Novel Molecular Targets for Immune Surveillance of Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. The Immuno System in HCC
2.1. Antigen Recognition
2.2. Tumour Microenvironment
2.2.1. Adoptive Cell Therapy
2.2.2. Lymphoid Inhibiting Cells
T Regulatory Lymphocytes
2.2.3. Myeloid Inhibiting Cells
Tumour-Associated Macrophages (TAMs)
Myeloid-Derived Suppressor Cells (MDSCs)
Dendritic Cells
2.2.4. Non-Parenchymal Hepatic Cells
3. Immune Classification
3.1. The Inflamed Class: Immune-Active, Immune-Exhausted and Immune-like
3.2. The Non-Inflamed Class: Immune-Intermediate and Immune-Excluded
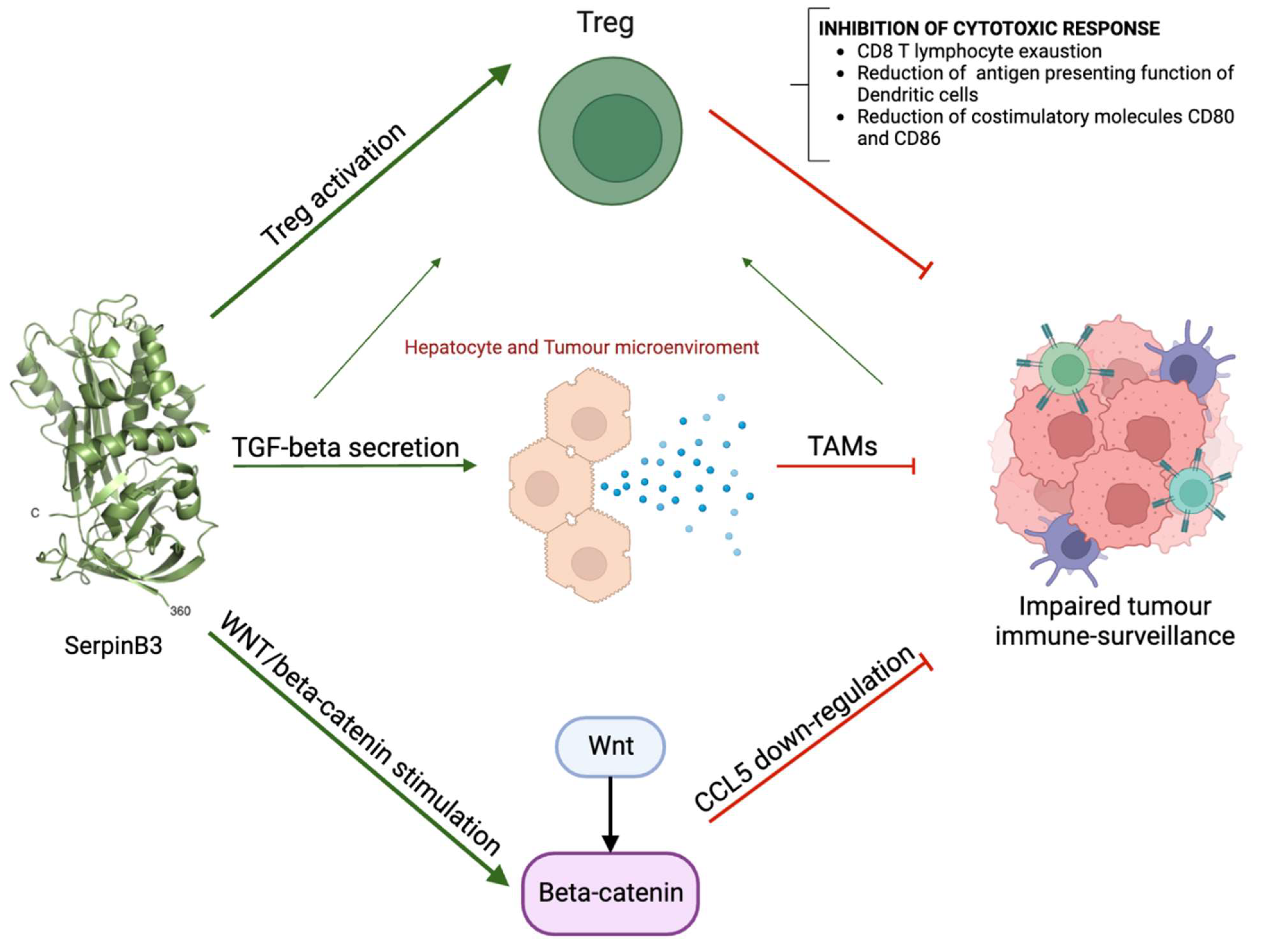
4. Role of SerpinB3 in the Immunosurveillance of HCC
Response to Hypoxic Conditions and HIF Induction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- Shah, P.A.; Patil, R.; Harrison, S.A. NAFLD-related Hepatocellular Carcinoma: The Growing Challenge. Hepatology 2023, 77, 323–338. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, T.; Tokushige, K. The Current View of Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma. Cancers 2021, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global Epidemiology of NAFLD-Related HCC: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Reig, M.; Villanueva, A. Emerging Tools for Hepatocellular Carcinoma Surveillance. Am. J. Gastroenterol. 2022, 117, 1948–1951. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Singal, A.G.; Zhang, E.; Narasimman, M.; Rich, N.E.; Waljee, A.K.; Hoshida, Y.; Yang, J.D.; Reig, M.; Cabibbo, G.; Nahon, P.; et al. HCC Surveillance Improves Early Detection, Curative Treatment Receipt, and Survival in Patients with Cirrhosis: A Meta-Analysis. J. Hepatol. 2022, 77, 128–139. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. Hepatology 2023. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma: A 2017 Update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Su, Q.; Fan, M.; Wang, J.; Ullah, A.; Ghauri, M.A.; Dai, B.; Zhan, Y.; Zhang, D.; Zhang, Y. Sanguinarine Inhibits Epithelial–Mesenchymal Transition via Targeting HIF-1α/TGF-β Feed-Forward Loop in Hepatocellular Carcinoma. Cell Death Dis. 2019, 10, 939. [Google Scholar] [CrossRef]
- Ullah, A.; Aziz, T.; Ullah, N.; Nawaz, T. Molecular Mechanisms of Sanguinarine in Cancer Prevention and Treatment. Anticancer Agents Med. Chem. 2023, 23, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Foerster, F.; Gairing, S.J.; Ilyas, S.I.; Galle, P.R. Emerging Immunotherapy for HCC: A Guide for Hepatologists. Hepatology 2022, 75, 1604–1626. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Gao, P.; Ding, J. Immune Checkpoint Inhibitor Resistance in Hepatocellular Carcinoma. Cancer Lett. 2023, 555, 216038. [Google Scholar] [CrossRef]
- Kim, T.K.; Vandsemb, E.N.; Herbst, R.S.; Chen, L. Adaptive Immune Resistance at the Tumour Site: Mechanisms and Therapeutic Opportunities. Nat. Rev. Drug Discov. 2022, 21, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Petrelli, F.; Ghidini, A.; Raimondi, A.; Smyth, E.; Pietrantonio, F. Efficacy and Activity of PD-1 Blockade in Patients with Advanced Esophageal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis with Focus on the Value of PD-L1 Combined Positive Score. ESMO Open 2022, 7, 100380. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Jorns, J.M.; Tozbikian, G. What’s New in Breast Pathology 2022: WHO 5th Edition and Biomarker Updates. J. Pathol. Transl. Med. 2022, 56, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1. [Google Scholar] [CrossRef]
- Roth, G.S.; Villeret, F.; Decaens, T.; Merle, P.; Nahon, P. Immunotherapy in Hepatocellular Carcinoma: How Does Underlying Liver Disease Influence Therapeutic Strategy and Outcomes? Liver Int. 2023, 43, 546–557. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated Efficacy and Safety Data from IMbrave150: Atezolizumab Plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Chan, L.S.; Kudo, M.; Sangro, B.; Kelley, R.K.; Furuse, J.; Park, J.-W.; Sunpaweravong, P.; Fasolo, A.; Yau, T.; Kawaoka, T.; et al. 714P—Impact of Viral Aetiology in the Phase 3 HIMALAYA Study of Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. Ann. Oncol. 2022, 33, S869–S870. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab Versus Sorafenib in Advanced Hepatocellular Carcinoma (CheckMate 459): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Finn, R.