Intratumoural Delivery of mRNA Loaded on a Cationic Hyper-Branched Cyclodextrin-Based Polymer Induced an Anti-Tumour Immunological Response in Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Polymer Synthesis
2.3. Polymer–DNA Complex Formation and Gel Retardation Assay
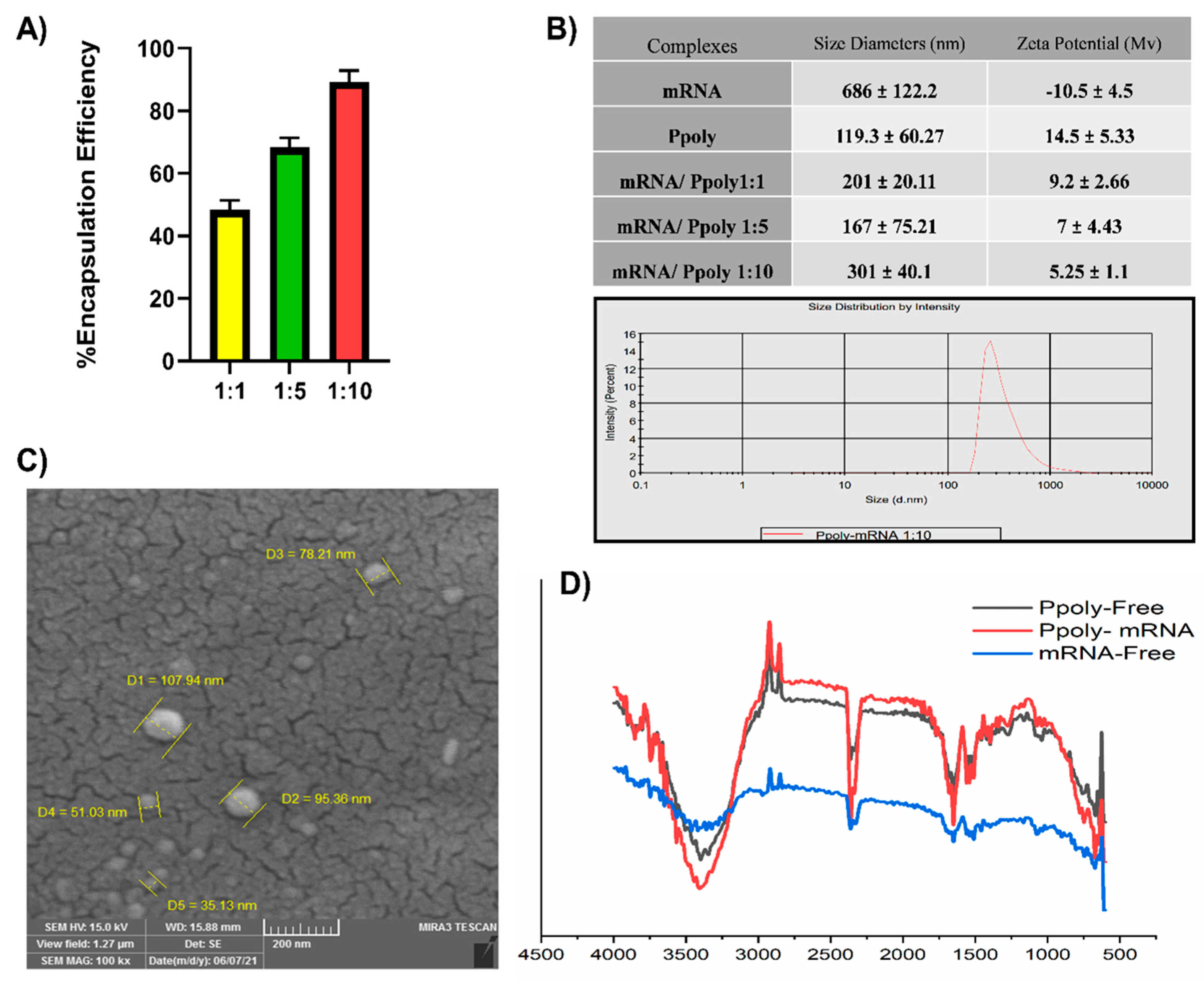
2.3.1. Complexes Characterisation
2.3.2. Determination of mRNA Encapsulation Efficiency
2.4. Cell Culture Protocol for 2D and 3D Spheroid Cell Culture
2.4.1. MTT (3-(4, 5-Dimethylthiazol-2-yl) 2, 5-Diphenyl Tetrazolium Bromide) Assay
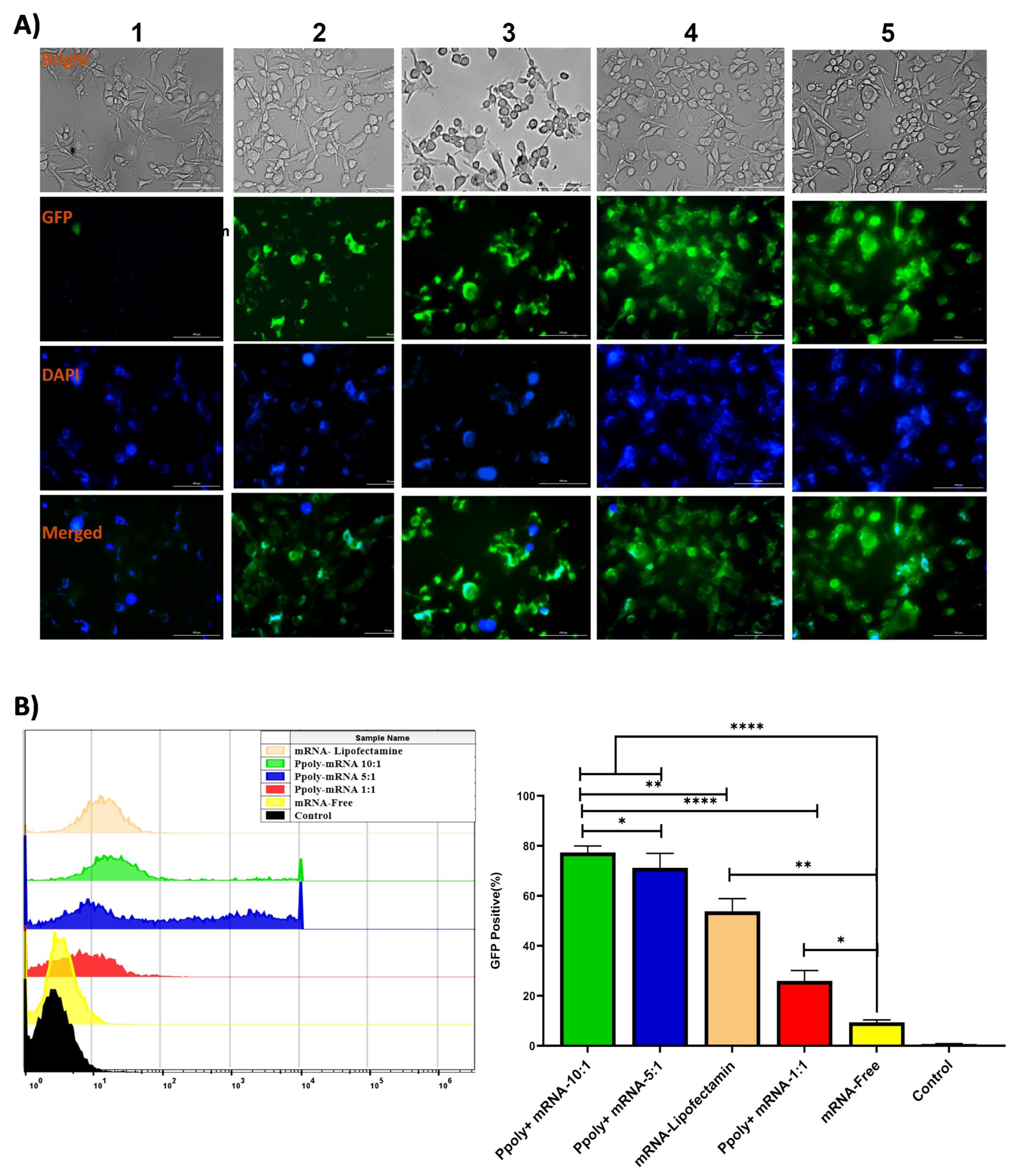
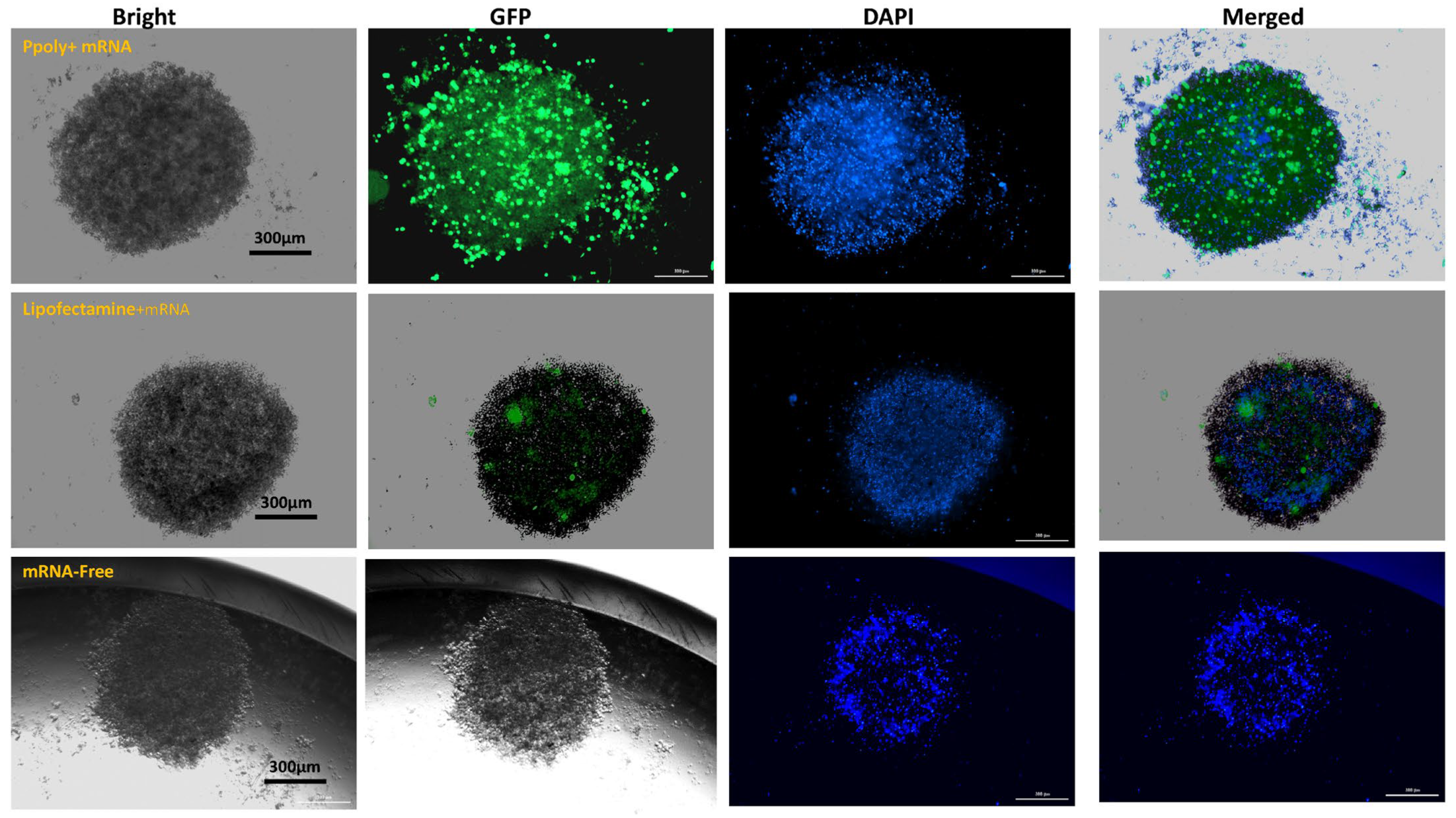
2.4.2. Measurement of EGFP (mRNA)-Polymer Transfection Using Fluorescence Microscopy in 2D and 3D
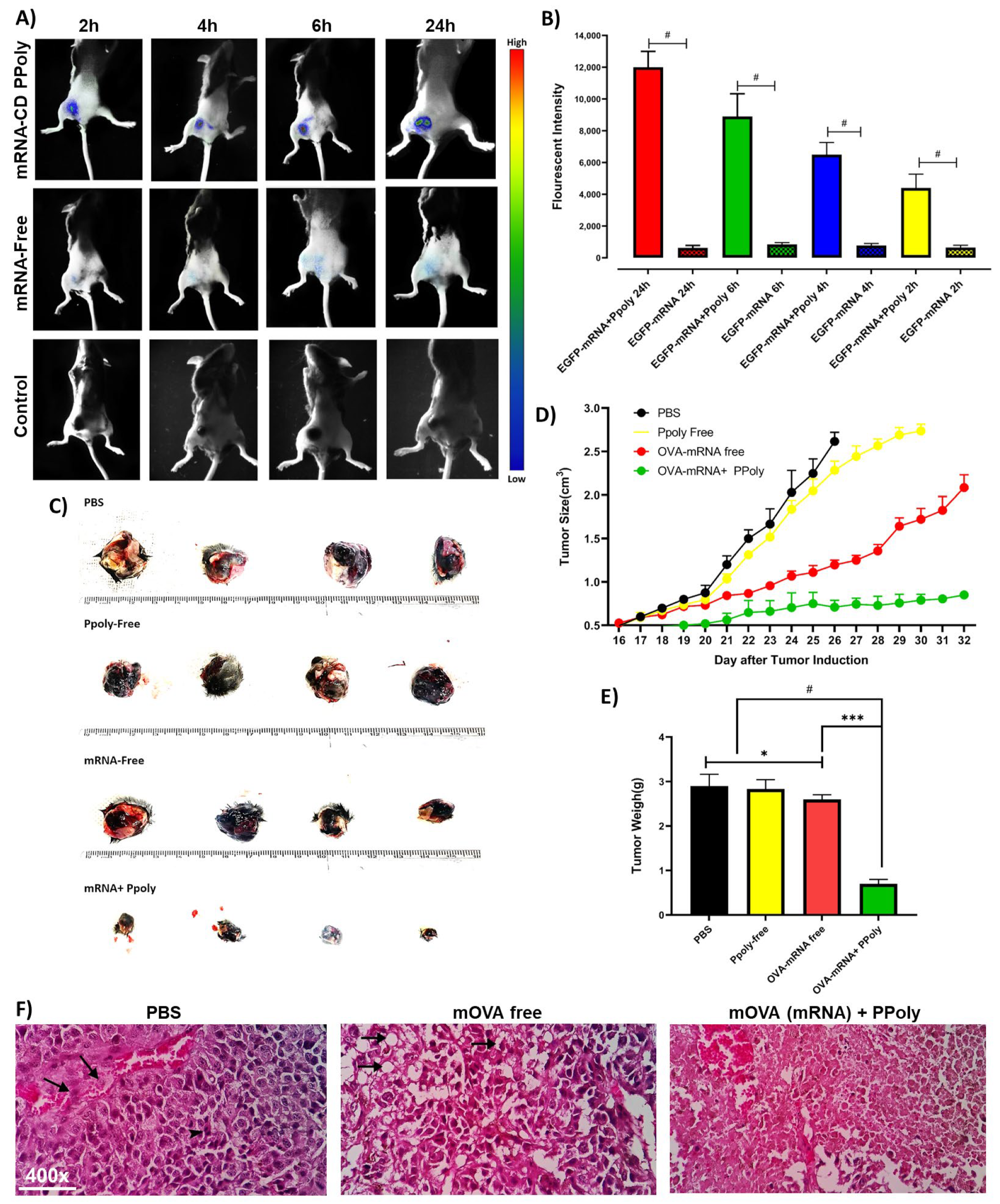
2.5. In Vivo Biodistribution of EGFP-mRNA Complexes with PPoly
2.6. Experimental Design and Tumour Induction
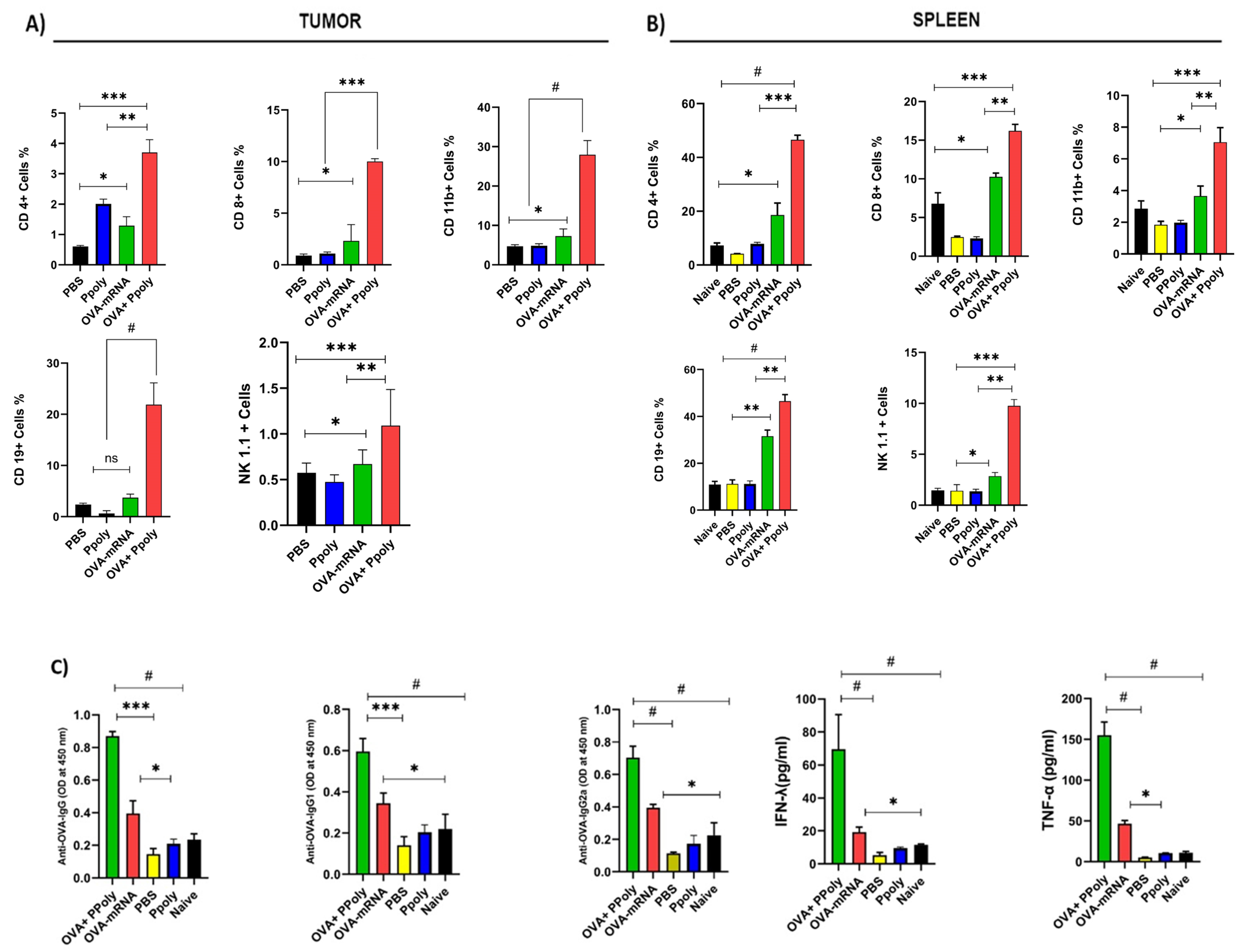
2.6.1. Flow Cytometry Detection of In Vivo Immune System Cell Responses in Tumour and Spleen
2.6.2. Antibodies and Flow Cytometry
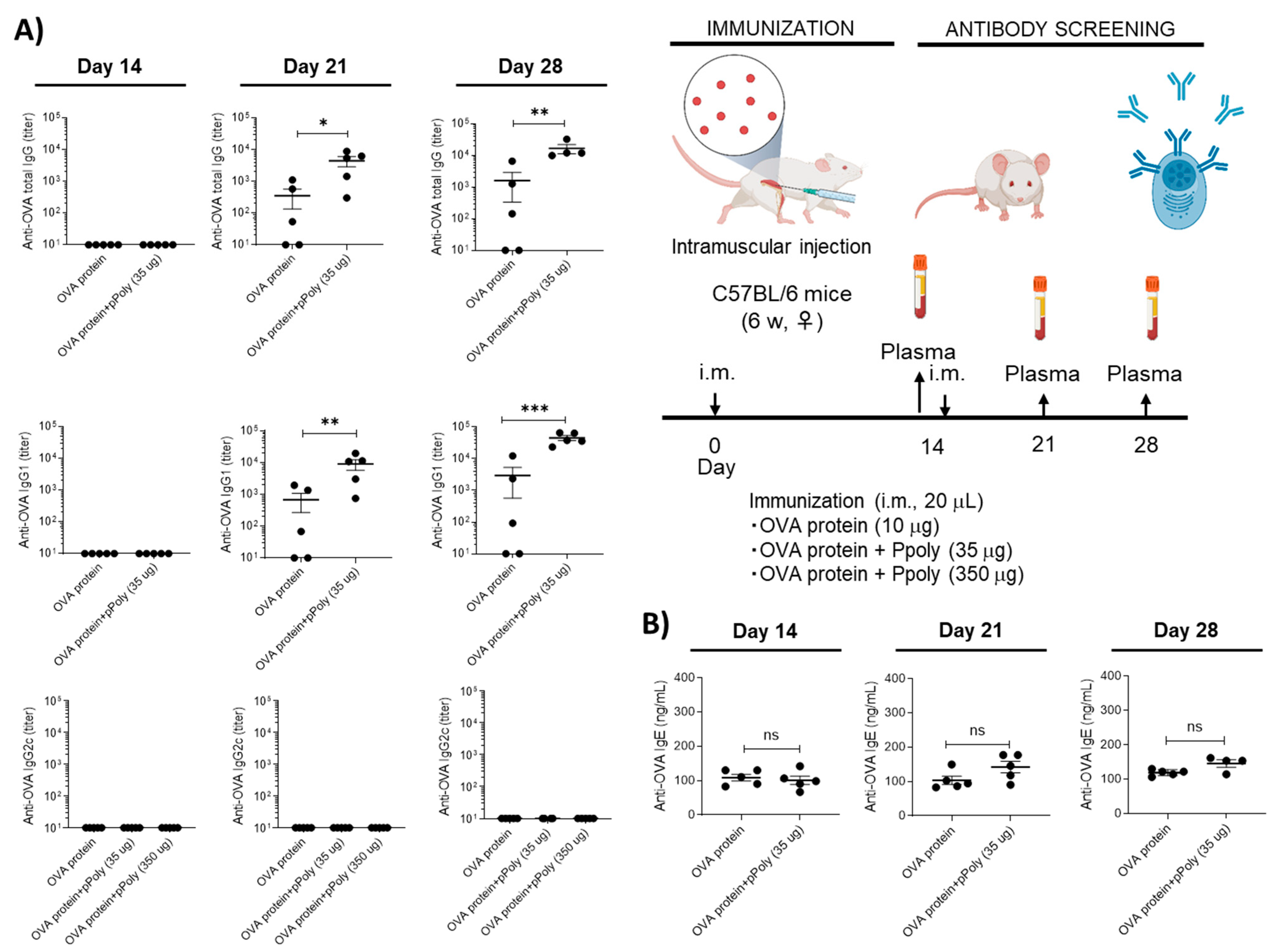
2.6.3. Determination of Antigen-Specific Antibodies in Serum
2.6.4. Histopathological Studies
2.6.5. Assessment of the Adjuvanticity of Ppoly
2.7. Statistical Analysis
3. Results and Discussion
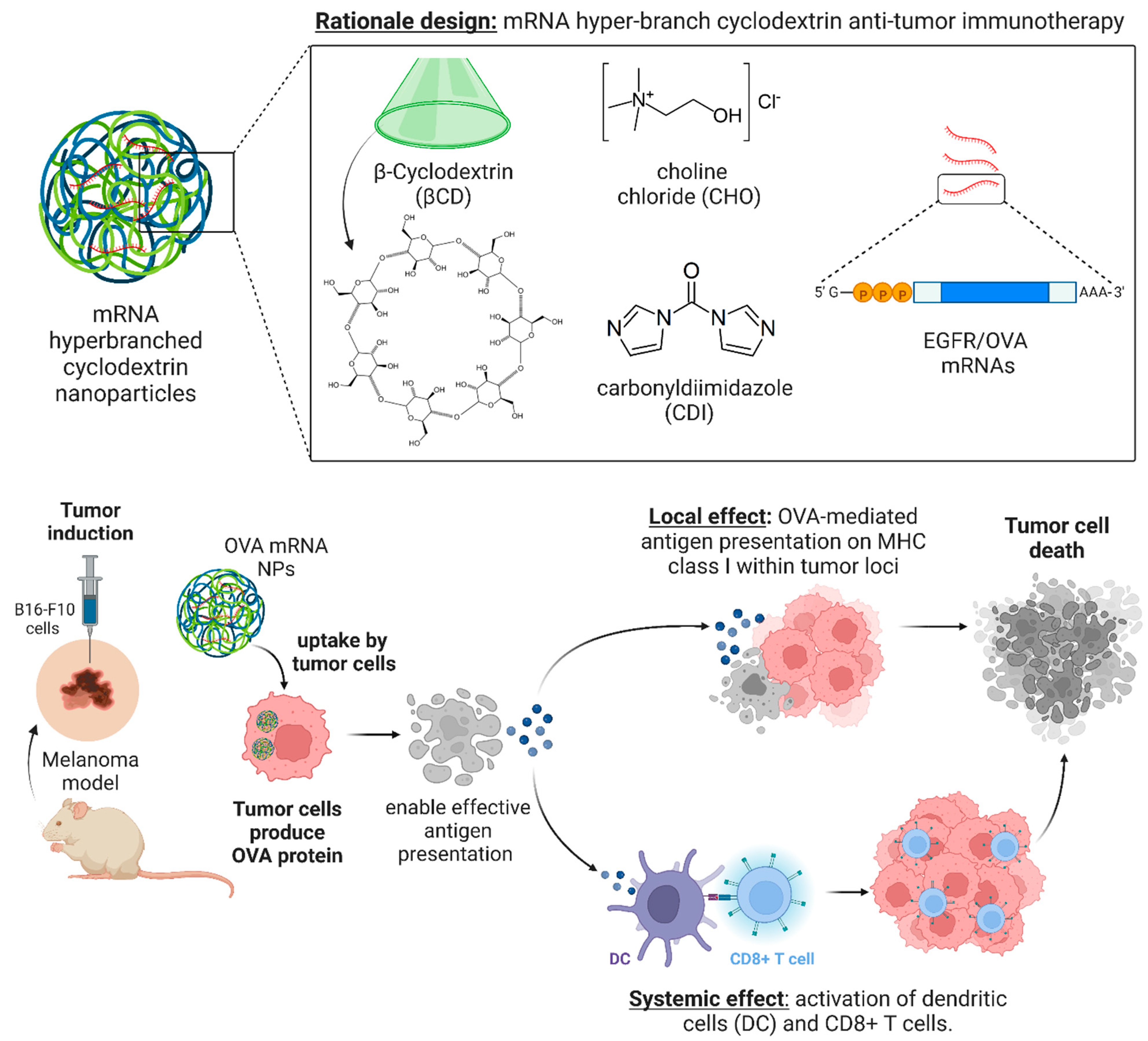
3.1. Design Rationale and Characterisation of mRNA Nanoparticles
3.2. Two-Dimensional (2D) and Three-Dimensional (3D) Spheroid Cytotoxicity and Uptake Analysis
3.3. In Vivo Immunotherapeutic Efficacy of mRNA Nanocomplexes
Cellular and Humoral Immune System Responses to mRNA Therapy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luke, J.J.; Hodi, F.S. Ipilimumab, vemurafenib, dabrafenib, and trametinib: Synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist 2013, 18, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Türeci, Ö.; Vormehr, M.; Kranz, L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, W.; Cole, J.; Zhu, G. Delivery of nucleic acid therapeutics for cancer immunotherapy. Med. Drug Discov. 