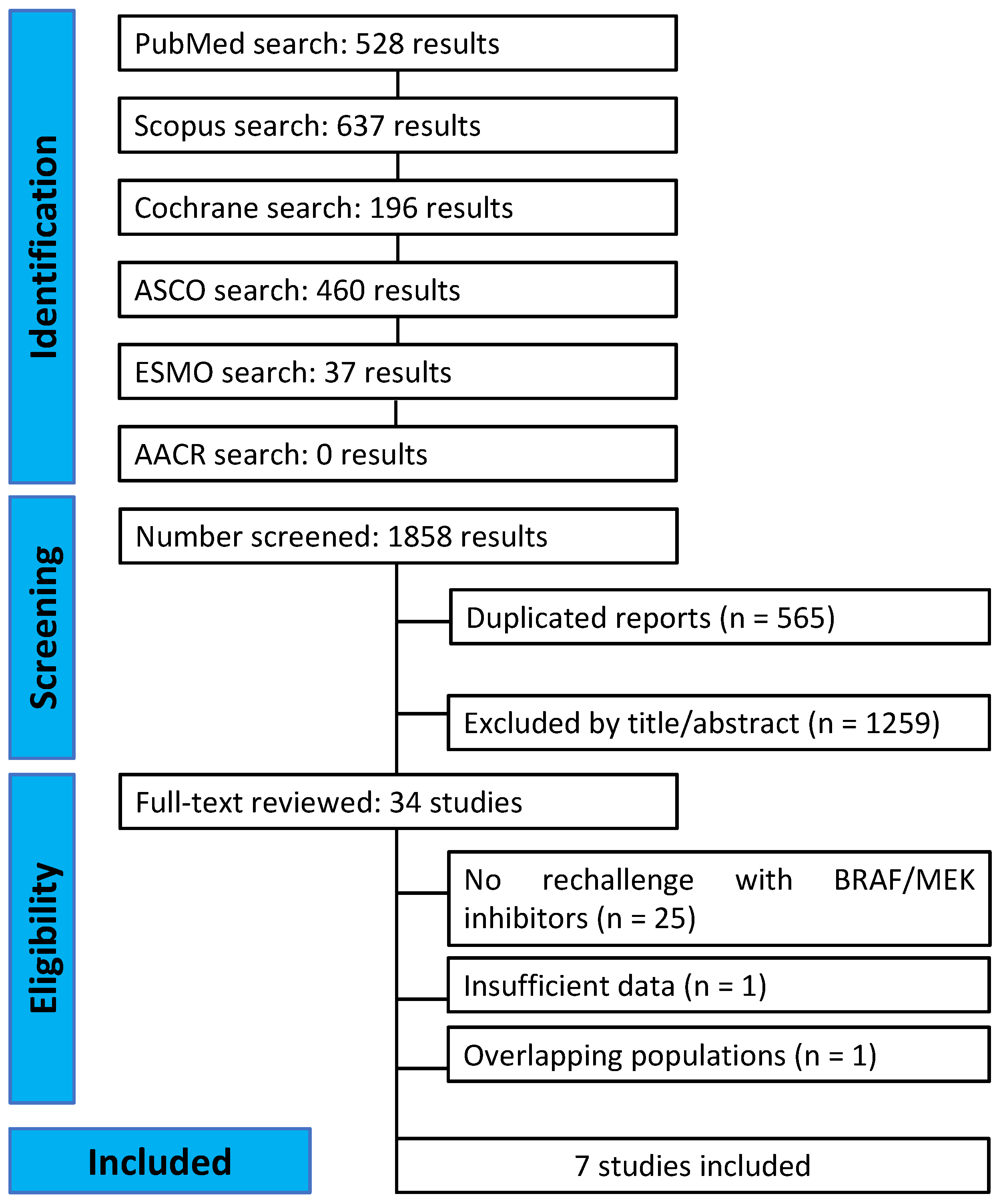
2.2. Search Strategy and Data Extraction
2.3. Endpoints and Subanalysis
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
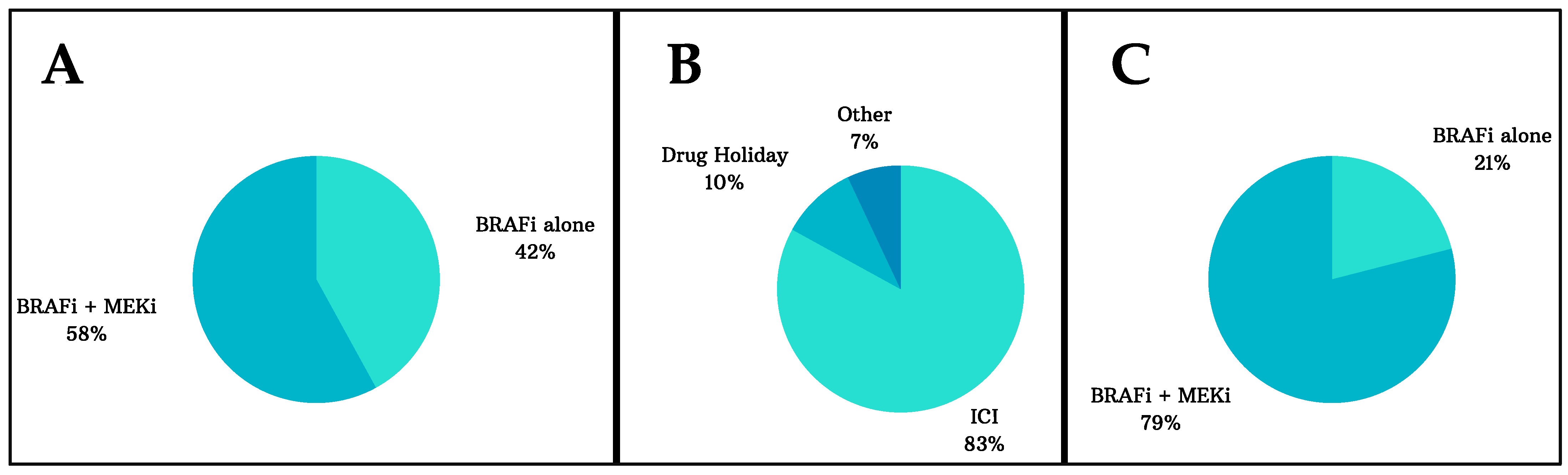
3.1. Characteristics of the Included Studies and Patients
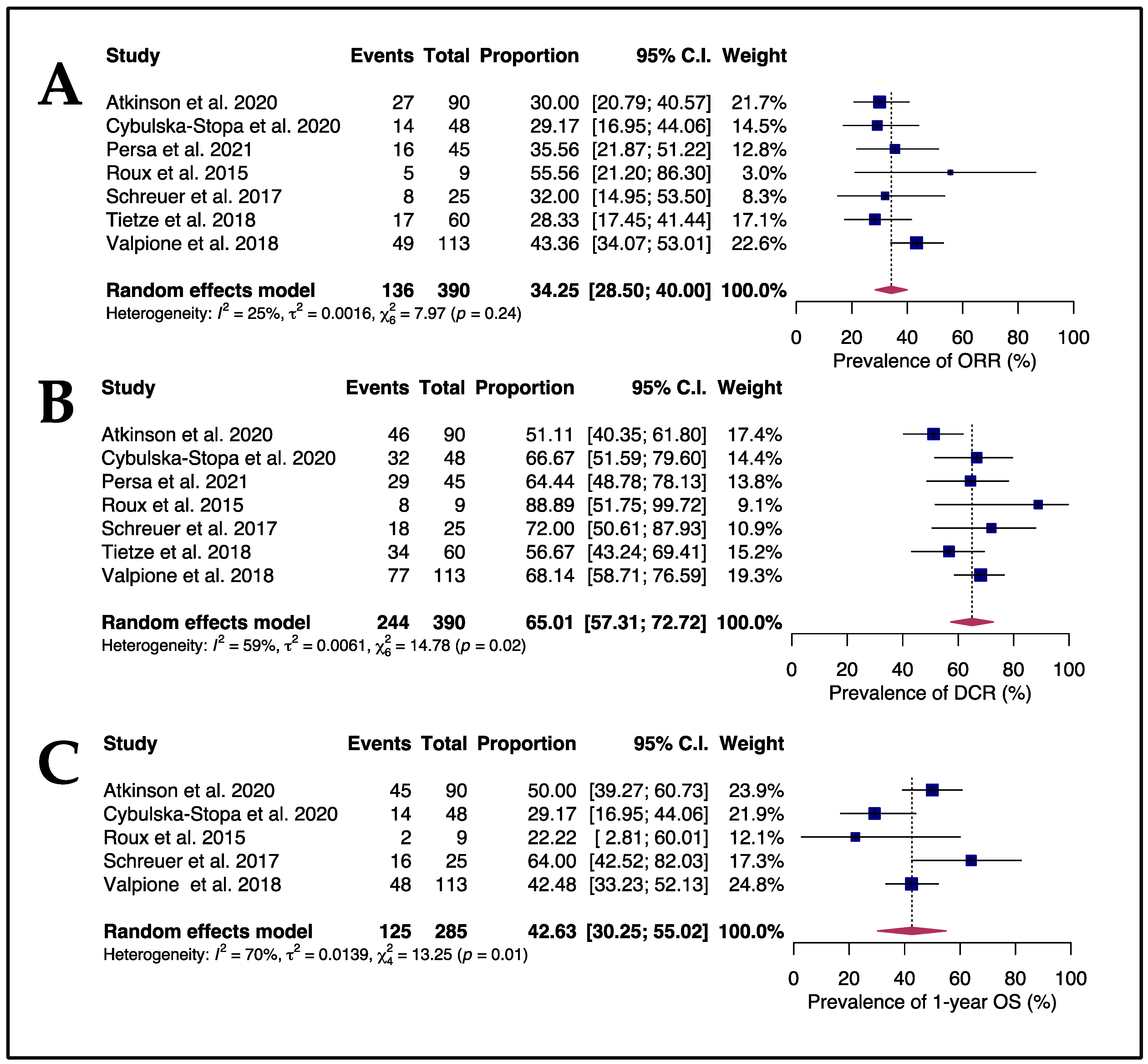
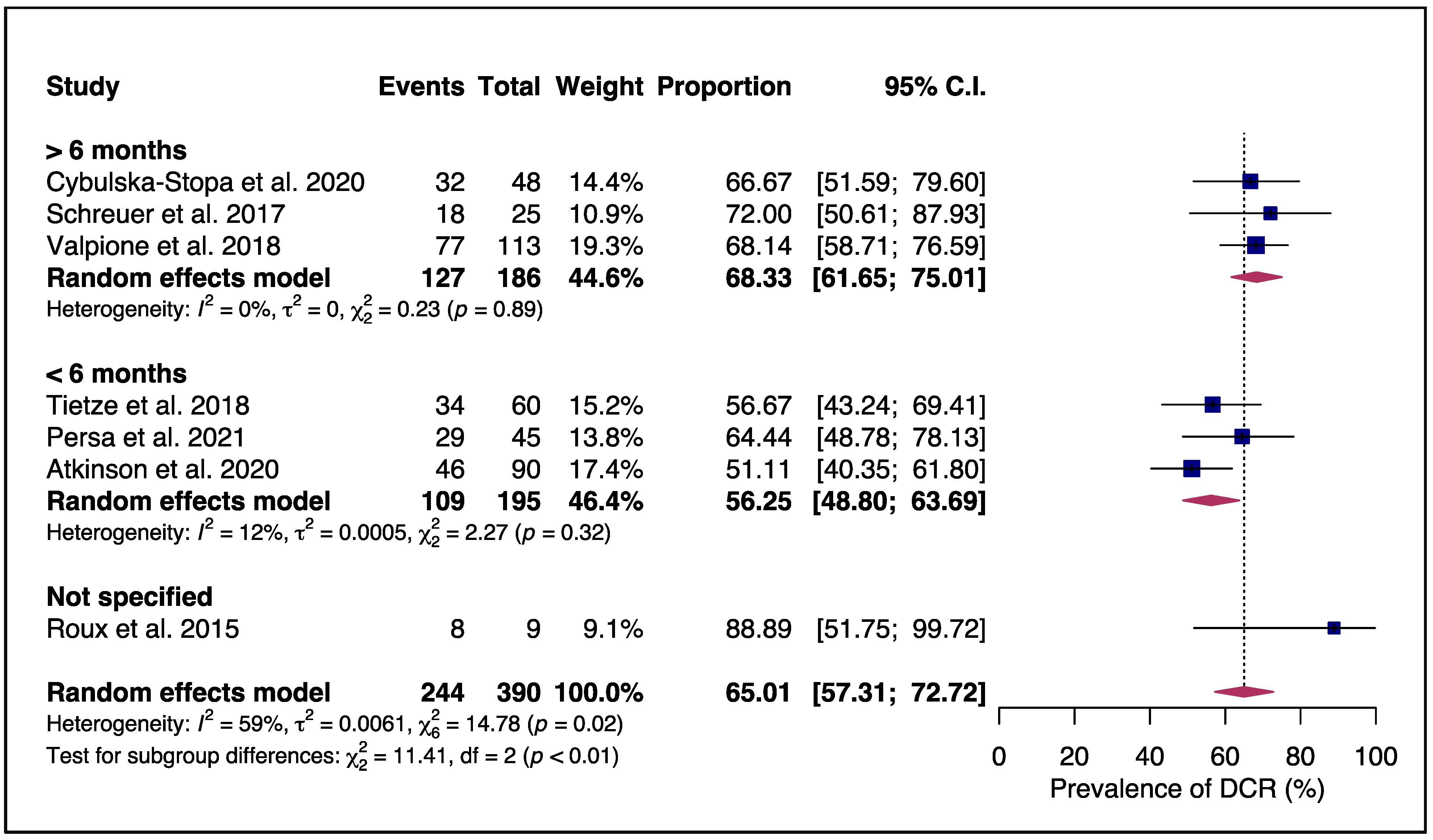
3.2. Efficacy and Safety of Rechallenging Advanced Melanoma Patients with Targeted Therapy
3.3. Quality Assessment
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Parra, L.M.; Webster, R.M. The Malignant Melanoma Market. Nat. Rev. Drug Discov. 2022, 21, 489–490. [Google Scholar] [CrossRef]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Scott, S.; Firth, A.U.; Sung, H.; Henley, S.J.; Sherman, R.L.; Siegel, R.L.; Anderson, R.N.; Kohler, B.A.; Benard, V.B.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. Cancer 2022, 128, 4251–4284. [Google Scholar] [CrossRef] [PubMed]
- Fundytus, A.; Sengar, M.; Lombe, D.; Hopman, W.; Jalink, M.; Gyawali, B.; Trapani, D.; Roitberg, F.; De Vries, E.G.E.; Moja, L.; et al. Access to Cancer Medicines Deemed Essential by Oncologists in 82 Countries: An International, Cross-Sectional Survey. Lancet Oncol. 2021, 22, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; Kubik, M.; Li, L.; et al. Screening for Skin Cancer. JAMA 2023, 329, 1290. [Google Scholar] [CrossRef]
- de Vere Hunt, I.; Lester, J.; Linos, E. Insufficient Evidence for Screening Reinforces Need for Primary Prevention of Skin Cancer. JAMA Intern. Med. 2023, 183, 509. [Google Scholar] [CrossRef] [PubMed]
- Melanoma Survival Rates|Melanoma Survival Statistics. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/survival-rates-for-melanoma-skin-cancer-by-stage.html (accessed on 23 February 2023).
- Sadeq, M.A.; Ashry, M.H.; Ghorab, R.M.F.; Afify, A.Y. Causes of Death among Patients with Cutaneous Melanoma: A US Population-Based Study. Sci. Rep. 2023, 13, 10257. [Google Scholar] [CrossRef] [PubMed]
- Berk-Krauss, J.; Stein, J.A.; Weber, J.; Polsky, D.; Geller, A.C. New Systematic Therapies and Trends in Cutaneous Melanoma Deaths Among US Whites, 1986–2016. Am. J. Public Health 2020, 110, 731–733. [Google Scholar] [CrossRef]
- Curti, B.D.; Faries, M.B. Recent Advances in the Treatment of Melanoma. N. Engl. J. Med. 2021, 384, 2229–2240. [Google Scholar] [CrossRef]
- Hersh, E.M.; Del Vecchio, M.; Brown, M.P.; Kefford, R.; Loquai, C.; Testori, A.; Bhatia, S.; Gutzmer, R.; Conry, R.; Haydon, A.; et al. A Randomized, Controlled Phase III Trial of Nab-Paclitaxel versus Dacarbazine in Chemotherapy-Naïve Patients with Metastatic Melanoma. Ann. Oncol. 2015, 26, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Falkson, C.I.; Ibrahim, J.; Kirkwood, J.M.; Coates, A.S.; Atkins, M.B.; Blum, R.H. Phase III Trial of Dacarbazine versus Dacarbazine with Interferon Alpha-2b versus Dacarbazine with Tamoxifen versus Dacarbazine with Interferon Alpha-2b and Tamoxifen in Patients with Metastatic Malignant Melanoma: An Eastern Cooperative Oncology Group Study. J. Clin. Oncol. 1998, 16, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Middleton, M.R.; Grob, J.J.; Aaronson, N.; Fierlbeck, G.; Tilgen, W.; Seiter, S.; Gore, M.; Aamdal, S.; Cebon, J.; Coates, A.; et al. Randomized Phase III Study of Temozolomide versus Dacarbazine in the Treatment of Patients with Advanced Metastatic Malignant Melanoma. J. Clin. Oncol. 2000, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Garbe, C.; Stolz, W.; Ellwanger, U.; Seiter, S.; Dummer, R.; Ugurel, S.; Sebastian, G.; Nashan, D.; Linse, R.; et al. Dacarbazine and Interferon α with or without Interleukin 2 in Metastatic Melanoma: A Randomized Phase III Multicentre Trial of the Dermatologic Cooperative Oncology Group (DeCOG). Br. J. Cancer 2001, 84, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Kefford, R.; Chiarion Sileni, V.; Nitti, D.; Rossi, C.R.; Pilati, P.; Mocellin, S. Systemic Treatments for Metastatic Cutaneous Melanoma. Cochrane Database Syst. Rev. 2014, 2, CD011123. [Google Scholar] [CrossRef]
