Daily Head and Neck Treatment Assessment for Optimal Proton Therapy Planning Robustness
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Robustness Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics and Treatment Characteristics
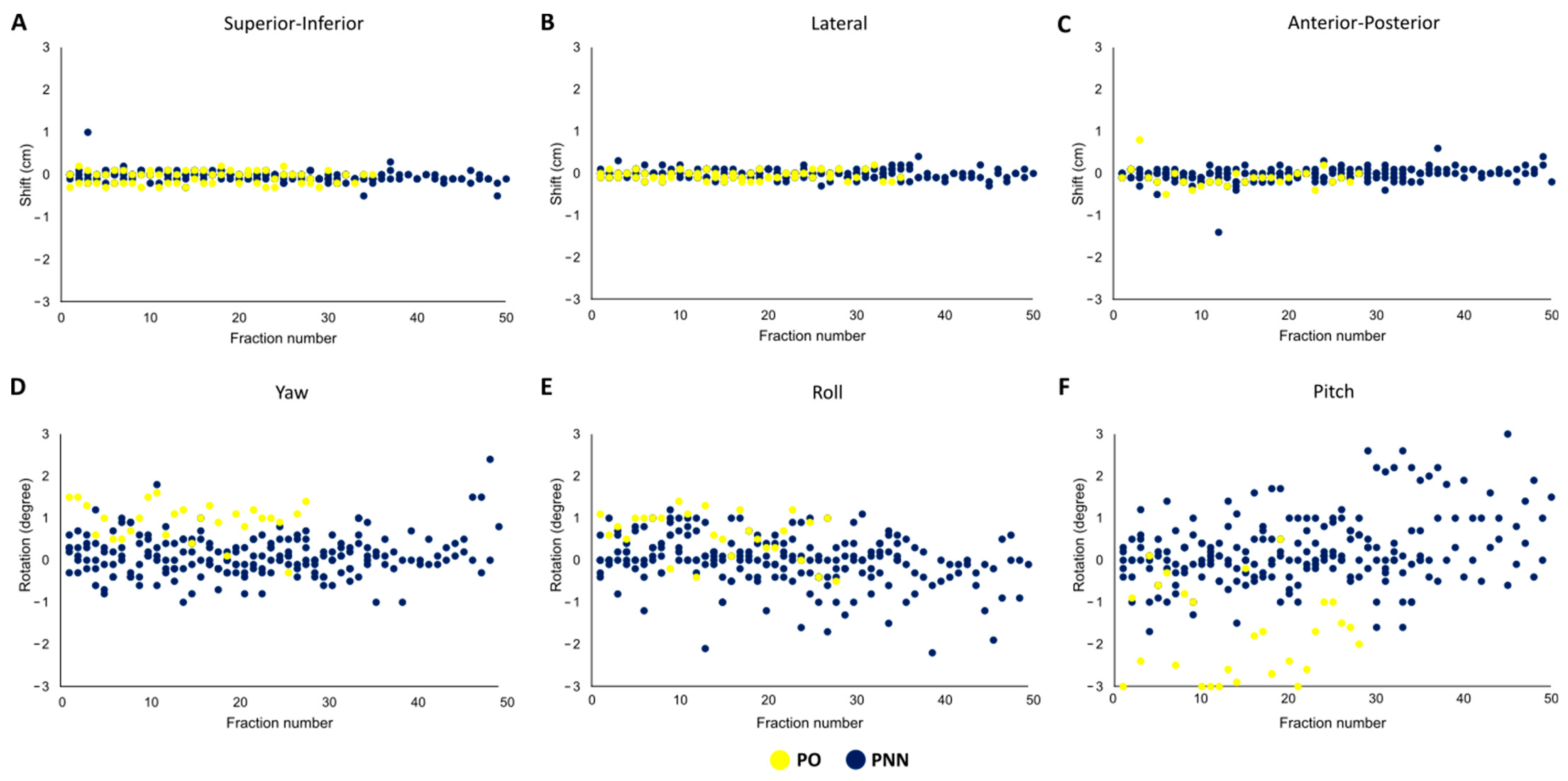
3.2. Daily Shifts
3.3. Fractional Uncertainty
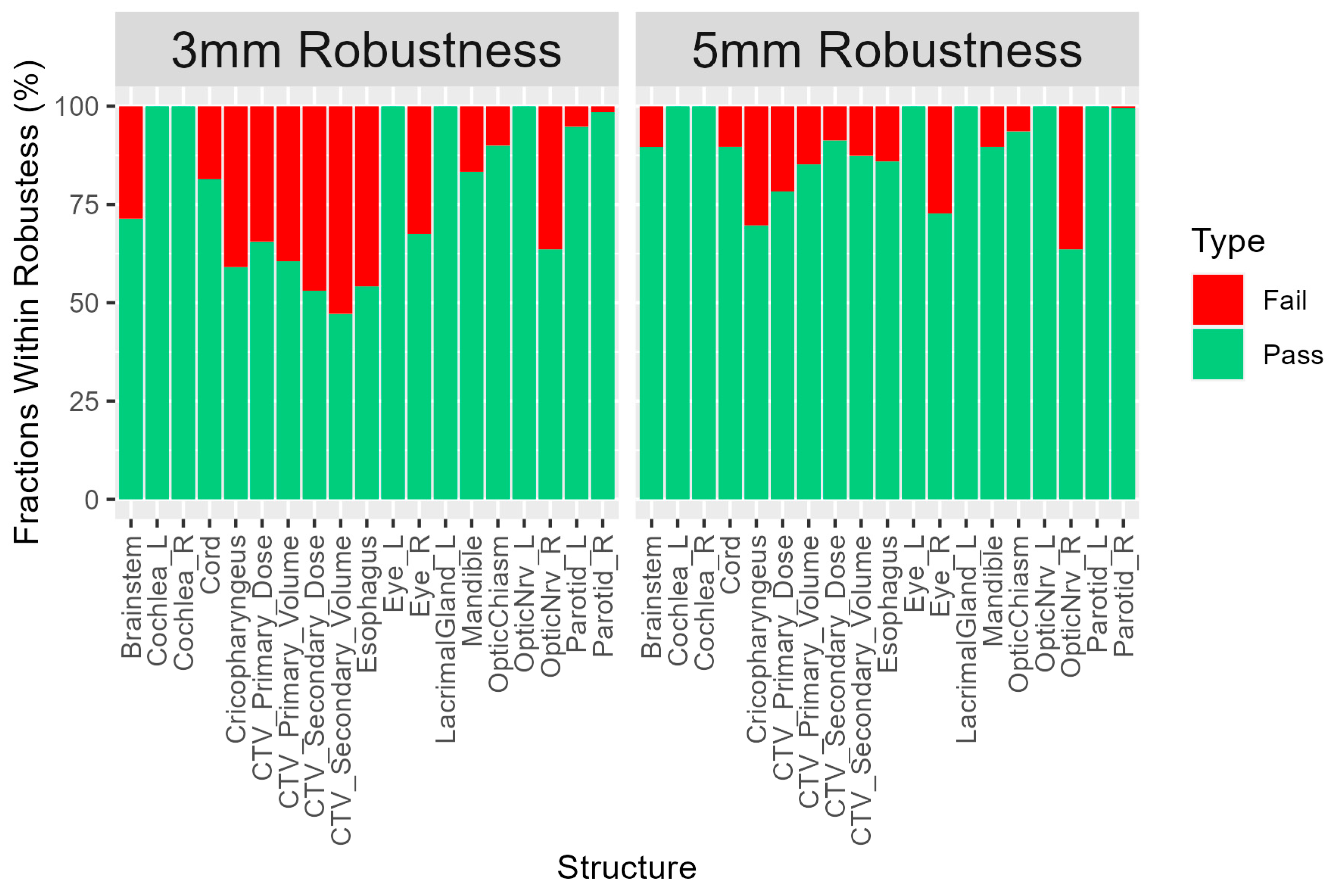
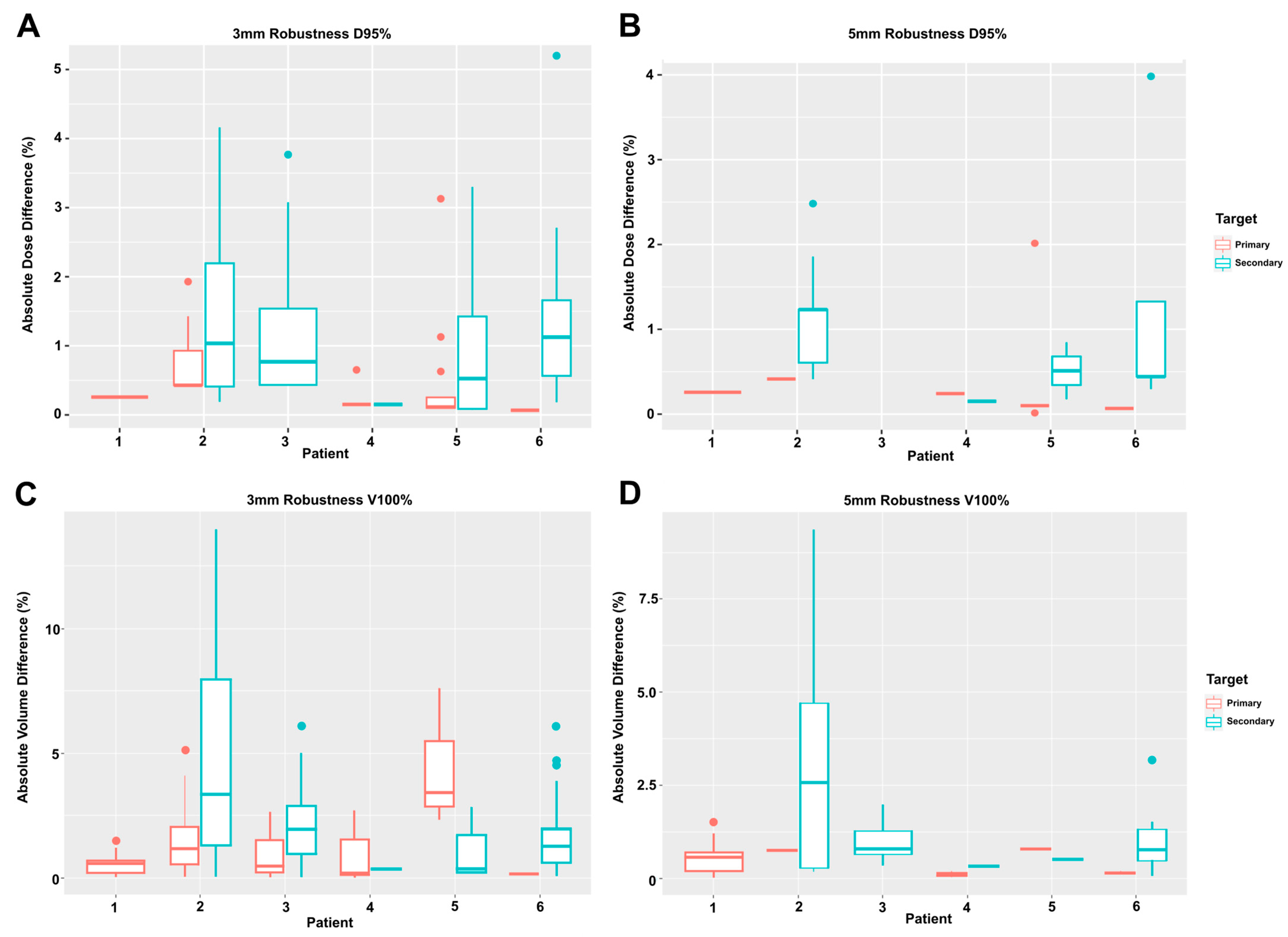
3.4. Fractional Dose Deviation
3.5. Fractional Volume Deviation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kramer, S.; Gelber, R.D.; Snow, J.B.; Marcial, V.A.; Lowry, L.D.; Davis, L.W.; Chandler, R. Combined radiation therapy and surgery in the management of advanced head and neck cancer: Final report of study 73–03 of the radiation therapy oncology group. Head Neck Surg. 1987, 10, 19–30. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Lomax, A.J. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: The potential effects of calculational uncertainties. Phys. Med. Biol. 2008, 53, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Lomax, A.J. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: The potential effects of inter-fraction and inter-field motions. Phys. Med. Biol. 2008, 53, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Seum, W.C.T.H.; Hunzeker, A.; Muller, O.; Foote, R.L.; Mundy, D.W. The effect of common dental fixtures on treatment planning and delivery for head and neck intensity modulated proton therapy. J. Appl. Clin. Med. Phys. 2023, 24, e13973. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, N.; Ishikawa, H.; Ohnishi, K.; Terunuma, T.; Mizumoto, M.; Numajiri, H.; Aihara, T.; Okumura, T.; Tsuboi, K.; Sakae, T.; et al. Dose distribution resulting from changes in aeration of nasal cavity or paranasal sinus cancer in the proton therapy. Radiother. Oncol. 2014, 113, 72–76. [Google Scholar] [CrossRef]
- Kraan, A.C.; Van De Water, S.; Teguh, D.N.; Al-Mamgani, A.; Madden, T.; Kooy, H.M.; Heijmen, B.J.M.; Hoogeman, M.S. Dose Uncertainties in IMPT for Oropharyngeal Cancer in the Presence of Anatomical, Range, and Setup Errors. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 888–896. [Google Scholar] [CrossRef]
- Müller, B.S.; Duma, M.N.; Kampfer, S.; Nill, S.; Oelfke, U.; Geinitz, H.; Wilkens, J.J. Impact of interfractional changes in head and neck cancer patients on the delivered dose in intensity modulated radiotherapy with protons and photons. Phys. Med. 2015, 31, 266–272. [Google Scholar] [CrossRef]
- Stuschke, M.; Kaiser, A.; Jawad, J.A.; Pöttgen, C.; Levegrün, S.; Farr, J. Multi-scenario based robust intensity-modulated proton therapy (IMPT) plans can account for set-up errors more effectively in terms of normal tissue sparing than planning target volume (PTV) based intensity-modulated photon plans in the head and neck regi. Radiat. Oncol. 2013, 8, 145. [Google Scholar] [CrossRef]