2020, 6, 100023. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- Anand, P.; Stahel, V.P. The safety of COVID-19 mRNA vaccines: A review. Patient Saf. Surg. 2021, 15, 20. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef]
- Servick, K. mRNA’s Next Challenge: Will It Work as a Drug; American Association for the Advancement of Science: Washington, DC, USA, 2020. [Google Scholar]
- Sokolova, V.; Rojas-Sánchez, L.; Białas, N.; Schulze, N.; Epple, M. Calcium phosphate nanoparticle-mediated transfection in 2D and 3D mono-and co-culture cell models. Acta Biomater. 2019, 84, 391–401. [Google Scholar] [CrossRef]
- Wei, T.; Cheng, Q.; Farbiak, L.; Anderson, D.G.; Langer, R.; Siegwart, D.J. Delivery of tissue-targeted scalpels: Opportunities and challenges for in vivo CRISPR/Cas-based genome editing. ACS Nano 2020, 14, 9243–9262. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid Nanoparticle–mRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef]
- Kong, N.; Tao, W.; Ling, X.; Wang, J.; Xiao, Y.; Shi, S.; Ji, X.; Shajii, A.; Gan, S.T.; Kim, N.Y. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019, 11, eaaw1565. [Google Scholar] [CrossRef]
- Islam, M.A.; Reesor, E.K.; Xu, Y.; Zope, H.R.; Zetter, B.R.; Shi, J. Biomaterials for mRNA delivery. Biomater. Sci. 2015, 3, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Rosenecker, J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017, 24, 133–143. [Google Scholar] [CrossRef]
- Haley, R.M.; Gottardi, R.; Langer, R.; Mitchell, M.J. Cyclodextrins in drug delivery: Applications in gene and combination therapy. Drug Deliv. Transl. Res. 2020, 10, 661. [Google Scholar] [CrossRef]
- Singh, R.P.; Hidalgo, T.; Cazade, P.-A.; Darcy, R.; Cronin, M.F.; Dorin, I.; O’Driscoll, C.o.M.; Thompson, D. Self-assembled cationic β-cyclodextrin nanostructures for siRNA delivery. Mol. Pharm. 2019, 16, 1358–1366. [Google Scholar] [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef]
