Advancements in the Treatment of CLL: The Rise of Zanubrutinib as a Preferred Therapeutic Option
Abstract
Simple Summary
Abstract
1. Introduction and Background
2. Earlier Studies of Zanubrutinib in CLL
- Consistent with the favorable oral bioavailability evident in preclinical studies, oral administration of zanu achieves therapeutic plasma drug concentrations using the recommended phase II dose of 160 mg twice daily, with maintenance of drug levels above the IC50 required for full occupancy of the BTK binding site [25,28].
- Zanu is less prone to pharmacological interactions with food, drug–drug interactions with strong or moderate CYP3A inhibitors, and proton pump inhibitors (PPIs) leading to more consistent, sustained therapeutic exposures and improved dosing convenience. In addition, the clinical use of zanu is less sensitive to impairments of liver function than ibrutinib [29].
3. Phase III Clinical Trials of Zanubrutinib in CLL
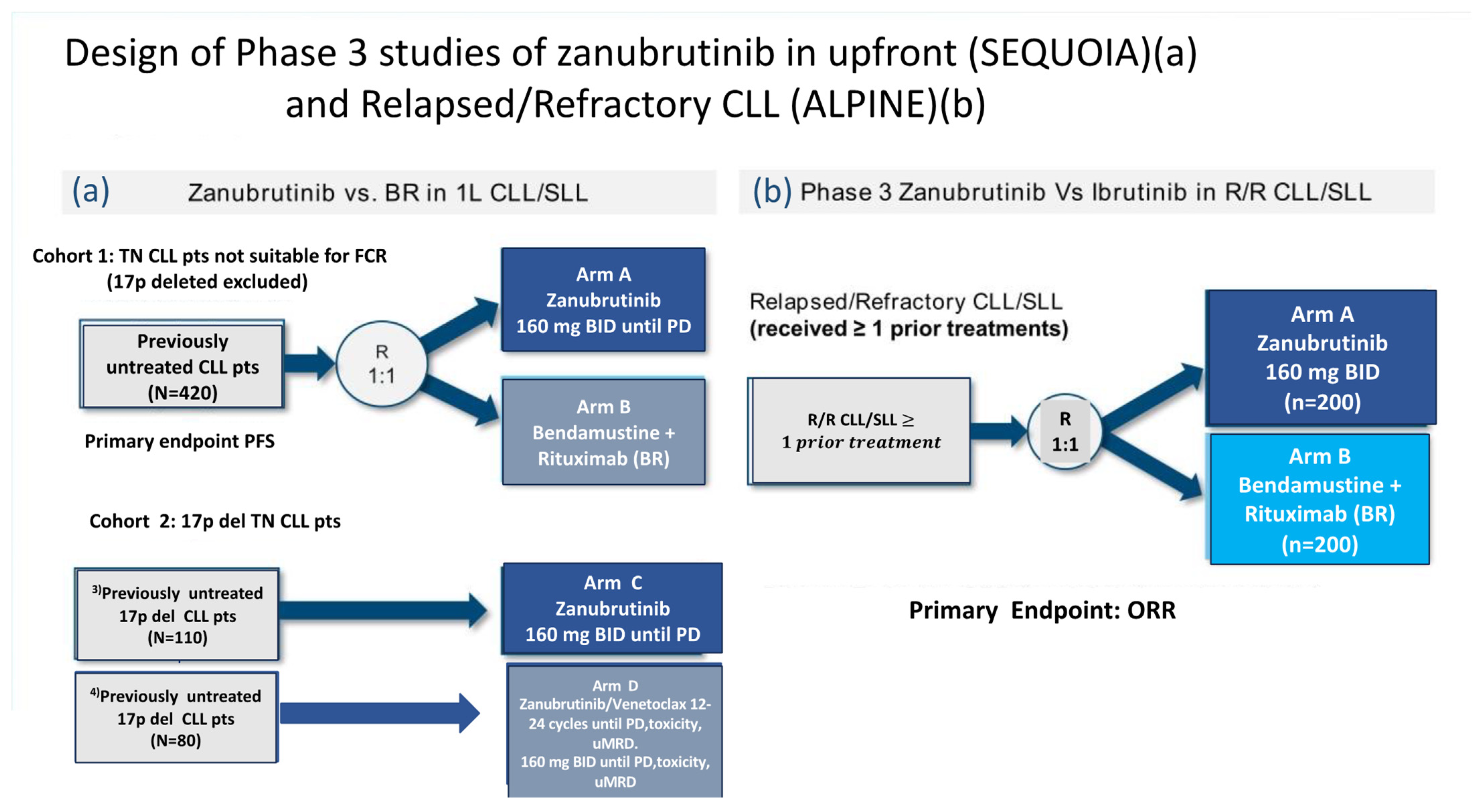
3.1. Sequoia Trial
- Cohort 1 comprised 479 patients without del(17p) who were randomized 1:1 and either assigned to receive zanu (n = 241)(until disease progression or unacceptable toxicity) or to bendamustine and rituximab (BR) (n = 238) for up to six cycles.
