Single-Cell Transcriptomics for Unlocking Personalized Cancer Immunotherapy: Toward Targeting the Origin of Tumor Development Immunogenicity
Abstract
Simple Summary
Abstract
1. Introduction
2. Current Status of Cancer Immunotherapy and Cancer Personalized Immunotherapy
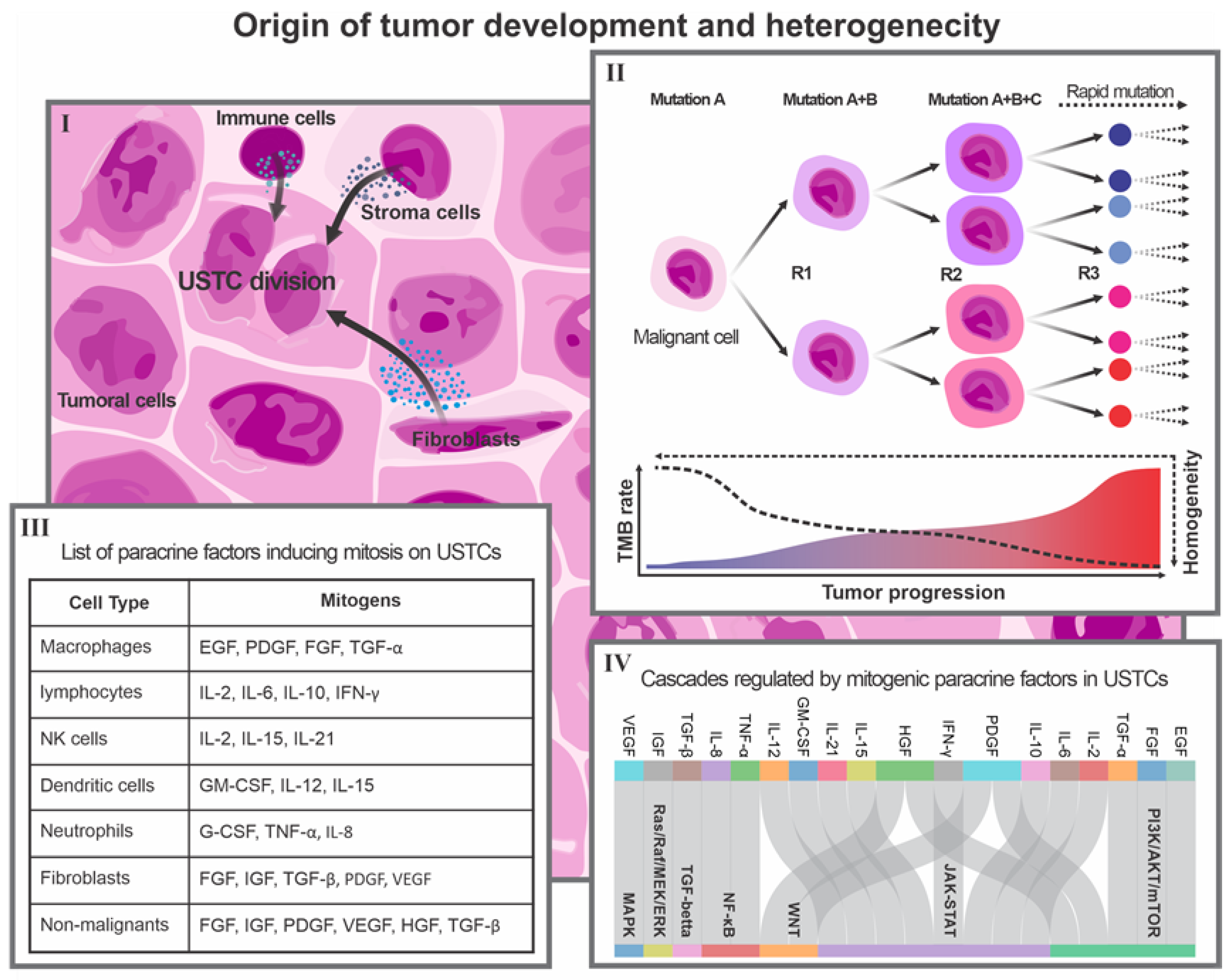