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z.; et al. LBA34—Primary Results from the Phase III LEAP-002 Study: Lenvatinib plus Pembrolizumab versus Lenvatinib as First-Line (1L) Therapy for Advanced Hepatocellular Carcinoma (AHCC). Ann. Oncol. 2022, 33, S1401. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH Limits Anti-Tumour Surveillance in Immunotherapy-Treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The Evolving Landscape of Biomarkers for Checkpoint Inhibitor Immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Haber, P.K.; Castet, F.; Torres-Martin, M.; Andreu-Oller, C.; Puigvehí, M.; Miho, M.; Radu, P.; Dufour, J.-F.; Verslype, C.; Zimpel, C.; et al. Molecular Markers of Response to Anti-PD1 Therapy in Advanced Hepatocellular Carcinoma. Gastroenterology 2023, 164, 72–88.e18. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and Functional Alterations of Tumor-Associated Antigen-Specific CD8+T-Cell Responses in Hepatocellular Carcinoma. Hepatology 2013, 59, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Okusaka, T.; Ohno, I.; Mitsunaga, S.; Kondo, S.; Ueno, H.; Morizane, C.; Gemmoto, K.; Suna, H.; Ushida, Y.; et al. Phase I Studies of Peptide Vaccine Cocktails Derived from GPC3, WDRPUH and NEIL3 for Advanced Hepatocellular Carcinoma. Immunotherapy 2021, 13, 371–385. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, H.; Zheng, J.; Liu, Y. Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J. Cancer 2020, 11, 2008–2021. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in Immunotherapy for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The Immune Contexture and Immunoscore in Cancer Prognosis and Therapeutic Efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Guerra, N.; Fessas, P.; Murphy, R.; Mineo, T.; Mauri, F.A.; Mukherjee, S.K.; Thursz, M.; Wong, C.N.; Sharma, R.; et al. Immune-Based Therapies for Hepatocellular Carcinoma. Oncogene 2020, 39, 3620–3637. [Google Scholar] [CrossRef] [PubMed]
- Ruf, B.; Heinrich, B.; Greten, T.F. Immunobiology and Immunotherapy of HCC: Spotlight on Innate and Innate-like Immune Cells. Cell. Mol. Immunol. 2021, 18, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Rochigneux, P.; Chanez, B.; De Rauglaudre, B.; Mitry, E.; Chabannon, C.; Gilabert, M. Adoptive Cell Therapy in Hepatocellular Carcinoma: Biological Rationale and First Results in Early Phase Clinical Trials. Cancers 2021, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Liu, S.; Yang, M. Regulatory T Cells and Their Associated Factors in Hepatocellular Carcinoma Development and Therapy. World J. Gastroenterol. 2022, 28, 3346–3358. [Google Scholar] [CrossRef]
- Cheng, K.; Cai, N.; Zhu, J.; Yang, X.; Liang, H.; Zhang, W. Tumor-Associated Macrophages in Liver Cancer: From Mechanisms to Therapy. Cancer Commun. 2022, 42, 1112–1140. [Google Scholar] [CrossRef]
- Serafini, P.; Meckel, K.; Kelso, M.; Noonan, K.; Califano, J.; Koch, W.; Dolcetti, L.; Bronte, V.; Borrello, I. Phosphodiesterase-5 Inhibition Augments Endogenous Antitumor Immunity by Reducing Myeloid-Derived Suppressor Cell Function. J. Exp. Med. 2006, 203, 2691–2702. [Google Scholar] [CrossRef]
- Jeng, L.-B.; Liao, L.-Y.; Shih, F.-Y.; Teng, C.-F. Dendritic-Cell-Vaccine-Based Immunotherapy for Hepatocellular Carcinoma: Clinical Trials and Recent Preclinical Studies. Cancers 2022, 14, 4380. [Google Scholar] [CrossRef]
- Li, X.; Yao, W.; Yuan, Y.; Chen, P.; Li, B.; Li, J.; Chu, R.; Song, H.; Xie, D.; Jiang, X.; et al. Targeting of Tumour-Infiltrating Macrophages via CCL2/CCR2 Signalling as a Therapeutic Strategy against Hepatocellular Carcinoma. Gut 2017, 66, 157–167. [Google Scholar] [CrossRef]
- Simon, T.G.; Duberg, A.-S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef]
- Ali, E.; Trailin, A.; Ambrozkiewicz, F.; Liška, V.; Hemminki, K. Activated Hepatic Stellate Cells in Hepatocellular Carcinoma: Their Role as a Potential Target for Future Therapies. Int. J. Mol. Sci. 2022, 23, 15292. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, X.; Chen, S.; Lai, Y.; Wei, X.; Li, B.; Lin, S.; Wang, S.; Wu, Q.; Liang, Q.; et al. Anti-GPC3-CAR T Cells Suppress the Growth of Tumor Cells in Patient-Derived Xenografts of Hepatocellular Carcinoma. Front. Immunol. 2017, 7, 690. [Google Scholar] [CrossRef]