- Scott, E.C.; Baines, A.C.; Gong, Y.; Moore, R.; Pamuk, G.E.; Saber, H.; Subedee, A.; Thompson, M.D.; Xiao, W.; Pazdur, R.; et al. Trends in the Approval of Cancer Therapies by the FDA in the Twenty-First Century. Nat. Rev. Drug Discov. 2023, 1–16. [Google Scholar] [CrossRef]
- Thompson, J.F.; Kefford, R.; Stevens, G.; Scolyer, R. Melanoma; Riker, A.I., Ed.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-78309-3. [Google Scholar]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers 2022, 14, 4652. [Google Scholar] [CrossRef]
- Gallicchio, L.; Devasia, T.P.; Tonorezos, E.; Mollica, M.A.; Mariotto, A. Estimation of the Number of Individuals Living with Metastatic Cancer in the United States. JNCI J. Natl. Cancer Inst. 2022, 114, 1476–1483. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Dankner, M.; Rose, A.A.N.; Rajkumar, S.; Siegel, P.M.; Watson, I.R. Classifying BRAF Alterations in Cancer: New Rational Therapeutic Strategies for Actionable Mutations. Oncogene 2018, 37, 3183–3199. [Google Scholar] [CrossRef]
- Alqathama, A. BRAF in Malignant Melanoma Progression and Metastasis: Potentials and Challenges. Am. J. Cancer Res. 2020, 10, 1103–1114. [Google Scholar]
- Proietti, I.; Skroza, N.; Bernardini, N.; Tolino, E.; Balduzzi, V.; Marchesiello, A.; Michelini, S.; Volpe, S.; Mambrin, A.; Mangino, G.; et al. Mechanisms of Acquired BRAF Inhibitor Resistance in Melanoma: A Systematic Review. Cancers 2020, 12, 2801. [Google Scholar] [CrossRef] [PubMed]
- Akbani, R.; Akdemir, K.C.; Aksoy, B.A.; Albert, M.; Ally, A.; Amin, S.B.; Arachchi, H.; Arora, A.; Auman, J.T.; Ayala, B.; et al. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, F.; Barruscotti, S.; Dominioni, T.; Zuccarini, A.; Pedrazzoli, P.; Chiellino, S. Treatment Following Progression in Metastatic Melanoma: The State of the Art from Scientific Literature to Clinical Need. Curr. Oncol. Rep. 2021, 23, 84. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [Green Version]
- Hauschild, A.; Grob, J.-J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H.; Kaempgen, E.; et al. Dabrafenib in BRAF-Mutated Metastatic Melanoma: A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Delord, J.-P.; Robert, C.; Nyakas, M.; McArthur, G.A.; Kudchakar, R.; Mahipal, A.; Yamada, Y.; Sullivan, R.; Arance, A.; Kefford, R.F.; et al. Phase I Dose-Escalation and -Expansion Study of the BRAF Inhibitor Encorafenib (LGX818) in Metastatic BRAF-Mutant Melanoma. Clin. Cancer Res. 2017, 23, 5339–5348. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [Green Version]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib Combined with Vemurafenib in Advanced BRAFV600-Mutant Melanoma (CoBRIM): Updated Efficacy Results from a Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef] [Green Version]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus Trametinib versus Dabrafenib Monotherapy in Patients with Metastatic BRAF V600E/K-Mutant Melanoma: Long-Term Survival and Safety Analysis of a Phase 3 Study. Ann. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib in Patients with BRAF-Mutant Melanoma (COLUMBUS): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall Survival in Patients with BRAF-Mutant Melanoma Receiving Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib (COLUMBUS): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef]