- Cohort 2 comprised 110 patients with del(17p) who were assigned to receive zanu monotherapy as it was deemed unethical to randomize patients with del(17p) to BR.
- Cohort 3 comprised 80 patients with del(17p) or TP53 aberrations who were assigned to receive zanu in combination with venetoclax (ZV). This cohort was opened when Cohort 2 was fully enrolled in order to provide non-randomized treatment for patients with del(17p).
3.2. Alpine Trial
4. Specific Aspects of Zanubrutinib Therapy in CLL
4.1. Is It Possible to Simplify the Zanubrutinib Treatment Schedule?
4.2. Zanubrutinib after Discontinuation of a Covalent BTKi Because of Toxicity
4.3. Combining Zanubrutinib with Monoclonal Antibodies or Anti-BCL2 Agents
4.4. Three Drug Zanubrutinib Combinations
4.5. Mechanisms of Zanubrutinib Resistance in CLL
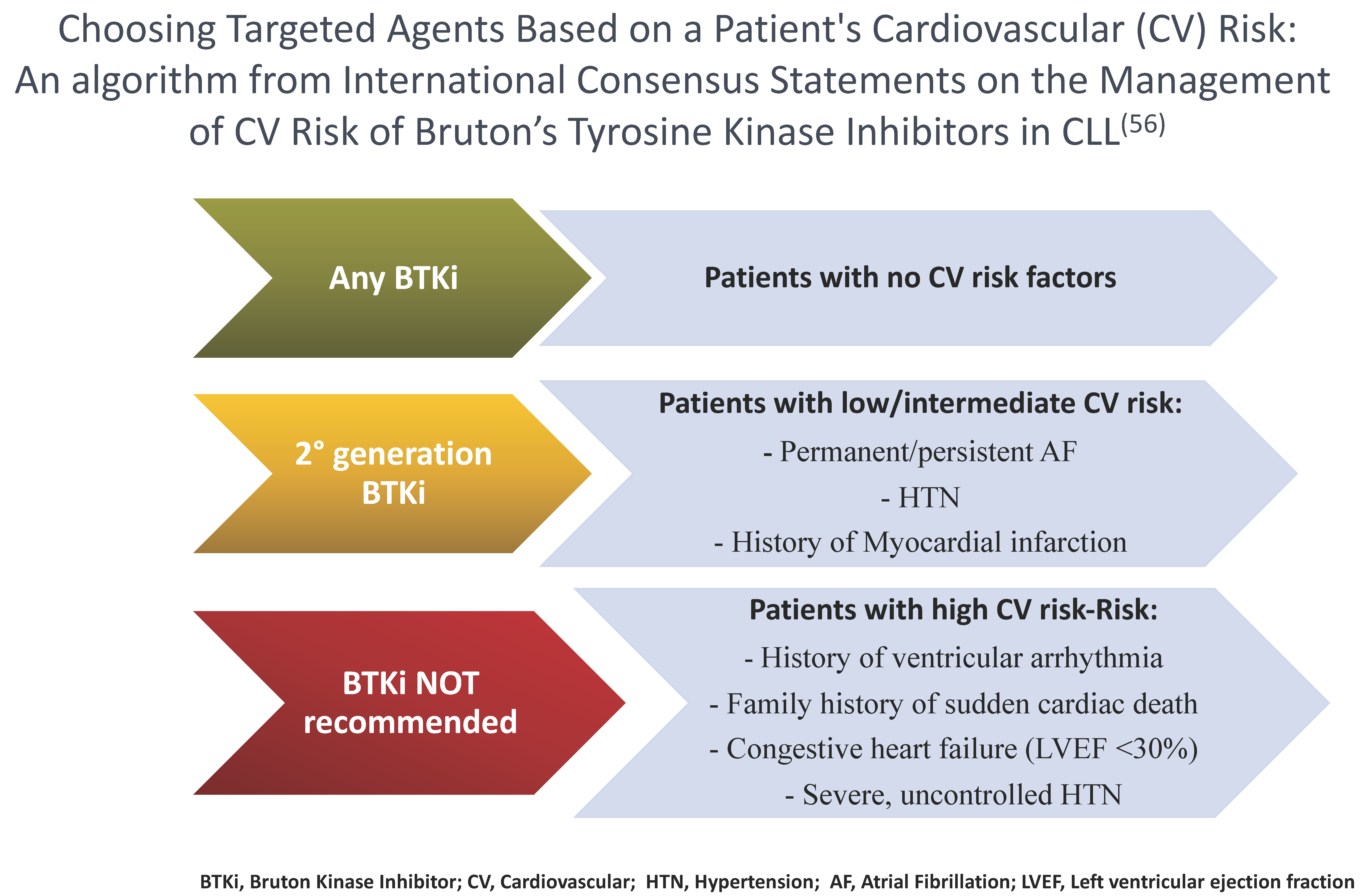
4.6. Zanubrutinib in Patients at Risk of Cardiovascular Complications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.A.; Grant, B.; Sharman, J.P.; Coleman, M.; Wierda, W.G.; et al. Targeting BTK with Ibrutinib in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2013, 369, 32–42. [Google Scholar] [CrossRef]
- Byrd, J.C.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Kay, N.E.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 2014, 371, 213–223. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib- Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, D.; et al. Ibrutinib plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicenter, randomized, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef]
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.E.; Dearden, C.; Grosicki, S.; et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022, 6, 5641–5654. [Google Scholar] [CrossRef]
- Molica, S.; Matutes, E.; Tam, C.; Polliack, A. Ibrutinib in the treatment of chronic lymphocytic leukemia: 5 years on. Hematol. Oncol. 2019, 38, 129–136. [Google Scholar] [CrossRef]