- Kolluri, A.; Li, D.; Li, N.; Duan, Z.; Roberts, L.R.; Ho, M. Human VH-Based Chimeric Antigen Receptor T Cells Targeting Glypican 3 Eliminate Tumors in Preclinical Models of HCC. Hepatol. Commun. 2023, 7, e0022. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, H.; Shi, B.; Di, S.; Sun, R.; Su, J.; Liu, Y.; Li, H.; Jiang, H.; Li, Z. Combined Antitumor Effects of Sorafenib and GPC3-CAR T Cells in Mouse Models of Hepatocellular Carcinoma. Mol. Ther. 2019, 27, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.A.; Hanafi, L.-A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and Biomarkers of Severe Cytokine Release Syndrome after CD19 Chimeric Antigen Receptor–Modified T-Cell Therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Qasim, W.; Brunetto, M.; Gehring, A.J.; Xue, S.-A.; Schurich, A.; Khakpoor, A.; Zhan, H.; Ciccorossi, P.; Gilmour, K.; Cavallone, D.; et al. Immunotherapy of HCC Metastases with Autologous T Cell Receptor Redirected T Cells, Targeting HBsAg in a Liver Transplant Patient. J. Hepatol. 2015, 62, 486–491. [Google Scholar] [CrossRef]
- Hafezi, M.; Lin, M.; Chia, A.; Chua, A.; Ho, Z.Z.; Fam, R.; Tan, D.; Aw, J.; Pavesi, A.; Krishnamoorthy, T.L.; et al. Immunosuppressive Drug-Resistant Armored T-Cell Receptor T Cells for Immune Therapy of HCC in Liver Transplant Patients. Hepatology 2021, 74, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhao, J.; Tan, A.T.; Hu, W.; Wang, S.-Y.; Jin, J.; Wu, J.; Li, Y.; Shi, L.; Fu, J.-L.; et al. Immunotherapy of HBV-Related Advanced Hepatocellular Carcinoma with Short-Term HBV-Specific TCR Expressed T Cells: Results of Dose Escalation, Phase I Trial. Hepatol. Int. 2021, 15, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Spear, T.T.; Callender, G.G.; Roszkowski, J.J.; Moxley, K.M.; Simms, P.E.; Foley, K.C.; Murray, D.C.; Scurti, G.M.; Li, M.; Thomas, J.T.; et al. TCR Gene-Modified T Cells Can Efficiently Treat Established Hepatitis C-Associated Hepatocellular Carcinoma Tumors. Cancer Immunol. Immunother. 2016, 65, 293–304. [Google Scholar] [CrossRef]
- Zhu, W.; Peng, Y.; Wang, L.; Hong, Y.; Jiang, X.; Li, Q.; Liu, H.; Huang, L.; Wu, J.; Celis, E.; et al. Identification of α-fetoprotein-specific T-cell Receptors for Hepatocellular Carcinoma Immunotherapy. Hepatology 2018, 68, 574–589. [Google Scholar] [CrossRef]
- Docta, R.Y.; Ferronha, T.; Sanderson, J.P.; Weissensteiner, T.; Pope, G.R.; Bennett, A.D.; Pumphrey, N.J.; Ferjentsik, Z.; Quinn, L.L.; Wiedermann, G.E.; et al. Tuning T-Cell Receptor Affinity to Optimize Clinical Risk-Benefit When Targeting Alpha-Fetoprotein–Positive Liver Cancer. Hepatology 2019, 69, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-J.; Lin, S.-Z.; Zhou, L.; Xie, H.-Y.; Zhou, W.-H.; Taki-Eldin, A.; Zheng, S.-S. Selective Recruitment of Regulatory T Cell through CCR6-CCL20 in Hepatocellular Carcinoma Fosters Tumor Progression and Predicts Poor Prognosis. PLoS ONE 2011, 6, e24671. [Google Scholar] [CrossRef] [PubMed]
- Bilate, A.M.; Lafaille, J.J. Induced CD4+Foxp3+Regulatory T Cells in Immune Tolerance. Annu. Rev. Immunol. 2012, 30, 733–758. [Google Scholar] [CrossRef]
- Cai, J.; Hu, M.; Chen, Z.; Ling, Z. The Roles and Mechanisms of Hypoxia in Liver Fibrosis. J. Transl. Med. 2021, 19, 186. [Google Scholar] [CrossRef]
- Lin, C.-A.; Chang, L.-L.; Zhu, H.; He, Q.-J.; Yang, B. Hypoxic Microenvironment and Hepatocellular Carcinoma Treatment. Hepatoma Res. 2018, 4, 26. [Google Scholar] [CrossRef]
- Noman, M.Z.; Hasmim, M.; Messai, Y.; Terry, S.; Kieda, C.; Janji, B.; Chouaib, S. Hypoxia: A Key Player in Antitumor Immune Response. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C569–C579. [Google Scholar] [CrossRef]
- Ren, L.; Yu, Y.; Wang, L.; Zhu, Z.; Lu, R.; Yao, Z. Hypoxia-Induced CCL28 Promotes Recruitment of Regulatory T Cells and Tumor Growth in Liver Cancer. Oncotarget 2016, 7, 75763–75773. [Google Scholar] [CrossRef] [PubMed]
- Suthen, S.D.; Lim, C.J.; Nguyen, P.H.D.; Dutertre, C.; Lai, H.L.H.; Wasser, M.; Chua, C.; Lim, T.K.H.; Leow, W.Q.; Loh, T.J.; et al. Hypoxia-Driven Immunosuppression by Treg and Type-2 Conventional Dendritic Cells in HCC. Hepatology 2022, 76, 1329–1344. [Google Scholar] [CrossRef]
- Capece, D.; Fischietti, M.; Verzella, D.; Gaggiano, A.; Cicciarelli, G.; Tessitore, A.; Zazzeroni, F.; Alesse, E. The Inflammatory Microenvironment in Hepatocellular Carcinoma: A Pivotal Role for Tumor-Associated Macrophages. BioMed Res. Int. 2013, 2013, 187204. [Google Scholar] [CrossRef]