- Matencio, A.; Hoti, G.; Monfared, Y.K.; Rezayat, A.; Pedrazzo, A.R.; Caldera, F.; Trotta, F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers 2021, 13, 1684. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hwang, S.J.; Davis, M. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug. Chem. 1999, 10, 1068–1074. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. J. Control. Release 2021, 330, 1046–1070. [Google Scholar] [CrossRef] [PubMed]
- Santana-Armas, M.L.; de Ilarduya, C.T. Strategies for cancer gene-delivery improvement by non-viral vectors. Int. J. Pharm. 2021, 596, 120291. [Google Scholar] [CrossRef] [PubMed]
- Wahane, A.; Waghmode, A.; Kapphahn, A.; Dhuri, K.; Gupta, A.; Bahal, R. Role of lipid-based and polymer-based non-viral vectors in nucleic acid delivery for next-generation gene therapy. Molecules 2020, 25, 2866. [Google Scholar] [CrossRef]
- Kircheis, R.; Wightman, L.; Schreiber, A.; Robitza, B.; Rössler, V.; Kursa, M.; Wagner, E. Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application. Gene Ther. 2001, 8, 28–40. [Google Scholar] [CrossRef]
- Marabelle, A.; Kohrt, H.; Caux, C.; Levy, R. Intratumoral immunization: A new paradigm for cancer therapy. Clin. Cancer Res. 2014, 20, 1747–1756. [Google Scholar] [CrossRef]
- Weide, B.; Carralot, J.-P.; Reese, A.; Scheel, B.; Eigentler, T.K.; Hoerr, I.; Rammensee, H.-G.; Garbe, C.; Pascolo, S. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J. Immunother. 2008, 31, 180–188. [Google Scholar] [CrossRef]
- Eggermont, A. Advances in systemic treatment of melanoma. Ann. Oncol. 2010, 21, vii339–vii344. [Google Scholar] [CrossRef]