- Pflugfelder, D.; Wilkens, J.J.; Oelfke, U. Worst case optimization: A method to account for uncertainties in the optimization of intensity modulated proton therapy. Phys. Med. Biol. 2008, 53, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, A.; Forsgren, A.; Hårdemark, B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med. Phys. 2011, 38, 1672–1684. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, E.W.; Habraken, S.J.M.; Scandurra, D.; Kierkels, R.G.J.; Unipan, M.; Eenink, M.G.C.; Steenbakkers, R.J.H.M.; Peeters, S.G.; Zindler, J.D.; Hoogeman, M.; et al. Practical robustness evaluation in radiotherapy—A photon and proton-proof alternative to PTV-based plan evaluation. Radiother. Oncol. 2019, 141, 267–274. [Google Scholar] [CrossRef] [PubMed]
- ICRU REPORT 93; Prescribing, Recording, and Reporting Light Ion Beam Therapy. ICRU: Bethesda, MD, USA, 2016.
- Veiga, C.; Janssens, G.; Baudier, T.; Hotoiu, L.; Brousmiche, S.; McClelland, J.; Teng, C.L.; Yin, L.; Royle, G.J.; Teo, B.-K. A comprehensive evaluation of the accuracy of CBCT and deformable registration based dose calculation in lung proton therapy. Biomed. Phys. Eng. Express 2017, 3, 015003. [Google Scholar] [CrossRef]