- Yeung, O.W.; Lo, C.-M.; Ling, C.-C.; Qi, X.; Geng, W.; Li, C.-X.; Ng, K.T.; Forbes, S.J.; Guan, X.-Y.; Poon, R.T.; et al. Alternatively Activated (M2) Macrophages Promote Tumour Growth and Invasiveness in Hepatocellular Carcinoma. J. Hepatol. 2014, 62, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, W.; Yin, S.; Zhou, Y.; Chen, T.; Qian, J.; Su, R.; Hong, L.; Lu, H.; Zhang, F.; et al. Blocking Triggering Receptor Expressed on Myeloid Cells-1-Positive Tumor-Associated Macrophages Induced by Hypoxia Reverses Immunosuppression and Anti-Programmed Cell Death Ligand 1 Resistance in Liver Cancer. Hepatology 2019, 70, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xun, Z.; Ma, K.; Liang, S.; Li, X.; Zhou, S.; Sun, L.; Liu, Y.; Du, Y.; Guo, X.; et al. Identification of a Tumour Immune Barrier in the HCC Microenvironment That Determines the Efficacy of Immunotherapy. J. Hepatol. 2023, 78, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Kalathil, S.; Lugade, A.A.; Miller, A.; Iyer, R.; Thanavala, Y. Higher Frequencies of GARP+CTLA-4+Foxp3+ T Regulatory Cells and Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients Are Associated with Impaired T-Cell Functionality. Cancer Res 2013, 73, 2435–2444. [Google Scholar] [CrossRef]
- Chhonker, S.K.; Rawat, D.; Koiri, R.K. Protective and Therapeutic Effects of Sildenafil and Tadalafil on Aflatoxin B1-Induced Hepatocellular Carcinoma. Mol. Cell. Biochem. 2021, 476, 1195–1209. [Google Scholar] [CrossRef]
- Chhonker, S.K.; Rawat, D.; Koiri, R.K. Repurposing PDE5 Inhibitor Tadalafil and Sildenafil as Anticancer Agent against Hepatocellular Carcinoma via Targeting Key Events of Glucose Metabolism and Multidrug Resistance. J. Biochem. Mol. Toxicol. 2022, 36, e23100. [Google Scholar] [CrossRef]
- Kong, D.; Jiang, Y.; Miao, X.; Wu, Z.; Liu, H.; Gong, W. Tadalafil Enhances the Therapeutic Efficacy of BET Inhibitors in Hepatocellular Carcinoma through Activating Hippo Pathway. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166267. [Google Scholar] [CrossRef]
- Zhong, M.; Zhong, C.; Cui, W.; Wang, G.; Zheng, G.; Li, L.; Zhang, J.; Ren, R.; Gao, H.; Wang, T.; et al. Induction of Tolerogenic Dendritic Cells by Activated TGF-β/Akt/Smad2 Signaling in RIG-I-Deficient Stemness-High Human Liver Cancer Cells. BMC Cancer 2019, 19, 439. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Z.; Yang, Y.; Jiang, Z.; Gu, Y.; Liu, Y.; Lin, C.; Pan, Z.; Yu, Y.; Jiang, M.; et al. Human CD14+CTLA-4+ Regulatory Dendritic Cells Suppress T-Cell Response by Cytotoxic T-Lymphocyte Antigen-4-Dependent IL-10 and Indoleamine-2,3-Dioxygenase Production in Hepatocellular Carcinoma. Hepatology 2014, 59, 567–579. [Google Scholar] [CrossRef]
- Lee, W.-C.; Wang, H.-C.; Hung, C.-F.; Huang, P.-F.; Lia, C.-R.; Chen, M.-F. Vaccination of Advanced Hepatocellular Carcinoma Patients with Tumor Lysate-Pulsed Dendritic Cells: A Clinical Trail. J. Immunother. 2005, 28, 496–504. [Google Scholar] [CrossRef]
- Palmer, D.H.; Midgley, R.S.; Mirza, N.; Torr, E.E.; Ahmed, F.; Steele, J.C.; Steven, N.M.; Kerr, D.J.; Young, L.S.; Adams, D.H. A Phase II Study of Adoptive Immunotherapy Using Dendritic Cells Pulsed with Tumor Lysate in Patients with Hepatocellular Carcinoma. Hepatology 2009, 49, 124–132. [Google Scholar] [CrossRef]
- Tada, F.; Abe, M.; Hirooka, M.; Ikeda, Y.; Hiasa, Y.; Lee, Y.; Jung, N.-C.; Lee, W.-B.; Lee, H.-S.; Bae, Y.-S.; et al. Phase I/II Study of Immunotherapy Using Tumor Antigen-Pulsed Dendritic Cells in Patients with Hepatocellular Carcinoma. Int. J. Oncol. 2012, 41, 1601–1609. [Google Scholar] [CrossRef]
- El Ansary, M.; Mogawer, S.; Elhamid, S.A.; Alwakil, S.; Aboelkasem, F.; El Sabaawy, H.; Abdelhalim, O. Immunotherapy by Autologous Dendritic Cell Vaccine in Patients with Advanced HCC. J. Cancer Res. Clin. Oncol. 2013, 139, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bayer, M.E.; Chen, X.; Fredrickson, C.; Cornforth, A.N.; Liang, G.; Cannon, J.; He, J.; Fu, Q.; Liu, J.; et al. Phase I Trial of Active Specific Immunotherapy with Autologous Dendritic Cells Pulsed with Autologous Irradiated Tumor Stem Cells in Hepatitis B-Positive Patients with Hepatocellular Carcinoma. J. Surg. Oncol. 2015, 111, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, Y.; Lee, M.; Heo, M.K.; Song, J.-S.; Kim, K.-H.; Lee, H.; Yi, N.-J.; Lee, K.-W.; Suh, K.-S.; et al. A Phase I/IIa Study of Adjuvant Immunotherapy with Tumour Antigen-Pulsed Dendritic Cells in Patients with Hepatocellular Carcinoma. Br. J. Cancer 2015, 113, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, L.-F.; Zou, Z.-Y.; Kong, W.-W.; Yan, J.; Meng, F.-Y.; Chen, F.-J.; Du, J.; Shao, J.; Xu, Q.-P.; et al. Phase I Clinical Study of Personalized Peptide Vaccination Combined with Radiotherapy for Advanced Hepatocellular Carcinoma. World J. Gastroenterol. 2017, 23, 5395–5404. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019, 25, 2116–2126. [Google Scholar] [CrossRef]