- Monfared, Y.K.; Mahmoudian, M.; Cecone, C.; Caldera, F.; Haiaty, S.; Heidari, H.R.; Rahbarghazi, R.; Matencio, A.; Zakeri-Milani, P.; Trotta, F. Hyper-Branched Cationic Cyclodextrin Polymers for Improving Plasmid Transfection in 2D and 3D Spheroid Cells. Pharmaceutics 2022, 14, 2690. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Sintra, T.E.; Nasirpour, M.; Siopa, F.; Rosatella, A.A.; Gonçalves, F.; Coutinho, J.A.; Afonso, C.A.; Ventura, S.P. Ecotoxicological evaluation of magnetic ionic liquids. Ecotoxicol. Environ. Saf. 2017, 143, 315–321. [Google Scholar] [CrossRef]
- Lee, P.P.; Fitzpatrick, D.R.; Beard, C.; Jessup, H.K.; Lehar, S.; Makar, K.W.; Pérez-Melgosa, M.; Sweetser, M.T.; Schlissel, M.S.; Nguyen, S. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 2001, 15, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Abdolahinia, E.D.; Nadri, S.; Rahbarghazi, R.; Barar, J.; Aghanejad, A.; Omidi, Y. Enhanced penetration and cytotoxicity of metformin and collagenase conjugated gold nanoparticles in breast cancer spheroids. Life Sci. 2019, 231, 116545. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, H.; Bonabi, E.; Zarghami, N. Stimulus-responsive drug/gene delivery system based on polyethylenimine cyclodextrin nanoparticles for potential cancer therapy. Carbohydr. Polym. 2022, 276, 118747. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, C.; Minelli, R.; Gigliotti, C.L.; Occhipinti, S.; Giovarelli, M.; Conti, L.; Boggio, E.; Shivakumar, Y.; Baldanzi, G.; Malacarne, V. B7h triggering inhibits the migration of tumor cell lines. J. Immunol. 2014, 192, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.X.; Zheng, L.W.; Ma, L.; Huang, H.Z.; Yu, R.Q.; Zwahlen, R.A. Tumorigenicity and validity of fluorescence labelled mesenchymal and epithelial human oral cancer cell lines in nude mice. BioMed Res. Int. 2016, 2016, 4897986. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Wojnilowicz, M.; Pan, S.; Ju, Y.; Zhang, W.; Liu, J.; Zhuo, R.; Jiang, X. Acid-sensitive poly (β-cyclodextrin)-based multifunctional supramolecular gene vector. Polym. Chem. 2018, 9, 450–462. [Google Scholar] [CrossRef]
- Khalil, I.A.; Kogure, K.; Akita, H.; Harashima, H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006, 58, 32–45. [Google Scholar] [CrossRef]