- Dummer, R.; Flaherty, K.T.; Robert, C.; Arance, A.; de Groot, J.W.B.; Garbe, C.; Gogas, H.J.; Gutzmer, R.; Krajsová, I.; Liszkay, G.; et al. COLUMBUS 5-Year Update: A Randomized, Open-Label, Phase III Trial of Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib in Patients With BRAF V600–Mutant Melanoma. J. Clin. Oncol. 2022, 40, 4178–4188. [Google Scholar] [CrossRef]
- Schadendorf, D.; Long, G.V.; Stroiakovski, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion-Sileni, V.; Schachter, J.; Garbe, C.; Dutriaux, C.; et al. Three-Year Pooled Analysis of Factors Associated with Clinical Outcomes across Dabrafenib and Trametinib Combination Therapy Phase 3 Randomised Trials. Eur. J. Cancer 2017, 82, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Cowey, C.L.; Offner, M.; Faries, M.; Carvajal, R.D. Efficacy, Safety, and Tolerability of Approved Combination BRAF and MEK Inhibitor Regimens for BRAF-Mutant Melanoma. Cancers 2019, 11, 1642. [Google Scholar] [CrossRef] [Green Version]
- Grob, J.J.; Amonkar, M.M.; Karaszewska, B.; Schachter, J.; Dummer, R.; Mackiewicz, A.; Stroyakovskiy, D.; Drucis, K.; Grange, F.; Chiarion-Sileni, V.; et al. Comparison of Dabrafenib and Trametinib Combination Therapy with Vemurafenib Monotherapy on Health-Related Quality of Life in Patients with Unresectable or Metastatic Cutaneous BRAF Val600-Mutation-Positive Melanoma (COMBI-v): Results of a Phase 3, Open-Label, Randomised Trial. Lancet Oncol. 2015, 16, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Amonkar, M.M.; Stroyakovskiy, D.; Levchenko, E.; Gogas, H.; de Braud, F.; Grob, J.-J.; Bondarenko, I.; Garbe, C.; Lebbe, C.; et al. Health-Related Quality of Life Impact in a Randomised Phase III Study of the Combination of Dabrafenib and Trametinib versus Dabrafenib Monotherapy in Patients with BRAF V600 Metastatic Melanoma. Eur. J. Cancer 2015, 51, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Lai-Kwon, J.; Inderjeeth, A.J.; Lisy, K.; Sandhu, S.; Rutherford, C.; Jefford, M. Impact of Immune Checkpoint Inhibitors and Targeted Therapy on Health-Related Quality of Life of People with Stage III and IV Melanoma: A Mixed-Methods Systematic Review. Eur. J. Cancer 2023, 184, 83–105. [Google Scholar] [CrossRef]
- Florent, L.; Saby, C.; Slimano, F.; Morjani, H. BRAF V600-Mutated Metastatic Melanoma and Targeted Therapy Resistance: An Update of the Current Knowledge. Cancers 2023, 15, 2607. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.; Xu, W.; Atkinson, V. Treatment Approaches for Melanomas That Relapse After Adjuvant or Neoadjuvant Therapy. Curr. Oncol. Rep. 2022, 24, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Ugurel, S.; Röhmel, J.; Ascierto, P.A.; Becker, J.C.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Livingstone, E.; Long, G.V.; et al. Survival of Patients with Advanced Metastatic Melanoma: The Impact of MAP Kinase Pathway Inhibition and Immune Checkpoint Inhibition—Update 2019. Eur. J. Cancer 2020, 130, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Ugurel, S.; Röhmel, J.; Ascierto, P.A.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Long, G.V.; Lorigan, P.; McArthur, G.A.; et al. Survival of Patients with Advanced Metastatic Melanoma: The Impact of Novel Therapies–Update 2017. Eur. J. Cancer 2017, 83, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, J.; Chhabra, G.; Singh, C.K.; Guzmán-Pérez, G.; Shirley, C.A.; Ahmad, N. Mechanisms of Immunotherapy Resistance in Cutaneous Melanoma: Recognizing a Shapeshifter. Front Oncol. 2022, 12, 880876. [Google Scholar] [CrossRef] [PubMed]
- Pham, J.P.; Joshua, A.M.; da Silva, I.P.; Dummer, R.; Goldinger, S.M. Chemotherapy in Cutaneous Melanoma: Is There Still a Role? Curr. Oncol. Rep. 2023, 25, 609–621. [Google Scholar] [CrossRef]