- Lipsky, A.; Lamanna, N. Managing toxicities of Bruton tyrosine kinase inhibitors. Am. Soc. Hematol. Educ. Program 2020, 2020, 336–345. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Project Orbis: FDA Approves Acalabrutinib for CLL and SLL. FDA Website. Posted 21 November 2019. Available online: https://bit.ly/35idpJM (accessed on 21 November 2019).
- Calquence Approved in the EU for the Treatment of Chronic Lymphocytic Leukaemia. News Release. AstraZeneca. 9 November 2020. Available online: https://bit.ly/3kiCkUT (accessed on 10 November 2020).
- Shirley, D. How does zanubrutinib fare in treatment of B-cell malignancies? Lancet Haematol. 2023, 10, e5–e6. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zanubrutinib-mantle-cell-lymphoma (accessed on 14 November 2019).
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zanubrutinib-marginal-zone-lymphoma (accessed on 14 September 2021).
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-waldenstroms-macroglobulinemia (accessed on 31 August 2021).
- Tam, C.S.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Sanz, R.G.; et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood 2020, 136, 2038–2050. [Google Scholar] [CrossRef]
- Tam, C.S.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Jurczak, W.; Šimkovič, M.; Shadman, M.; Österborg, A.; Laurenti, L.; et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lym-phoma (SEQUOIA): A randomised, controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma (accessed on 19 January 2023).
- Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/brukinsa (accessed on 21 November 2022).
- Wierda, W.G.; Byrd, J.C.; Abramson, J.S.; Bilgrami, S.F.; Bociek, G.; Brander, D.; Brown, J.; Chanan-Khan, A.A.; Chavez, J.C.; Coutre, S.E.; et al. NCCN Guidelines Insights: Chronic lymphocytic leukemia/small lymphocytic lymphoma, version 1.2023. J. Natl. Compr. Cancer Netw. 2022, 20, 622–634. [Google Scholar] [CrossRef]
- Available online: https://www.onkopedia.com/de/onkopedia/guidelines/chronische-lymphatische-leukaemie-cll/@@guideline/html/index.html (accessed on 19 January 2023).