- Tan, L.; Zheng, T.; Li, M.; Zhong, X.; Tang, Y.; Qin, M.; Sun, X. Optimization of an mRNA vaccine assisted with cyclodextrin-polyethyleneimine conjugates. Drug Deliv. Transl. Res. 2020, 10, 678–689. [Google Scholar] [CrossRef]
- Akbarzadeh, F.; Khoshgard, K.; Arkan, E.; Hosseinzadeh, L.; Hemati Azandaryani, A. Evaluating the photodynamic therapy efficacy using 5-aminolevulinic acid and folic acid-conjugated bismuth oxide nanoparticles on human nasopharyngeal carcinoma cell line. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S514–S523. [Google Scholar] [CrossRef]
- Chen, K.; Chen, Q.; Wang, K.; Zhu, J.; Li, W.; Li, W.; Qiu, L.; Guan, G.; Qiao, M.; Zhao, X. Synthesis and characterization of a PAMAM-OH derivative containing an acid-labile β-thiopropionate bond for gene delivery. Int. J. Pharm. 2016, 509, 314–327. [Google Scholar] [CrossRef]
- Canatella, P.J.; Black, M.M.; Bonnichsen, D.M.; McKenna, C.; Prausnitz, M.R. Tissue electroporation: Quantification and analysis of heterogeneous transport in multicellular environments. Biophys. J. 2004, 86, 3260–3268. [Google Scholar] [CrossRef]
- Marrero, B.; Heller, R. The use of an in vitro 3D melanoma model to predict in vivo plasmid transfection using electroporation. Biomaterials 2012, 33, 3036–3046. [Google Scholar] [CrossRef]
- Kong, Q.; Wu, G.; Han, L.; Zhang, Z.; Du, J.; Sun, W.; Cao, L. A transfection method of PS-asODNs targeting ANGPTL4 in multicellular structures of hepatocarcinoma cell line. Cancer Gene Ther. 2015, 22, 285–290. [Google Scholar] [CrossRef]
- Mellor, H.; Davies, L.; Caspar, H.; Pringle, C.; Hyde, S.; Gill, D.; Callaghan, R. Optimising non-viral gene delivery in a tumour spheroid model. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Clin. Appl. 2006, 8, 1160–1170. [Google Scholar] [CrossRef]
- Desoize, B.; Jardillier, J.-C. Multicellular resistance: A paradigm for clinical resistance? Crit. Rev. Oncol. Hematol. 2000, 36, 193–207. [Google Scholar] [CrossRef]