- de Galarreta, M.R.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti–PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103+ Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef]
- Montironi, C.; Castet, F.; Haber, P.K.; Pinyol, R.; Torres-Martin, M.; Torrens, L.; Mesropian, A.; Wang, H.; Puigvehi, M.; Maeda, M.; et al. Inflamed and Non-Inflamed Classes of HCC: A Revised Immunogenomic Classification. Gut 2023, 72, 129–140. [Google Scholar] [CrossRef]
- Mehrfeld, C.; Zenner, S.; Kornek, M.; Lukacs-Kornek, V. The Contribution of Non-Professional Antigen-Presenting Cells to Immunity and Tolerance in the Liver. Front. Immunol. 2018, 9, 635. [Google Scholar] [CrossRef]
- Perdiguero, E.G.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; De Bruijn, M.; Geissmann, F.; et al. Tissue-Resident Macrophages Originate from Yolk Sac-Derived Erythro-Myeloid Progenitors. Exp. Hematol. 2015, 43, S64. [Google Scholar] [CrossRef]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver Sinusoidal Endothelial Cells—Gatekeepers of Hepatic Immunity. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Knolle, P.A.; Uhrig, A.; Hegenbarth, S.; Löser, E.; Schmitt, E.; Gerken, G.; Lohse, A.W. IL-10 Down-Regulates T Cell Activation by Antigen-Presenting Liver Sinusoidal Endothelial Cells through Decreased Antigen Uptake via the Mannose Receptor and Lowered Surface Expression of Accessory Molecules. Clin. Exp. Immunol. 1998, 114, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yang, L.; van Rooijen, N.; Ohnishi, H.; Seki, E.; Zimmerman, K.A.; Song, C.J.; Gonzalez-Mize, N.; Li, Z.; Yoder, B.K.; et al. Hepatic Recruitment of Macrophages Promotes Nonalcoholic Steatohepatitis through CCR2. Am. J. Physiol. Liver Physiol. 2012, 302, G1310–G1321. [Google Scholar] [CrossRef]
- Tran, S.; Baba, I.; Poupel, L.; Dussaud, S.; Moreau, M.; Gélineau, A.; Marcelin, G.; Magréau-Davy, E.; Ouhachi, M.; Lesnik, P.; et al. Impaired Kupffer Cell Self-Renewal Alters the Liver Response to Lipid Overload during Non-Alcoholic Steatohepatitis. Immunity 2020, 53, 627–640.e5. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Seyedkazemi, S.; Francque, S.; Sanyal, A.; Rinella, M.; Charlton, M.; Loomba, R.; Ratziu, V.; Kochuparampil, J.; Fischer, L.; et al. A Randomized, Double-Blind, Multicenter, Phase 2b Study to Evaluate the Safety and Efficacy of a Combination of Tropifexor and Cenicriviroc in Patients with Nonalcoholic Steatohepatitis and Liver Fibrosis: Study Design of the TANDEM Trial. Contemp. Clin. Trials 2020, 88, 105889. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Puengel, T.; Govaere, O.; Abdallah, A.T.; Mossanen, J.C.; Kohlhepp, M.; Liepelt, A.; Lefebvre, E.; Luedde, T.; Hellerbrand, C.; et al. Therapeutic Inhibition of Inflammatory Monocyte Recruitment Reduces Steatohepatitis and Liver Fibrosis. Hepatology 2018, 67, 1270–1283. [Google Scholar] [CrossRef]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα Is a Mediator and Potential Interventional Target for NASH and Subsequent Liver Cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.-P.; Schwabe, R.F. Fate Tracing Reveals Hepatic Stellate Cells as Dominant Contributors to Liver Fibrosis Independent of Its Aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef] [PubMed]
- Filliol, A.; Saito, Y.; Nair, A.; Dapito, D.H.; Yu, L.-X.; Ravichandra, A.; Bhattacharjee, S.; Affo, S.; Fujiwara, N.; Su, H.; et al. Opposing Roles of Hepatic Stellate Cell Subpopulations in Hepatocarcinogenesis. Nature 2022, 610, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; de Moura, M.C.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-Specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Silverman, G.A.; Bird, P.I.; Carrell, R.W.; Church, F.C.; Coughlin, P.B.; Gettins, P.G.; Irving, J.A.; Lomas, D.A.; Luke, C.J.; Moyer, R.W.; et al. The Serpins Are an Expanding Superfamily of Structurally Similar but Functionally Diverse Proteins. Evolution, Mechanism of Inhibition, Novel Functions, and a Revised Nomenclature. J. Biol. Chem. 2001, 276, 33293–33296. [Google Scholar] [CrossRef]