- Seghers, A.C.; Wilgenhof, S.; Lebbé, C.; Neyns, B. Successful Rechallenge in Two Patients with BRAF-V600-Mutant Melanoma Who Experienced Previous Progression during Treatment with a Selective BRAF Inhibitor. Melanoma Res. 2012, 22, 466–472. [Google Scholar] [CrossRef]
- Romano, E.; Pradervand, S.; Paillusson, A.; Weber, J.; Harshman, K.; Muehlethaler, K.; Speiser, D.; Peters, S.; Rimoldi, D.; Michielin, O. Identification of Multiple Mechanisms of Resistance to Vemurafenib in a Patient with BRAFV600E-Mutated Cutaneous Melanoma Successfully Rechallenged after Progression. Clin. Cancer Res. 2013, 19, 5749–5757. [Google Scholar] [CrossRef] [Green Version]
- Mackiewicz-Wysocka, M.; Krokowicz, Ł.; Kocur, J.; Mackiewicz, J. Resistance to Vemurafenib Can Be Reversible after Treatment Interruption. Medicine 2014, 93, e157. [Google Scholar] [CrossRef]
- Amann, V.C.; Hoffmann, D.; Mangana, J.; Dummer, R.; Goldinger, S.M. Successful Retreatment with Combined BRAF/MEK Inhibition in Metastatic BRAFV600-Mutated Melanoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1638–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reschke, R.; Simon, J.; Ziemer, M. Rechallenge of Targeted Therapy in Metastatic Melanoma. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Viñal, D.; Martinez, D.; Espinosa, E. Efficacy of Rechallenge with BRAF Inhibition Therapy in Patients with Advanced BRAFV600 Mutant Melanoma. Clin. Transl. Oncol. 2019, 21, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 16 June 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Migliavaca, C.B.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting Proportional Meta-Analysis in Different Types of Systematic Reviews: A Guide for Synthesisers of Evidence. BMC Med. Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef]
- McGrath, S.; Sohn, H.; Steele, R.; Benedetti, A. Meta-analysis of the Difference of Medians. Biom. J. 2020, 62, 69–98. [Google Scholar] [CrossRef]
- Atkinson, V.; Batty, K.; Long, G.V.; Carlino, M.S.; Peters, G.D.; Bhave, P.; Moore, M.A.; Xu, W.; Brown, L.J.; Arneil, M.; et al. Activity and Safety of Third-Line BRAF-Targeted Therapy (TT) Following First-Line TT and Second-Line Immunotherapy (IT) in Advanced Melanoma. J. Clin. Oncol. 2020, 38, 10049. [Google Scholar] [CrossRef]
- Cybulska-Stopa, B.; Rogala, P.; Czarnecka, A.M.; Galus, Ł.; Dziura, R.; Rajczykowski, M.; Kubiatowski, T.; Wiśniewska, M.; Gęga-Czarnota, A.; Teterycz, P.; et al. BRAF and MEK Inhibitors Rechallenge as Effective Treatment for Patients with Metastatic Melanoma. Melanoma Res. 2020, 30, 465–471. [Google Scholar] [CrossRef]
- Persa, O.D.; Mauch, C. Outcomes after Retreatment with MAPK Inhibitors and Immune Checkpoint Inhibitors in Melanoma Patients. Future Oncol. 2021, 17, 3809–3817. [Google Scholar] [CrossRef]
- Roux, J.; Pages, C.; Malouf, D.; Basset Seguin, N.; Madjlessi, N.; Baccard, M.; Comte, C.; Archimbaud, A.; Battistella, M.; Viguier, M.; et al. BRAF Inhibitor Rechallenge in Patients with Advanced BRAF V600-Mutant Melanoma. Melanoma Res. 2015, 25, 559–563. [Google Scholar] [CrossRef]
- Schreuer, M.; Jansen, Y.; Planken, S.; Chevolet, I.; Seremet, T.; Kruse, V.; Neyns, B. Combination of Dabrafenib plus Trametinib for BRAF and MEK Inhibitor Pretreated Patients with Advanced BRAFV600-Mutant Melanoma: An Open-Label, Single Arm, Dual-Centre, Phase 2 Clinical Trial. Lancet Oncol. 2017, 18, 464–472. [Google Scholar] [CrossRef]
- Tietze, J.K.; Forschner, A.; Loquai, C.; Mitzel-Rink, H.; Zimmer, L.; Meiss, F.; Rafei-Shamsabadi, D.; Utikal, J.; Bergmann, M.; Meier, F.; et al. The Efficacy of Re-Challenge with BRAF Inhibitors after Previous Progression to BRAF Inhibitors in Melanoma: A Retrospective Multicenter Study. Oncotarget 2018, 9, 34336–34346. [Google Scholar] [CrossRef] [Green Version]