- Sheikh, K.; Liu, D.; Li, H.; Acharya, S.; Ladra, M.M.; Hrinivich, W.T. Dosimetric evaluation of cone-beam CT-based synthetic CTs in pediatric patients undergoing intensity-modulated proton therapy. J. Appl. Clin. Med. Phys. 2022, 23, e13604. [Google Scholar] [CrossRef]
- Kurz, C.; Dedes, G.; Resch, A.; Reiner, M.; Ganswindt, U.; Nijhuis, R.; Thieke, C.; Belka, C.; Parodi, K.; Landry, G. Comparing cone-beam CT intensity correction methods for dose recalculation in adaptive intensity-modulated photon and proton therapy for head and neck cancer. Acta Oncol. 2015, 54, 1651–1657. [Google Scholar] [CrossRef]
- Hague, C.; Aznar, M.; Dong, L.; Fotouhi-Ghiam, A.; Lee, L.W.; Li, T.; Lin, A.; Lowe, M.; Lukens, J.N.; McPartlin, A.; et al. Inter-fraction robustness of intensity-modulated proton therapy in the post-operative treatment of oropharyngeal and oral cavity squamous cell carcinomas. Br. J. Radiol. 2020, 93, 20190638. [Google Scholar] [CrossRef]
- Anand, A.; Bues, M.; Gamez, M.E.; Stefan, C.; Patel, S.H. Individual Field Simultaneous Optimization (IFSO) in spot scanning proton therapy of head and neck cancers. Med. Dosim. 2019, 44, 375–378. [Google Scholar] [CrossRef]
- Raystation. Deformable Registration in Raystation (White Paper). Available online: https://www.raysearchlabs.com/siteassets/media/publications/white-papers/wp-pdfs/synthetic_ct_whitepaper.pdf (accessed on 1 April 2023).