- Kato, H.; Torigoe, T. Radioimmunoassay for Tumor Antigen of Human Cervical Squamous Cell Carcinoma. Cancer 1977, 40, 1621–1628. [Google Scholar] [CrossRef]
- Cataltepe, S.; Gornstein, E.R.; Schick, C.; Kamachi, Y.; Chatson, K.; Fries, J.; Silverman, G.A.; Upton, M.P. Co-Expression of the Squamous Cell Carcinoma Antigens 1 and 2 in Normal Adult Human Tissues and Squamous Cell Carcinomas. J. Histochem. Cytochem. 2000, 48, 113–122. [Google Scholar] [CrossRef]
- Turato, C.; Vitale, A.; Fasolato, S.; Ruvoletto, M.; Terrin, L.; Quarta, S.; Morales, R.R.; Biasiolo, A.; Zanus, G.; Zali, N.; et al. SERPINB3 Is Associated with TGF-β1 and Cytoplasmic β-Catenin Expression in Hepatocellular Carcinomas with Poor Prognosis. Br. J. Cancer 2014, 110, 2708–2715. [Google Scholar] [CrossRef]
- Correnti, M.; Cappon, A.; Pastore, M.; Piombanti, B.; Lori, G.; Oliveira, D.V.P.N.; Munoz-Garrido, P.; Lewinska, M.; Andersen, J.B.; Coulouarn, C.; et al. The Protease-Inhibitor SerpinB3 as a Critical Modulator of the Stem-like Subset in Human Cholangiocarcinoma. Liver Int. 2022, 42, 233–248. [Google Scholar] [CrossRef]
- Turato, C.; Buendia, M.A.; Fabre, M.; Redon, M.J.; Branchereau, S.; Quarta, S.; Ruvoletto, M.; Perilongo, G.; Grotzer, M.A.; Gatta, A.; et al. Over-Expression of SERPINB3 in Hepatoblastoma: A Possible Insight into the Genesis of This Tumour? Eur. J. Cancer 2012, 48, 1219–1226. [Google Scholar] [CrossRef]
- Pontisso, P. Role of SERPINB3 in Hepatocellular Carcinoma. Ann. Hepatol. 2014, 13, 722–727. [Google Scholar] [CrossRef]
- Tolomeo, A.M.; Quarta, S.; Biasiolo, A.; Ruvoletto, M.; Pozzobon, M.; De Lazzari, G.; Malvicini, R.; Turato, C.; Arrigoni, G.; Pontisso, P.; et al. Engineered EVs for Oxidative Stress Protection. Pharmaceuticals 2021, 14, 703. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Suminami, Y.; Hirakawa, H.; Nawata, S.; Numa, F.; Kato, H. Squamous Cell Carcinoma Antigen Suppresses Radiation-Induced Cell Death. Br. J. Cancer 2001, 84, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, C.; Nakanishi, J.; Kadoya, K.; Hibino, T. Serpin Squamous Cell Carcinoma Antigen Inhibits UV-Induced Apoptosis via Suppression of c-JUN NH2-Terminal Kinase. J. Cell Biol. 2006, 172, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Ciscato, F.; Sciacovelli, M.; Villano, G.; Turato, C.; Bernardi, P.; Rasola, A.; Pontisso, P. SERPINB3 Protects from Oxidative Damage by Chemotherapeutics through Inhibition of Mitochondrial Respiratory Complex I. Oncotarget 2013, 5, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Turato, C.; Fornari, F.; Pollutri, D.; Fassan, M.; Quarta, S.; Villano, G.; Ruvoletto, M.; Bolondi, L.; Gramantieri, L.; Pontisso, P. MiR-122 Targets SerpinB3 and Is Involved in Sorafenib Resistance in Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 171. [Google Scholar] [CrossRef]
- Quarta, S.; Vidalino, L.; Turato, C.; Ruvoletto, M.; Calabrese, F.; Valente, M.; Cannito, S.; Fassina, G.; Parola, M.; Gatta, A.; et al. SERPINB3 Induces Epithelial-Mesenchymal Transition. J. Pathol. 2010, 221, 343–356. [Google Scholar] [CrossRef]
- Chen, L.; Shi, V.; Wang, S.; Sun, L.; Freeman, R.N.; Yang, J.; Inkman, M.J.; Ghosh, S.; Ruiz, F.; Jayachandran, K.; et al. SCCA1/SERPINB3 Suppresses Anti-Tumor Immunity and Blunts Therapy-Induced T Cell Responses via STAT-Dependent Chemokine Production. J. Clin. Investig. 2023. [Google Scholar] [CrossRef]
- Turato, C.; Calabrese, F.; Biasiolo, A.; Quarta, S.; Ruvoletto, M.; Tono, N.; Paccagnella, D.; Fassina, G.; Merkel, C.; Harrison, T.J.; et al. SERPINB3 Modulates TGF-β Expression in Chronic Liver Disease. Lab. Investig. 2010, 90, 1016–1023. [Google Scholar] [CrossRef]
- Turato, C.; Biasiolo, A.; Pengo, P.; Frecer, V.; Quarta, S.; Fasolato, S.; Ruvoletto, M.; Beneduce, L.; Zuin, J.; Fassina, G.; et al. Increased Antiprotease Activity of the SERPINB3 Polymorphic Variant SCCA-PD. Exp. Biol. Med. 2011, 236, 281–290. [Google Scholar] [CrossRef]
- Lunardi, F.; Villano, G.; Perissinotto, E.; Agostini, C.; Rea, F.; Gnoato, M.; Bradaschia, A.; Valente, M.; Pontisso, P.; Calabrese, F. Overexpression of SERPIN B3 Promotes Epithelial Proliferation and Lung Fibrosis in Mice. Lab. Investig. 2011, 91, 945–954. [Google Scholar] [CrossRef]