- Tam, C.S.; Trotman, J.; Opat, S.; Burger, J.A.; Cull, G.; Gottlieb, D.; Harrup, R.; Johnston, P.B.; Marlton, P.; Munoz, J.; et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019, 134, 851–859. [Google Scholar] [CrossRef]
- Brander, D.M. The use of zanubrutinib in chronic lymphocytic leukemia. Clin. Adv. Hematol. Oncol. 2022, 20, 705–708. [Google Scholar] [PubMed]
- Cull, G.; Burger, J.A.; Opat, S.; Gottlieb, D.; Verner, E.; Trotman, J.; Marlton, P.; Munoz, J.; Johnston, P.; Simpson, D.; et al. Zanubrutinib for treatment-naïve and relapsed/refractory chronic lymphocytic leukaemia: Long-term follow-up of the phase I/II AU-003 study. Br. J. Haematol. 2022, 196, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; Wang, Y.; Jain, P.; Wang, M. Zanubrutinib in lymphoproliferative disorders: A comprehensive review. Ther. Adv. Hematol. 2022, 13, 20406207221093980. [Google Scholar] [CrossRef]
- Ou, Y.C.; Tang, Z.; Novotny, W.; Tawashi, M.; Li, T.; Coleman, H.A.; Sahasranaman, S. Evaluation of drug interaction potential of zanubrutinib with cocktail probes representative of CYP3A4, CYP2C9, CYP2C19, P-gp and BCRP. Br. J. Clin. Pharmacol. 2020, 87, 2926–2936. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Jurczak, W.; Šimkovič, M.; Shadman, M.; Österborg, A.; Laurenti, L.; et al. SEQUOIA: Results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab in patients with treat-ment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood 2021, 138 (Suppl. S1), 396. [Google Scholar] [CrossRef]
- Tam, C.S.; Robak, T.; Ghia, P.; Kahl, B.S.; Walker, P.; Janowski, W.; Simpson, D.; Shadman, M.; Ganly, P.S.; Laurenti, L.; et al. Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica 2020, 106, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Ferrant, E.; Flinn, I.W.; Tam, C.S.; Ghia, P.; Robak, T.; Brown, J.R.; Ramakrishnan, V.; Tian, T.; Kuwahara, S.B.; et al. Zanubrutinib in Combination with Venetoclax for Patients with Treatment-Naïve (TN) Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) with del(17p): Early Results from Arm D of the SEQUOIA (BGB-3111-304) Trial. In Proceedings of the American Society of Hematology Annual Meeting, Atlanta, GA, USA, 11–14 December 2021. [Google Scholar]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 2022, 36, 1171–1175. [Google Scholar] [CrossRef]
- Ghia, P.; Barnes, G.; Yang, K.; Tam, C.; Hillmen, P.; Robak, T.; Brown, J.; Kahl, B.; Tian, T.; Szeto, A.; et al. P662: Patient-Reported Outcomes from a Phase 3 Randomized Study of Zanubrutinib versus Bendamustine Plus Rituximab (Br) in Patients with Treatment-Naïve (TN) CLL/SLL. Hemasphere 2022, 6, 560–561. [Google Scholar] [CrossRef]
- Hillmen, P.; Eichhorst, B.; Brown, J.R.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Kazmierczak, M.; Zhou, K.; Šimkovič, M.; et al. Zanubrutinib Versus Ibrutinib in Relapsed/Refractory Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: Interim Analysis of a Randomized Phase III Trial. J. Clin. Oncol. 2023, 41, 1035–1045. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Kaźmierczak, L.; Jurczak, W.; Zhou, K.; et al. Zanubrutinib demonstrates superior progression-free survival (PFS) compared with ibrutinib for treatment of re-lapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma (R/R CLL/SLL): Results from final analysis of ALPINE randomized phase 3 study. Blood 2022, 140 (Suppl. S1), LBA-6. [Google Scholar]
- Tam, C.S.; Ou, Y.C.; Trotman, J.; Opat, S. Clinical pharmacology and PK/PD translation of the second-generation Bruton’s ty-rosine kinase inhibitor, zanubrutinib. Expert Rev. Clin. Pharmacol. 2021, 14, 1329–1344. [Google Scholar] [CrossRef]