- Valpione, S.; Carlino, M.S.; Mangana, J.; Mooradian, M.J.; McArthur, G.; Schadendorf, D.; Hauschild, A.; Menzies, A.M.; Arance, A.; Ascierto, P.A.; et al. Rechallenge with BRAF-Directed Treatment in Metastatic Melanoma: A Multi-Institutional Retrospective Study. Eur. J. Cancer 2018, 91, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Chapman, P.B.; Robert, C.; Larkin, J.; Haanen, J.B.; Ribas, A.; Hogg, D.; Hamid, O.; Ascierto, P.A.; Testori, A.; Lorigan, P.C.; et al. Vemurafenib in Patients with BRAFV600 Mutation-Positive Metastatic Melanoma: Final Overall Survival Results of the Randomized BRIM-3 Study. Ann. Oncol. 2017, 28, 2581–2587. [Google Scholar] [CrossRef]
- Hauschild, A.; Ascierto, P.A.; Schadendorf, D.; Grob, J.J.; Ribas, A.; Kiecker, F.; Dutriaux, C.; Demidov, L.V.; Lebbé, C.; Rutkowski, P.; et al. Long-Term Outcomes in Patients with BRAF V600-Mutant Metastatic Melanoma Receiving Dabrafenib Monotherapy: Analysis from Phase 2 and 3 Clinical Trials. Eur. J. Cancer 2020, 125, 114–120. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.-J.; et al. Dabrafenib and Trametinib versus Dabrafenib and Placebo for Val600 BRAF-Mutant Melanoma: A Multicentre, Double-Blind, Phase 3 Randomised Controlled Trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Savoia, P.; Zavattaro, E.; Cremona, O. Clinical Implications of Acquired BRAF Inhibitors Resistance in Melanoma. Int. J. Mol. Sci. 2020, 21, 9730. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Dummer, R.; Gogas, H.J.; Flaherty, K.T.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; et al. Update on Tolerability and Overall Survival in COLUMBUS: Landmark Analysis of a Randomised Phase 3 Trial of Encorafenib plus Binimetinib vs. Vemurafenib or Encorafenib in Patients with BRAF V600–Mutant Melanoma. Eur. J. Cancer 2020, 126, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Guo, W. A Review of the Molecular Pathways Involved in Resistance to BRAF Inhibitors in Patients with Advanced-Stage Melanoma. Med. Sci. Monit. 2020, 26, e920957-1. [Google Scholar] [CrossRef]
- Nazarian, R.; Shi, H.; Wang, Q.; Kong, X.; Koya, R.C.; Lee, H.; Chen, Z.; Lee, M.-K.; Attar, N.; Sazegar, H.; et al. Melanomas Acquire Resistance to B-RAF(V600E) Inhibition by RTK or N-RAS Upregulation. Nature 2010, 468, 973–977. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.B.; Menzies, A.M.; Zimmer, L.; Eroglu, Z.; Ye, F.; Zhao, S.; Rizos, H.; Sucker, A.; Scolyer, R.A.; Gutzmer, R.; et al. Acquired BRAF Inhibitor Resistance: A Multicenter Meta-Analysis of the Spectrum and Frequencies, Clinical Behaviour, and Phenotypic Associations of Resistance Mechanisms. Eur. J. Cancer 2015, 51, 2792–2799. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wang, L.; Huang, S.; Heynen, G.J.J.E.; Prahallad, A.; Robert, C.; Haanen, J.; Blank, C.; Wesseling, J.; Willems, S.M.; et al. Reversible and Adaptive Resistance to BRAF(V600E) Inhibition in Melanoma. Nature 2014, 508, 118–122. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Chen, J.Y.; Slominski, A.T. How Cancer Hijacks the Body’s Homeostasis through the Neuroendocrine System. Trends Neurosci. 2023, 46, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Smith, L.K.; Parmenter, T.; Kleinschmidt, M.; Kusnadi, E.P.; Kang, J.; Martin, C.A.; Lau, P.; Patel, R.; Lorent, J.; Papadopoli, D.; et al. Adaptive Translational Reprogramming of Metabolism Limits the Response to Targeted Therapy in BRAFV600 Melanoma. Nat. Commun. 2022, 13, 1100. [Google Scholar] [CrossRef]
- Yan, C.; Chen, S.C.; Ayers, G.D.; Nebhan, C.A.; Roland, J.T.; Weiss, V.L.; Johnson, D.B.; Richmond, A. Proximity of Immune and Tumor Cells Underlies Response to BRAF/MEK-Targeted Therapies in Metastatic Melanoma Patients. NPJ Precis. Oncol. 2022, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Liu, J.; Huang, L.; Qin, Y.; Hawley, T.; Seo, C.; Merlino, G.; Yu, Y. AXL/AKT Axis Mediated-Resistance to BRAF Inhibitor Depends on PTEN Status in Melanoma. Oncogene 2018, 37, 3275–3289. [Google Scholar] [CrossRef] [PubMed]