- Calabrese, F.; Lunardi, F.; Giacometti, C.; Marulli, G.; Gnoato, M.; Pontisso, P.; Saetta, M.; Valente, M.; Rea, F.; Perissinotto, E.; et al. Overexpression of Squamous Cell Carcinoma Antigen in Idiopathic Pulmonary Fibrosis: Clinicopathological Correlations. Thorax 2008, 63, 795–802. [Google Scholar] [CrossRef]
- Terrin, L.; Agostini, M.; Ruvoletto, M.; Martini, A.; Pucciarelli, S.; Bedin, C.; Nitti, D.; Pontisso, P. SerpinB3 Upregulates the Cyclooxygenase-2 / β-Catenin Positive Loop in Colorectal Cancer. Oncotarget 2017, 8, 15732–15743. [Google Scholar] [CrossRef]
- Quarta, S.; Cappon, A.; Turato, C.; Ruvoletto, M.; Cannito, S.; Villano, G.; Biasiolo, A.; Maggi, M.; Protopapa, F.; Bertazza, L.; et al. SerpinB3 Upregulates Low-Density Lipoprotein Receptor-Related Protein (LRP) Family Members, Leading to Wnt Signaling Activation and Increased Cell Survival and Invasiveness. Biology 2023, 12, 771. [Google Scholar] [CrossRef]
- Novo, E.; Cappon, A.; Villano, G.; Quarta, S.; Cannito, S.; Bocca, C.; Turato, C.; Guido, M.; Maggiora, M.; Protopapa, F.; et al. SerpinB3 as a Pro-Inflammatory Mediator in the Progression of Experimental Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 910526. [Google Scholar] [CrossRef]
- Xiong, X.; Kuang, H.; Ansari, S.; Liu, T.; Gong, J.; Wang, S.; Zhao, X.-Y.; Ji, Y.; Li, C.; Guo, L.; et al. Landscape of Intercellular Crosstalk in Healthy and NASH Liver Revealed by Single-Cell Secretome Gene Analysis. Mol. Cell 2019, 75, 644–660.e5. [Google Scholar] [CrossRef] [PubMed]
- Turato, C.; Scarpa, M.; Kotsafti, A.; Cappon, A.; Quarta, S.; Biasiolo, A.; Cavallin, F.; Trevellin, E.; Guzzardo, V.; Fassan, M.; et al. Squamous Cell Carcinoma Antigen 1 Is Associated to Poor Prognosis in Esophageal Cancer through Immune Surveillance Impairment and Reduced Chemosensitivity. Cancer Sci. 2019, 110, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.; Luisetto, R.; Ghirardello, A.; Cavicchioli, L.; Codolo, G.; Biasiolo, A.; Maggioni, G.; Saccon, F.; Beggio, M.; Cappon, A.; et al. SERPINB3 Delays Glomerulonephritis and Attenuates the Lupus-Like Disease in Lupus Murine Models by Inducing a More Tolerogenic Immune Phenotype. Front. Immunol. 2018, 9, 2081. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in Cancer: Significance and Impact on Clinical Outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Chiche, J.; Pouysségur, J. Hypoxia and Cancer. J. Mol. Med. 2007, 85, 1301–1307. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Giaccia, A.J. The Role of Hypoxia-Inducible Factors in Tumorigenesis. Cell Death Differ. 2008, 15, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.P.; Marie-Egyptienne, D.T.; Hedley, D.W. Cancer Stem Cells, Hypoxia and Metastasis. Semin. Radiat. Oncol. 2009, 19, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Foglia, B.; Sutti, S.; Cannito, S.; Rosso, C.; Maggiora, M.; Autelli, R.; Novo, E.; Bocca, C.; Villano, G.; Ramavath, N.N.; et al. Hepatocyte-Specific Deletion of HIF2α Prevents NASH-Related Liver Carcinogenesis by Decreasing Cancer Cell Proliferation. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 459–482. [Google Scholar] [CrossRef]
- Cannito, S.; Novo, E.; Compagnone, A.; Valfrè di Bonzo, L.; Busletta, C.; Zamara, E.; Paternostro, C.; Povero, D.; Bandino, A.; Bozzo, F.; et al. Redox Mechanisms Switch on Hypoxia-Dependent Epithelial-Mesenchymal Transition in Cancer Cells. Carcinogenesis 2008, 29, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.K.; Tennant, D.A.; McKeating, J.A. Hypoxia Inducible Factors in Liver Disease and Hepatocellular Carcinoma: Current Understanding and Future Directions. J. Hepatol. 2014, 61, 1397–1406. [Google Scholar] [CrossRef]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Chen, C.; Lou, T. Hypoxia Inducible Factors in Hepatocellular Carcinoma. Oncotarget 2017, 8, 46691–46703. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining Normoxia, Physoxia and Hypoxia in Tumours—Implications for Treatment Response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- Luo, D.; Wang, Z.; Wu, J.; Jiang, C.; Wu, J. The Role of Hypoxia Inducible Factor-1 in Hepatocellular Carcinoma. BioMed. Res. Int. 2014, 2014, 409272. [Google Scholar] [CrossRef]
- Menrad, H.; Werno, C.; Schmid, T.; Copanaki, E.; Deller, T.; Dehne, N.; Brüne, B. Roles of Hypoxia-Inducible Factor-1α (HIF-1α) Versus HIF-2α in the Survival of Hepatocellular Tumor Spheroids. Hepatology 2010, 51, 2183–2192. [Google Scholar] [CrossRef]