- Hillmen, P.; Eichhorst, B.; Brown, J.R.; Lamanna, N.; O’Brien, S.; Tam, C.S.; Qiu, L.; Kazmierczak, M.; Zhou, K.; Šimkovič, M.; et al. Health-related quality of life outcomes associated with zanubrutinib vs ibrutinib monotherapy in patients with re-lapsed/refractory (RR) CLL/SLL: Results from the randomized phase 3 ALPINE trial. HemaSphere 2022, 6 (Suppl. S3), 663. [Google Scholar] [CrossRef]
- Ou, Y.C.; Tang, Z.; Novotny, W.; Cohen, A.; Wang, K.; Liu, L.; Gao, Y.; Sahasranaman, S. Rationale for once-daily or twice-daily dosing of zanubrutinib in patients with mantle cell lymphoma. Leuk. Lymphoma 2021, 62, 2612–2624. [Google Scholar] [CrossRef]
- Shadman, M.; Flinn, I.W.; Levy, M.Y.; Porter, R.F.; Burke, J.M.; Zafar, S.F.; Misleh, J.; Kingsley, E.C.; Yimer, H.A.; Freeman, B.; et al. Zanubrutinib in patients with previously treated B-cell malignancies intolerant of previous Bruton tyrosine kinase inhibitors in the USA: A phase 2, open-label, single-arm study. Lancet Haematol. 2023, 10, e35–e45. [Google Scholar] [CrossRef]
- Mato, A.R.; Nabhan, C.; Thompson, M.C.; Lamanna, N.; Brander, D.M.; Hill, B.; Howlett, C.; Skarbnik, A.; Cheson, B.D.; Zent, C.; et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: A real-world analysis. Haematologica 2018, 103, 874–879. [Google Scholar] [CrossRef]
- Byrd, J.C.; Woyach, J.A.; Furman, R.R.; Martin, P.; O’Brien, S.; Brown, J.R.; Stephens, D.M.; Barrientos, J.C.; Devereux, S.; Hillmen, P.; et al. Acalabrutinib in treatment-naive chronic lymphocytic leukemia. Blood 2021, 137, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.A.; Thompson, P.A.; Allan, J.N.; Coleman, M.; Sharman, J.P.; Cheson, B.D.; Jones, D.; Izumi, R.; Frigault, M.M.; Quah, C.; et al. Phase II study of acalabrutinib in ibrutinib-intolerant patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica 2021, 106, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Sivina, M.; Jain, N.; Kim, E.; Kadia, T.; Estrov, Z.; Nogueras-Gonzalez, G.M.; Huang, X.; Jorgensen, J.; Li, J.; et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood 2019, 133, 1011–1019. [Google Scholar] [CrossRef]
- Tam, C.S.; Quach, H.; Nicol, A.; Badoux, X.; Rose, H.; Prince, H.M.; Leahy, M.F.; Eek, R.; Wickham, N.; Patil, S.S.; et al. Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv. 2020, 4, 4802–4811. [Google Scholar] [CrossRef] [PubMed]
- Soumerai, J.D.; Mato, A.R.; Dogan, A.; Seshan, V.E.; Joffe, E.; Flaherty, K.; Carter, J.; Hochberg, E.; Barnes, J.A.; Hamilton, A.M.; et al. Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: A multicentre, single-arm, phase 2 trial. Lancet Haematol. 2021, 8, e879–e890. [Google Scholar] [CrossRef]
- Davids, M.S.; Lampson, B.L.; Tyekucheva, S.; Wang, Z.; Lowney, J.C.; Pazienza, S.; Montegaard, J.; Patterson, V.; Weinstock, M.; Crombie, J.L.; et al. Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: A single-arm, open-label, phase 2 study. Lancet Oncol. 2021, 22, 1391–1402. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Guinn, D.; Lehman, A.; Blachly, J.S.; Lozanski, A.; Heerema, N.A.; Zhao, W.; Coleman, J.; Jones, D.; et al. BTKC481S-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2017, 35, 1437–1443. [Google Scholar] [CrossRef]
- Zhu, H.; Sha, Y.; Miao, Y.; Qin, S.; Jiang, R.; Wu, W.; Xia, Y.; Qiu, T.; Wang, L.; Fan, L.; et al. Integrating Multi-Omics to Reveal the Clonal Evolutionary Characteristics in CLL Patients with Zanubrutinib Resistance. Blood 2022, 140 (Suppl. S1), 6985–6987. [Google Scholar] [CrossRef]