- McGill, G.G.; Horstmann, M.; Widlund, H.R.; Du, J.; Motyckova, G.; Nishimura, E.K.; Lin, Y.-L.; Ramaswamy, S.; Avery, W.; Ding, H.-F.; et al. Bcl2 Regulation by the Melanocyte Master Regulator Mitf Modulates Lineage Survival and Melanoma Cell Viability. Cell 2002, 109, 707–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konieczkowski, D.J.; Johannessen, C.M.; Abudayyeh, O.; Kim, J.W.; Cooper, Z.A.; Piris, A.; Frederick, D.T.; Barzily-Rokni, M.; Straussman, R.; Haq, R.; et al. A Melanoma Cell State Distinction Influences Sensitivity to MAPK Pathway Inhibitors. Cancer Discov. 2014, 4, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Khaliq, M.; Manikkam, M.; Martinez, E.D.; Fallahi-Sichani, M. Epigenetic Modulation Reveals Differentiation State Specificity of Oncogene Addiction. Nat. Commun. 2021, 12, 1536. [Google Scholar] [CrossRef] [PubMed]
- Karras, P.; Bordeu, I.; Pozniak, J.; Nowosad, A.; Pazzi, C.; Van Raemdonck, N.; Landeloos, E.; Van Herck, Y.; Pedri, D.; Bervoets, G.; et al. A Cellular Hierarchy in Melanoma Uncouples Growth and Metastasis. Nature 2022, 610, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Centeno, P.P.; Pavet, V.; Marais, R. The Journey from Melanocytes to Melanoma. Nat. Rev. Cancer 2023, 23, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Frederick, D.T.; Piris, A.; Cogdill, A.P.; Cooper, Z.A.; Lezcano, C.; Ferrone, C.R.; Mitra, D.; Boni, A.; Newton, L.P.; Liu, C.; et al. BRAF Inhibition Is Associated with Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients with Metastatic Melanoma. Clin. Cancer Res. 2013, 19, 1225–1231. [Google Scholar] [CrossRef] [Green Version]
- Wilmott, J.S.; Long, G.V.; Howle, J.R.; Haydu, L.E.; Sharma, R.N.; Thompson, J.F.; Kefford, R.F.; Hersey, P.; Scolyer, R.A. Cancer Therapy: Clinical Selective BRAF Inhibitors Induce Marked T-Cell Infiltration into Human Metastatic Melanoma. Clin. Cancer Res. 2012, 18, 1386–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stege, H.; Haist, M.; Nikfarjam, U.; Schultheis, M.; Heinz, J.; Pemler, S.; Loquai, C.; Grabbe, S. The Status of Adjuvant and Neoadjuvant Melanoma Therapy, New Developments and Upcoming Challenges. Target. Oncol. 2021, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.; Dearden, H.C.; Nguyen, B.; Soon, J.A.; Smith, J.L.; Randhawa, M.; Mant, A.; Warburton, L.; Lo, S.; Meniawy, T.; et al. Combined Ipilimumab and Nivolumab First-line and after BRAF-targeted Therapy in Advanced Melanoma. Pigment. Cell Melanoma Res. 2020, 33, 358–365. [Google Scholar] [CrossRef]
- Haas, L.; Elewaut, A.; Gerard, C.L.; Umkehrer, C.; Leiendecker, L.; Pedersen, M.; Krecioch, I.; Hoffmann, D.; Novatchkova, M.; Kuttke, M.; et al. Acquired Resistance to Anti-MAPK Targeted Therapy Confers an Immune-Evasive Tumor Microenvironment and Cross-Resistance to Immunotherapy in Melanoma. Nat. Cancer 2021, 2, 693–708. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yang, Z.; Algazi, A.P.; Lomeli, S.H.; Wang, Y.; Othus, M.; Hong, A.; Wang, X.; Randolph, C.E.; et al. Anti-PD-1/L1 Lead-in before MAPK Inhibitor Combination Maximizes Antitumor Immunity and Efficacy. Cancer Cell 2021, 39, 1375–1387.e6. [Google Scholar] [CrossRef]
- Chandra, S.; Choi, J.S.; Sosman, J.A. Melanoma: Does Sequencing Really Matter? J. Clin. Oncol. 2023, 41, 167–169. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; Rutkowski, P.; Ferraresi, V.; Arance, A.; Guida, M.; Maiello, E.; Gogas, H.; et al. Sequencing of Ipilimumab Plus Nivolumab and Encorafenib plus Binimetinib for Untreated BRAF -Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J. Clin. Oncol. 2023, 41, 212–221. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial—ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