- Carver, K.; Ming, X.; Juliano, R.L. Multicellular tumor spheroids as a model for assessing delivery of oligonucleotides in three dimensions. Mol. Ther. Nucleic Acids 2014, 3, e153. [Google Scholar] [CrossRef]
- Galadanci, H.; Idris, S.; Sadauki, H.; Yakasai, I. Programs and policies for reducing maternal mortality in Kano State, Nigeria: A review. Afr. J. Reprod. Health 2010, 14, 31–36. [Google Scholar]
- Lugowska, I.; Teterycz, P.; Rutkowski, P. Immunotherapy of melanoma. Contemp. Oncol. /Współczesna Onkol. 2018, 2018, 61–67. [Google Scholar] [CrossRef]
- Ma, K.; Mi, C.-L.; Cao, X.-X.; Wang, T.-Y. Progress of cationic gene delivery reagents for non-viral vector. Appl. Microbiol. Biotechnol. 2021, 105, 525–538. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Yu, X.; Cheng, Q.; Johnson, L.T.; Chatterjee, S.; Zhang, D.; Lee, S.M.; Sun, Y.; Lin, T.-C. Zwitterionic phospholipidation of cationic polymers facilitates systemic mRNA delivery to spleen and lymph nodes. J. Am. Chem. Soc. 2021, 143, 21321–21330. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, J.; Zhou, H.; Zeng, X.; Ruan, Z.; Pu, Z.; Jiang, X.; Matsui, A.; Zhu, L.; Amoozgar, Z. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 2022, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Yoon, H.Y.; Shin, J.M.; Saravanakumar, G.; Noh, K.H.; Song, K.-H.; Jeon, J.-H.; Kim, D.-W.; Lee, K.-M.; Kim, K. A polymeric conjugate foreignizing tumor cells for targeted immunotherapy in vivo. J. Control. Release 2015, 199, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Rice, J.; Reesor, E.; Zope, H.; Tao, W.; Lim, M.; Ding, J.; Chen, Y.; Aduluso, D.; Zetter, B.R. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials 2021, 266, 120431. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Case, M.; Brooks, C. Palmitic acid conjugation of a protein antigen enhances major histocompatibility complex class II-restricted presentation to T cells. Immunology 1992, 76, 593. [Google Scholar]