- Blombery, P.; Thompson, E.R.; Lew, T.E.; Tiong, I.S.; Bennett, R.; Cheah, C.Y.; Lewis, K.L.; Handunnetti, S.M.; Tang, C.P.S.; Roberts, A.; et al. Enrichment of BTK Leu528Trp mutations in patients with CLL on zanubrutinib: Potential for pirtobrutinib cross-resistance. Blood Adv. 2022, 6, 5589–5592. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.E.; Brown, J.R. Selecting initial therapy in CLL. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Shadman, M. Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Review. JAMA 2023, 329, 918–932. [Google Scholar] [CrossRef]
- Gordon, M.J.; Kaempf, A.; Sitlinger, A.; Shouse, G.; Mei, M.; Brander, D.M.; Salous, T.; Hill, B.T.; Alqahtani, H.; Choi, M.; et al. The Chronic Lymphocytic Leukemia Comorbidity Index (CLL-CI): A Three-Factor Comorbidity Model. Clin. Cancer Res. 2021, 27, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, T.; Wiczer, T.; Waller, A.; Philippon, J.; Porter, K.; Haddad, D.; Guha, A.; Rogers, K.A.; Bhat, S.; Byrd, J.C.; et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019, 134, 1919–1928. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Parikh, S.A.; Noseworthy, P.A.; Goede, V.; Chaffee, K.G.; Bahlo, J.; Call, T.G.; Schwager, S.M.; Ding, W.; Eichhorst, B.; et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk. Lymphoma 2017, 58, 1630–1639. [Google Scholar] [CrossRef]
- Awan, F.T.; Addison, D.; Alfraih, F.; Baratta, S.J.; Campos, R.N.; Cugliari, M.S.; Goh, Y.T.; Ionin, V.A.; Mundnich, S.; Sverdlov, A.L.; et al. In-ternational consensus statement on the management of cardiovascular risk of Bruton’s tyrosine kinase inhibitors in CLL. Blood Adv. 2022, 6, 5516–5525. [Google Scholar] [CrossRef]
- Bhat, S.A.; Gambril, J.; Azali, L.; Chen, S.T.; Rosen, L.; Palettas, M.; Wiczer, T.E.; Kalathoor, S.; Zhao, Q.; Rogers, K.A.; et al. Ventricular ar-rhythmias and sudden death events following acalabrutinib initiation. Blood 2022, 140, 2142–2145. [Google Scholar] [CrossRef]
- Lampson, B.L.; Yu, L.; Glynn, R.J.; Barrientos, J.C.; Jacobsen, E.D.; Banerji, V.; Jones, J.A.; Walewska, R.; Savage, K.J.; Michaud, G.F.; et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood 2017, 129, 2581–2584. [Google Scholar] [CrossRef]
- Hwang, S.; Wang, J.; Tian, Z.; Qi, X.; Jiang, Y.; Zhang, S.; Godby, R.C.; Parikh, S.; Ding, W.; Hampel, P.; et al. Comparison of treat-ment-emergent adverse events of acalabrutinib and zanubrutinib in clinical trials in B-cell malignancies: A systematic review and meta-anaysis. EHA 2023, P632, 386461. Available online: https://library.ehaweb.org/eha/2023/eha2023-congress/386461/steven.hwang.comparison.of.treatment-emergent.adverse.events.of.acalabrutinib.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D2%2Asearch%3Dacalabrutinib (accessed on 18 May 2023).
- Hampel, P.J.; Parikh, S.A. Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer J. 2022, 12, 161. [Google Scholar] [CrossRef]
- Kuss, B.; Nagarajan, C.; Hsieh, W.S.; Cheah, C.Y. Practical management of chronic lymphocytic leukemia with acalabrutinib. Leuk. Lymphoma 2022, 63, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pepin, X.; Burri, H.; Zheng, L.; Kuptsova-Clarkson, N.; de Jong, A.; Yu, T.; MacArthur, H.L.; Majewski, M.; Byrd, J.C.; et al. Bi-oequivalence and Relative Bioavailability Studies to Assess a New Acalabrutinib Formulation That Enables Coadministration With Proton-Pump Inhibitors. Clin. Pharmacol. Drug Dev. 2022, 11, 1294–1307. [Google Scholar] [CrossRef]
- Molica, S.; Giannarelli, D.; Visentin, A.; Reda, G.; Sportoletti, P.; Frustaci, A.M.; Chiarenza, A.; Ciolli, S.; Vitale, C.; Laurenti, L.; et al. Prediction of outcomes in chronic lymphocytic leukemia patients treated with ibrutinib: Validation of current prognostic models and development of a simplified three-factor model. Am. J. Hematol. 2022, 97, E176–E180. [Google Scholar] [CrossRef]