- He, C.; Sun, X.-P.; Qiao, H.; Jiang, X.; Wang, D.; Jin, X.; Dong, X.; Wang, J.; Jiang, H.; Sun, X. Downregulating Hypoxia-Inducible Factor-2α Improves the Efficacy of Doxorubicin in the Treatment of Hepatocellular Carcinoma. Cancer Sci. 2012, 103, 528–534. [Google Scholar] [CrossRef]
- Sun, H.-X.; Xu, Y.; Yang, X.-R.; Wang, W.-M.; Bai, H.; Shi, R.-Y.; Nayar, S.K.; Devbhandari, R.P.; He, Y.-Z.; Zhu, Q.; et al. Hypoxia Inducible Factor 2 alpha Inhibits Hepatocellular Carcinoma Growth through the Transcription Factor Dimerization Partner 3/ E2F Transcription Factor 1-Dependent Apoptotic Pathway. Hepatology 2013, 57, 1088–1097. [Google Scholar] [CrossRef]
- Zhao, D.; Zhai, B.; He, C.; Tan, G.; Jiang, X.; Pan, S.; Dong, X.; Wei, Z.; Ma, L.; Qiao, H.; et al. Upregulation of HIF-2α Induced by Sorafenib Contributes to the Resistance by Activating the TGF-α/EGFR Pathway in Hepatocellular Carcinoma Cells. Cell. Signal. 2014, 26, 1030–1039. [Google Scholar] [CrossRef]
- Yang, S.-L.; Liu, L.-P.; Niu, L.; Sun, Y.-F.; Yang, X.-R.; Fan, J.; Ren, J.-W.; Chen, G.G.; Lai, P.B. Downregulation and Pro-Apoptotic Effect of Hypoxia-Inducible Factor 2 Alpha in Hepatocellular Carcinoma. Oncotarget 2016, 7, 34571–34581. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Huang, J.; Li, Z.; Gong, Y.; Zou, B.; Liu, X.; Ding, L.; Li, P.; Zhu, Z.; et al. HIF-2α Upregulation Mediated by Hypoxia Promotes NAFLD-HCC Progression by Activating Lipid Synthesis via the PI3K-AKT-mTOR Pathway. Aging 2019, 11, 10839–10860. [Google Scholar] [CrossRef] [PubMed]
- Cannito, S.; Turato, C.; Paternostro, C.; Biasiolo, A.; Colombatto, S.; Cambieri, I.; Quarta, S.; Novo, E.; Morello, E.; Villano, G.; et al. Hypoxia Up-Regulates SERPINB3 through HIF-2α in Human Liver Cancer Cells. Oncotarget 2015, 6, 2206–2221. [Google Scholar] [CrossRef] [PubMed]
- Turato, C.; Cannito, S.; Simonato, D.; Villano, G.; Morello, E.; Terrin, L.; Quarta, S.; Biasiolo, A.; Ruvoletto, M.; Martini, A.; et al. SerpinB3 and Yap Interplay Increases Myc Oncogenic Activity. Sci. Rep. 2015, 5, 17701. [Google Scholar] [CrossRef] [PubMed]
- Cannito, S.; Foglia, B.; Villano, G.; Turato, C.; Delgado, T.C.; Morello, E.; Pin, F.; Novo, E.; Napione, L.; Quarta, S.; et al. SerpinB3 Differently Up-Regulates Hypoxia Inducible Factors-1α and -2α in Hepatocellular Carcinoma: Mechanisms Revealing Novel Potential Therapeutic Targets. Cancers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
| Target Cell | Mechanism of Surveillance | Suggested Therapy | References | |
|---|---|---|---|---|
| Adoptive Cell Therapy | CAR-T | [45] | ||
| TCR Engineered T cells | [41] | |||
| Lymphoid cells | Tregs | Secretion of TGF-beta and IL-10. Inhibition of M1 activity. | Immune Checkpoint Inhibitors | [46] |
| Myeloid cells | TAMs | Inhibition of CD8+. Stimulation of Tregs. Production of proangiogenic and pro-proliferation cytokines | Eliminating production | [47] |
| Remodelling M2 polarization | ||||
| Blocking communication with cancer | ||||
| MDSCs | L-Arginine and nitric oxide synthase-2 pathway-dependent inhibition of cytotoxic response | PDE5 inhibitors | [48] | |
| DCs | Antigen-presenting function. Boosting cytotoxic activity | Vaccine strategies | [49] | |
| Non-parenchymal cells | KCs | Macrophages chemotaxis and polarization. Up-regulation of PD-1/PD-L1. | Blocking CCL2/CCR2 axis | [50] |
| Platelets inhibition | [51] | |||
| HSCs | Promotion of fibrosis and oncogenesis | Targeting molecular pathways of activation (e.g., TGF-beta) | [52] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, P.; Martini, A.; Pontisso, P.; Angeli, P. Novel Molecular Targets for Immune Surveillance of Hepatocellular Carcinoma. Cancers 2023, 15, 3629. https://doi.org/10.3390/cancers15143629
Guerra P, Martini A, Pontisso P, Angeli P. Novel Molecular Targets for Immune Surveillance of Hepatocellular Carcinoma. Cancers. 2023; 15(14):3629. https://doi.org/10.3390/cancers15143629
Chicago/Turabian StyleGuerra, Pietro, Andrea Martini, Patrizia Pontisso, and Paolo Angeli. 2023. "Novel Molecular Targets for Immune Surveillance of Hepatocellular Carcinoma" Cancers 15, no. 14: 3629. https://doi.org/10.3390/cancers15143629
APA StyleGuerra, P., Martini, A., Pontisso, P., & Angeli, P. (2023). Novel Molecular Targets for Immune Surveillance of Hepatocellular Carcinoma. Cancers, 15(14), 3629. https://doi.org/10.3390/cancers15143629