- Welti, M.; Dimitriou, F.; Gutzmer, R.; Dummer, R. Triple Combination of Immune Checkpoint Inhibitors and BRAF/MEK Inhibitors in BRAF V600 Melanoma: Current Status and Future Perspectives. Cancers 2022, 14, 5489. [Google Scholar] [CrossRef]
- Switzer, B.; Puzanov, I.; Skitzki, J.J.; Hamad, L.; Ernstoff, M.S. Managing Metastatic Melanoma in 2022: A Clinical Review. JCO Oncol. Pract. 2022, 18, 335–351. [Google Scholar] [CrossRef]
- van Breeschoten, J.; Wouters, M.W.J.M.; Hilarius, D.L.; Haanen, J.B.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; de Groot, J.W.B.; Hospers, G.A.P.; Kapiteijn, E.; et al. First-Line BRAF/MEK Inhibitors versus Anti-PD-1 Monotherapy in BRAFV600-Mutant Advanced Melanoma Patients: A Propensity-Matched Survival Analysis. Br. J. Cancer 2021, 124, 1222–1230. [Google Scholar] [CrossRef]
- Adeleke, S.; Okoli, S.; Augustine, A.; Galante, J.; Agnihotri, A.; Uccello, M.; Ghose, A.; Moschetta, M.; Boussios, S. Melanoma—The Therapeutic Considerations in the Clinical Practice. Ann. Palliat. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.F.; Choi, J.; Sosman, J. New Approaches to Targeted Therapy in Melanoma. Cancers 2023, 15, 3224. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ramírez, D.; Fernández-Orland, A.; Ferrándiz, L. Immunotherapy and Targeted Therapy in Patients With Advanced Melanoma and the V600 BRAF Mutation: Which One First? Actas. Dermosifiliogr. 2023. [Google Scholar] [CrossRef]
- Das Thakur, M.; Salangsang, F.; Landman, A.S.; Sellers, W.R.; Pryer, N.K.; Levesque, M.P.; Dummer, R.; McMahon, M.; Stuart, D.D. Modelling Vemurafenib Resistance in Melanoma Reveals a Strategy to Forestall Drug Resistance. Nature 2013, 494, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Kuczynski, E.A.; Sargent, D.J.; Grothey, A.; Kerbel, R.S. Drug Rechallenge and Treatment beyond Progression—Implications for Drug Resistance. Nat. Rev. Clin. Oncol. 2013, 10, 571–587. [Google Scholar] [CrossRef] [Green Version]
- Gebhardt, C.; Ascierto, P.; Atkinson, V.; Corrie, P.; Dummer, R.; Schadendorf, D. The Concepts of Rechallenge and Retreatment in Melanoma: A Proposal for Consensus Definitions. Eur. J. Cancer 2020, 138, 68–76. [Google Scholar] [CrossRef]
- Devji, T.; Levine, O.; Neupane, B.; Beyene, J.; Xie, F. Systemic Therapy for Previously Untreated Advanced BRAF-Mutated Melanoma. JAMA Oncol. 2017, 3, 366. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Messersmith, H.; Kaur, V.; Kirkwood, J.M.; Kudchadkar, R.; McQuade, J.L.; Provenzano, A.; Swami, U.; Weber, J.; Alluri, K.C.; et al. Systemic Therapy for Melanoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3947–3970. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U. Cutaneous Melanoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, Y.; Ito, T.; Kato, H.; Irie, H.; Kaji, T.; Maekawa, T.; Asai, J.; Yamamoto, Y.; Fujimura, T.; Nakai, Y.; et al. Outcome of Combination Therapy Using BRAF and MEK Inhibitors among Asian Patients with Advanced Melanoma: An Analysis of 112 Cases. Eur. J. Cancer 2021, 145, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Colombino, M.; Capone, M.; Lissia, A.; Cossu, A.; Rubino, C.; De Giorgi, V.; Massi, D.; Fonsatti, E.; Staibano, S.; Nappi, O.; et al. BRAF/NRAS Mutation Frequencies Among Primary Tumors and Metastases in Patients With Melanoma. J. Clin. Oncol. 2012, 30, 2522–2529. [Google Scholar] [CrossRef]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of Brain Metastases According to Molecular Subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ishitsuka, Y.; Tanaka, R.; Okiyama, N.; Watanabe, R.; Saito, A.; Furuta, J.; Fujisawa, Y. Frequent Brain Metastases during Treatment with BRAF/MEK Inhibitors: A Retrospective Single Institutional Study. J. Dermatol. 2020, 47, 1191–1194. [Google Scholar] [CrossRef]