- Zaman, M.; Toth, I. Immunostimulation by synthetic lipopeptide-based vaccine candidates: Structure-activity relationships. Front. Immunol. 2013, 4, 318. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Midoux, P.; Pichon, C. Lipid-based mRNA vaccine delivery systems. Expert Rev. Vaccines 2015, 14, 221–234. [Google Scholar] [CrossRef]
- McKinlay, C.J.; Benner, N.L.; Haabeth, O.A.; Waymouth, R.M.; Wender, P.A. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc. Natl. Acad. Sci. USA 2018, 115, E5859–E5866. [Google Scholar] [CrossRef]
- Fenton, O.S.; Kauffman, K.J.; Kaczmarek, J.C.; McClellan, R.L.; Jhunjhunwala, S.; Tibbitt, M.W.; Zeng, M.D.; Appel, E.A.; Dorkin, J.R.; Mir, F.F. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv. Mater. 2017, 29, 1606944. [Google Scholar] [CrossRef]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Théry, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012, 12, 269–281. [Google Scholar] [CrossRef]
- Abbott, M.; Ustoyev, Y. Cancer and the immune system: The history and background of immunotherapy. In Seminars in Oncology Nursing; WB Saunders: Philadelphia, PA, USA, 2019; p. 150923. [Google Scholar]
- Moyer, T.J.; Kato, Y.; Abraham, W.; Chang, J.Y.; Kulp, D.W.; Watson, N.; Turner, H.L.; Menis, S.; Abbott, R.K.; Bhiman, J.N. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat. Med. 2020, 26, 430–440. [Google Scholar] [CrossRef]
- Watanabe, A.; Nishida, S.; Burcu, T.; Shibahara, T.; Kusakabe, T.; Kuroda, E.; Ishii, K.J.; Kumanogoh, A. Safety and immunogenicity of a quadrivalent seasonal influenza vaccine adjuvanted with hydroxypropyl-β-cyclodextrin: A phase 1 clinical trial. Vaccine 2022, 40, 4150–4159. [Google Scholar] [CrossRef]
- Onishi, M.; Ozasa, K.; Kobiyama, K.; Ohata, K.; Kitano, M.; Taniguchi, K.; Homma, T.; Kobayashi, M.; Sato, A.; Katakai, Y. Hydroxypropyl-β-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. J. Immunol. 2015, 194, 2673–2682. [Google Scholar] [CrossRef]