- Davids, M.S.; Sharman, J.P.; Eyre, T.A.; Woyach, J.A.; de Miranda, P.A.P.; Shahkarami, M.; Butturini, A.; Emeribe, U.; Byrd, J.C. Contribution of Obinutuzumab to Acalabrutinib Therapy in Patients with Treatment-Naive Chronic Lymphocytic Leukemia: Analysis of Survival Outcomes By Genomic Features. Blood 2022, 140, 4173–4175. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Šimkovič, M.; Kriachok, I.; Illés, Á.; de la Serna, J.; Dolan, S.; Campbell, P.; et al. Acalabrutinib Versus Investigator’s Choice in Relapsed/Refractory Chronic Lymphocytic Leukemia: Final ASCEND Trial Results. Hemasphere 2022, 6, e801. [Google Scholar] [CrossRef]
- Skarbnik, A.; Kittai, A.; Miranda, M.; Yong, A.; Roos, J.; Hettle, R.; Palazuelos-Munoz, S.; Shetty, V.; Ghia, P. A Matching-Adjusted Indirect Comparison of the Efficacy and Safety of Acalabrutinib Versus Zanubrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. Hematol. Oncol. 2023, 41, 742–743. [Google Scholar] [CrossRef]
- Tam, C.S.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.W.; Opat, S.; Barr, P.M.; Tedeschi, A.; Trentin, L.; Bannerji, R.; et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: Primary analysis of the CAPTIVATE FD cohort. Blood 2022, 139, 3278–3289. [Google Scholar] [CrossRef]
- Kater, A.P.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.-D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Robak, T.; et al. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. NEJM Évid. 2022, 1, EVIDoa2200006. [Google Scholar] [CrossRef]
| Reference | Schedule |
N of Pts (Tx Status) | 17p(del)/TP53 Mut (%) | ORR (%) | Survival Outcome | G ≥ 3 AEs (%) | Toxicity-related Discontinuations (%) | Any Grade/(G ≥ 3) AF (%,) | Any Grade Bleeding/ (G ≥ 3) (%) | Any Grade Hypertension/ (G ≥ 3) (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cull et al. [27] | Zanu 160 mg bid or 320 mg/d or 160 mg/d | 22 (TN) 101 (R/R) | 5.6 (TN) 6.0 (R/R) | 100 (TN) 95 (R/R) | 3-YEAR PFS: 90 (TN) 81 (R/R) | 73.2 | 9.8 | 4.9 (3.3) | 38.2 (3.3) | 19.5 (8.9) |
| Tam et al. [19] (SEQUOIA Group A) | Zanu 160 mg bid | 241 (TN) | 1 | 94.6 | 2-YEAR PFS: 85.5% | 53 | 8 | 3 | 41 (3.7) | 6 (6) |
| Tam et al. [31] (SEQUOIA Group C) | Zanu 160 mg bid | 111 (TN) | 99 | 90 | 2-YEAR PFS: 88.9% | 55 | 5 | 48 (5) | 5 (5) | |
| Brown et al. [20] 2022 |
Zanu 160 mg bid Ibrutinib: 420 mg/d | 327 (R/R) 325 (R/R) | 13.8 15.4 | 2-YEAR PFS: 78.4 65.9 | 67.3 70.4 | 16.2 22.2 | 6.2 (2.5) 13.3 (4) | 42.3 (3.4) 41.4 (3.7) | 23.5 (15.1) 22.8 (13.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molica, S.; Tam, C.; Allsup, D.; Polliack, A. Advancements in the Treatment of CLL: The Rise of Zanubrutinib as a Preferred Therapeutic Option. Cancers 2023, 15, 3737. https://doi.org/10.3390/cancers15143737
Molica S, Tam C, Allsup D, Polliack A. Advancements in the Treatment of CLL: The Rise of Zanubrutinib as a Preferred Therapeutic Option. Cancers. 2023; 15(14):3737. https://doi.org/10.3390/cancers15143737
Chicago/Turabian StyleMolica, Stefano, Constantine Tam, David Allsup, and Aaron Polliack. 2023. "Advancements in the Treatment of CLL: The Rise of Zanubrutinib as a Preferred Therapeutic Option" Cancers 15, no. 14: 3737. https://doi.org/10.3390/cancers15143737
APA StyleMolica, S., Tam, C., Allsup, D., & Polliack, A. (2023). Advancements in the Treatment of CLL: The Rise of Zanubrutinib as a Preferred Therapeutic Option. Cancers, 15(14), 3737. https://doi.org/10.3390/cancers15143737