- Wagner, N.B.; Forschner, A.; Leiter, U.; Garbe, C.; Eigentler, T.K. S100B and LDH as Early Prognostic Markers for Response and Overall Survival in Melanoma Patients Treated with Anti-PD-1 or Combined Anti-PD-1 plus Anti-CTLA-4 Antibodies. Br. J. Cancer 2018, 119, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Internò, V.; Sergi, M.C.; Metta, M.E.; Guida, M.; Trerotoli, P.; Strippoli, S.; Circelli, S.; Porta, C.; Tucci, M. Melanoma Brain Metastases: A Retrospective Analysis of Prognostic Factors and Efficacy of Multimodal Therapies. Cancers 2023, 15, 1542. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Ribas, A.; Larkin, J.; McArthur, G.A.; Lewis, K.D.; Hauschild, A.; Flaherty, K.T.; McKenna, E.; Zhu, Q.; Mun, Y.; et al. Impact of Initial Treatment and Prognostic Factors on Postprogression Survival in BRAF-Mutated Metastatic Melanoma Treated with Dacarbazine or Vemurafenib ± Cobimetinib: A Pooled Analysis of Four Clinical Trials. J. Transl. Med. 2020, 18, 294. [Google Scholar] [CrossRef]
- Heinzerling, L.; Eigentler, T.K.; Fluck, M.; Hassel, J.C.; Heller-Schenck, D.; Leipe, J.; Pauschinger, M.; Vogel, A.; Zimmer, L.; Gutzmer, R. Tolerability of BRAF/MEK Inhibitor Combinations: Adverse Event Evaluation and Management. ESMO Open 2019, 4, e000491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study ID | N | Age †, y | TT in 1st/2nd Line with BRAFi + MEKi (%) | IT with ICI (%) | Interval between TT and RC † (Months) | RC with BRAFi + MEKi (%) | ECOG (0–1, ≥2) (%) | LDH (Normal, ≥ULN) (%) | CNS Disease at RC (yes, no) (%) |
|---|---|---|---|---|---|---|---|---|---|
| Atkinson (2020) [60] | 90 | 61 | 80 | 100 | NA | 93 | 60, 28 | 30, 51 | 49, 46 |
| Cybulska-Stopa (2020) [61] | 51 | 56 | 68 | 100 | 8.6 | 96 | 78, 22 | 22, 76 | 59, 41 |
| Persa (2021) [62] | 48 | 57 | 79 | 75 | 4 | 83 | NA | 40, 60 | 50, 50 |
| Roux (2015) [63] | 10 | 52.4 | 0 | 80 | NA | 10 | 50, 50 | 70, 30 | 60, 40 |
| Schreuer (2017) [64] | 25 | 54.7 | 64 | 100 | 6.1 | 100 | 80, 20 | 72, 28 | 68, 32 |
| Tietze (2018) [65] | 60 | 56 | 32 | 67 | 3.4 | 68 | 63, 32 | 43, 57 | 60, 40 |
| Valpione (2017) [66] | 116 | 51.9 | 35 | 71 | 7.7 | 66 | 61, 22 | 39, 55 | 44, 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priantti, J.N.; Vilbert, M.; Madeira, T.; Moraes, F.C.A.; Hein, E.C.K.; Saeed, A.; Cavalcante, L. Efficacy and Safety of Rechallenge with BRAF/MEK Inhibitors in Advanced Melanoma Patients: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3754. https://doi.org/10.3390/cancers15153754
Priantti JN, Vilbert M, Madeira T, Moraes FCA, Hein ECK, Saeed A, Cavalcante L. Efficacy and Safety of Rechallenge with BRAF/MEK Inhibitors in Advanced Melanoma Patients: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(15):3754. https://doi.org/10.3390/cancers15153754
Chicago/Turabian StylePriantti, Jonathan N., Maysa Vilbert, Thiago Madeira, Francisco Cezar A. Moraes, Erica C. Koch Hein, Anwaar Saeed, and Ludimila Cavalcante. 2023. "Efficacy and Safety of Rechallenge with BRAF/MEK Inhibitors in Advanced Melanoma Patients: A Systematic Review and Meta-Analysis" Cancers 15, no. 15: 3754. https://doi.org/10.3390/cancers15153754
APA StylePriantti, J. N., Vilbert, M., Madeira, T., Moraes, F. C. A., Hein, E. C. K., Saeed, A., & Cavalcante, L. (2023). Efficacy and Safety of Rechallenge with BRAF/MEK Inhibitors in Advanced Melanoma Patients: A Systematic Review and Meta-Analysis. Cancers, 15(15), 3754. https://doi.org/10.3390/cancers15153754