- Hayashi, T.; Momota, M.; Kuroda, E.; Kusakabe, T.; Kobari, S.; Makisaka, K.; Ohno, Y.; Suzuki, Y.; Nakagawa, F.; Lee, M.S. DAMP-inducing adjuvant and PAMP adjuvants parallelly enhance protective type-2 and type-1 immune responses to influenza split vaccination. Front. Immunol. 2018, 9, 2619. [Google Scholar] [CrossRef]
- Lorentzen, C.L.; Haanen, J.B.; Met, Ö.; Svane, I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khazaei Monfared, Y.; Mahmoudian, M.; Zakeri-Milani, P.; Cecone, C.; Hayashi, T.; Ishii, K.J.; Conde, J.; Matencio, A.; Trotta, F. Intratumoural Delivery of mRNA Loaded on a Cationic Hyper-Branched Cyclodextrin-Based Polymer Induced an Anti-Tumour Immunological Response in Melanoma. Cancers 2023, 15, 3748. https://doi.org/10.3390/cancers15143748
Khazaei Monfared Y, Mahmoudian M, Zakeri-Milani P, Cecone C, Hayashi T, Ishii KJ, Conde J, Matencio A, Trotta F. Intratumoural Delivery of mRNA Loaded on a Cationic Hyper-Branched Cyclodextrin-Based Polymer Induced an Anti-Tumour Immunological Response in Melanoma. Cancers. 2023; 15(14):3748. https://doi.org/10.3390/cancers15143748
Chicago/Turabian StyleKhazaei Monfared, Yousef, Mohammad Mahmoudian, Parvin Zakeri-Milani, Claudio Cecone, Tomoya Hayashi, Ken J. Ishii, João Conde, Adrián Matencio, and Francesco Trotta. 2023. "Intratumoural Delivery of mRNA Loaded on a Cationic Hyper-Branched Cyclodextrin-Based Polymer Induced an Anti-Tumour Immunological Response in Melanoma" Cancers 15, no. 14: 3748. https://doi.org/10.3390/cancers15143748
APA StyleKhazaei Monfared, Y., Mahmoudian, M., Zakeri-Milani, P., Cecone, C., Hayashi, T., Ishii, K. J., Conde, J., Matencio, A., & Trotta, F. (2023). Intratumoural Delivery of mRNA Loaded on a Cationic Hyper-Branched Cyclodextrin-Based Polymer Induced an Anti-Tumour Immunological Response in Melanoma. Cancers, 15(14), 3748. https://doi.org/10.3390/cancers15143748