- Zumsteg, Z.; DeMarco, J.; Lee, S.P.; Steinberg, M.L.; Lin, C.S.; McBride, W.; Lin, K.; Wang, P.C.; Kupelian, P.; Lee, P. Image guidance during head-and-neck cancer radiation therapy: Analysis of alignment trends with in-room cone-beam computed tomography scans. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 712–719. [Google Scholar] [CrossRef]
- ICRU (International Commission on Radiation Units and Measurements). Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). J. ICRU 2010, 10. [Google Scholar] [CrossRef]
- Góra, J.; Kuess, P.; Stock, M.; Andrzejewski, P.; Knäusl, B.; Paskeviciute, B.; Altorjai, G.; Georg, D. ART for head and neck patients: On the difference between VMAT and IMPT. Acta Oncol. 2015, 54, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Stanforth, A.; Lin, L.; Beitler, J.J.; Janopaul-Naylor, J.R.; Chang, C.W.; Press, R.H.; Patel, S.A.; Zhao, J.; Eaton, B.; Schreibmann, E.E.; et al. Onboard cone-beam CT-based replan evaluation for head and neck proton therapy. J. Appl. Clin. Med. Phys. 2022, 23, e13550. [Google Scholar] [CrossRef] [PubMed]
- Thummerer, A.; Zaffino, P.; Meijers, A.; Marmitt, G.G.; Seco, J.; Steenbakkers, R.J.H.M.; Langendijk, J.A.; Both, S.; Spadea, M.F.; Knopf, A.C. Comparison of CBCT based synthetic CT methods suitable for proton dose calculations in adaptive proton therapy. Phys. Med. Biol. 2020, 65, 095002. [Google Scholar] [CrossRef] [PubMed]
- Zechner, A.; Ziegler, I.; Hug, E.; Lütgendorf-Caucig, C.; Stock, M. Evaluation of the inter- and intrafraction displacement for head patients treated at the particle therapy centre MedAustron based on the comparison of different commercial immobilisation devices. Z. Med. Phys. 2022, 32, 39–51. [Google Scholar] [CrossRef]
| Cases (n = 6) | |
|---|---|
| Age at treatment (yrs), mean (SD) | 50.5 (22.7) |
| Sex: | |
| Male | 3 (50.0%) |
| Female | 3 (50.0%) |
| Location: | |
| Sinonasal | 2 (33.3%) |
| Tonsil | 2 (33.3%) |
| Schwannoma | 1 (23.8%) |
| Salivary Gland | 1 (23.8%) |
| T Stage: | |
| T1 | 1 (23.8%) |
| T2 | 1 (23.8%) |
| T3 | 1 (23.8%) |
| T4 | 3 (50.0%) |
| N Stage: | |
| N0 | 2 (33.3%) |
| N1 | 2 (33.3%) |
| N2 | 2 (33.3%) |
| Primary prescription (Gy), mean (SD) | 67.1 (8.7) |
| Secondary prescription (Gy), mean (SD) | 63.0 (2.8) |
| Weight loss (kg), mean (SD) | −2.0 (3.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.; Shaaban, S.G.; Gogineni, E.; Page, B.; Quon, H.; Li, H.; Ger, R. Daily Head and Neck Treatment Assessment for Optimal Proton Therapy Planning Robustness. Cancers 2023, 15, 3719. https://doi.org/10.3390/cancers15143719
Chang L, Shaaban SG, Gogineni E, Page B, Quon H, Li H, Ger R. Daily Head and Neck Treatment Assessment for Optimal Proton Therapy Planning Robustness. Cancers. 2023; 15(14):3719. https://doi.org/10.3390/cancers15143719
Chicago/Turabian StyleChang, Leslie, Sherif G. Shaaban, Emile Gogineni, Brandi Page, Harry Quon, Heng Li, and Rachel Ger. 2023. "Daily Head and Neck Treatment Assessment for Optimal Proton Therapy Planning Robustness" Cancers 15, no. 14: 3719. https://doi.org/10.3390/cancers15143719
APA StyleChang, L., Shaaban, S. G., Gogineni, E., Page, B., Quon, H., Li, H., & Ger, R. (2023). Daily Head and Neck Treatment Assessment for Optimal Proton Therapy Planning Robustness. Cancers, 15(14), 3719. https://doi.org/10.3390/cancers15143719