3. Individual Origin of Tumor Development: Concepts and Facts
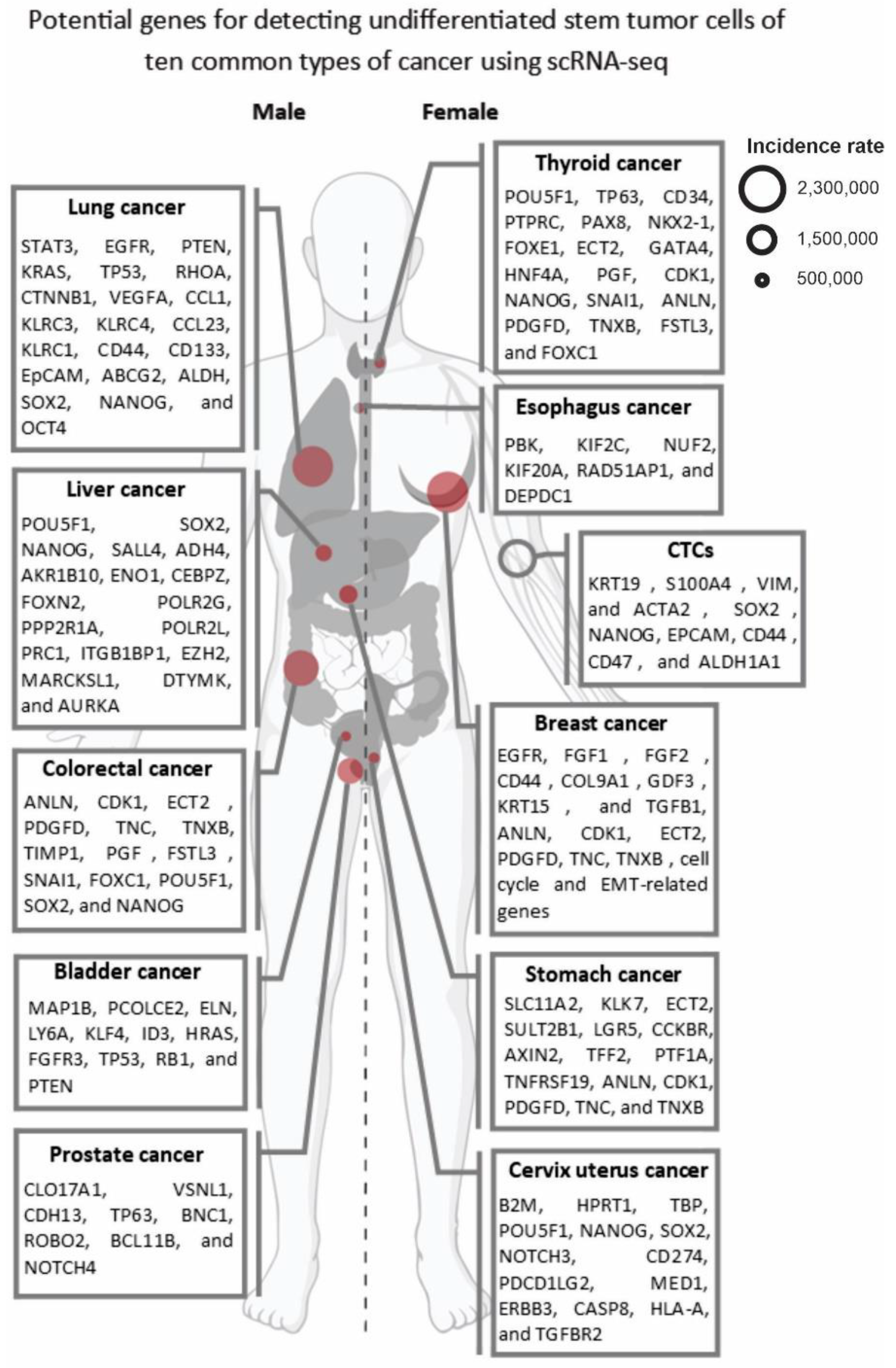
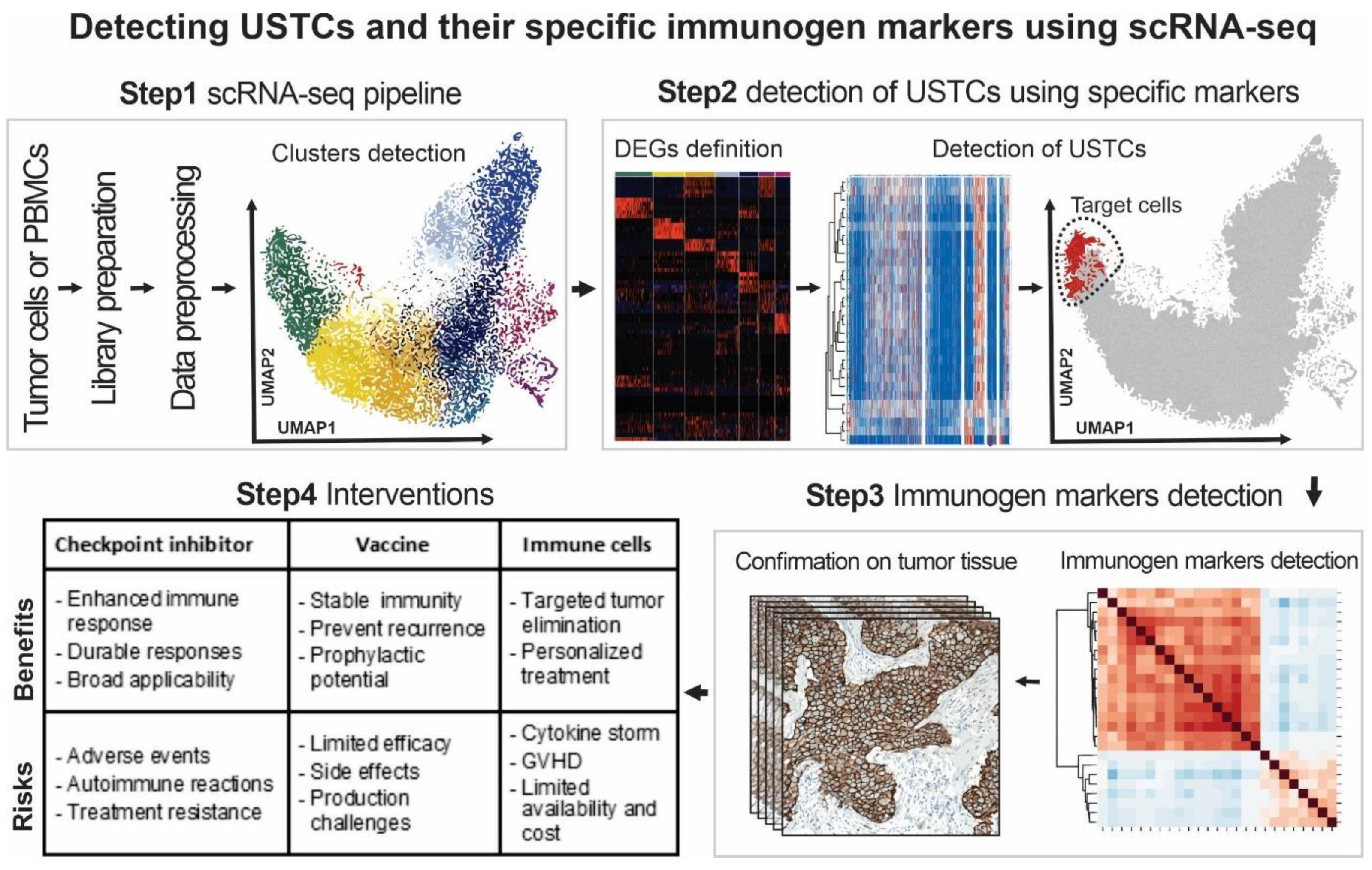
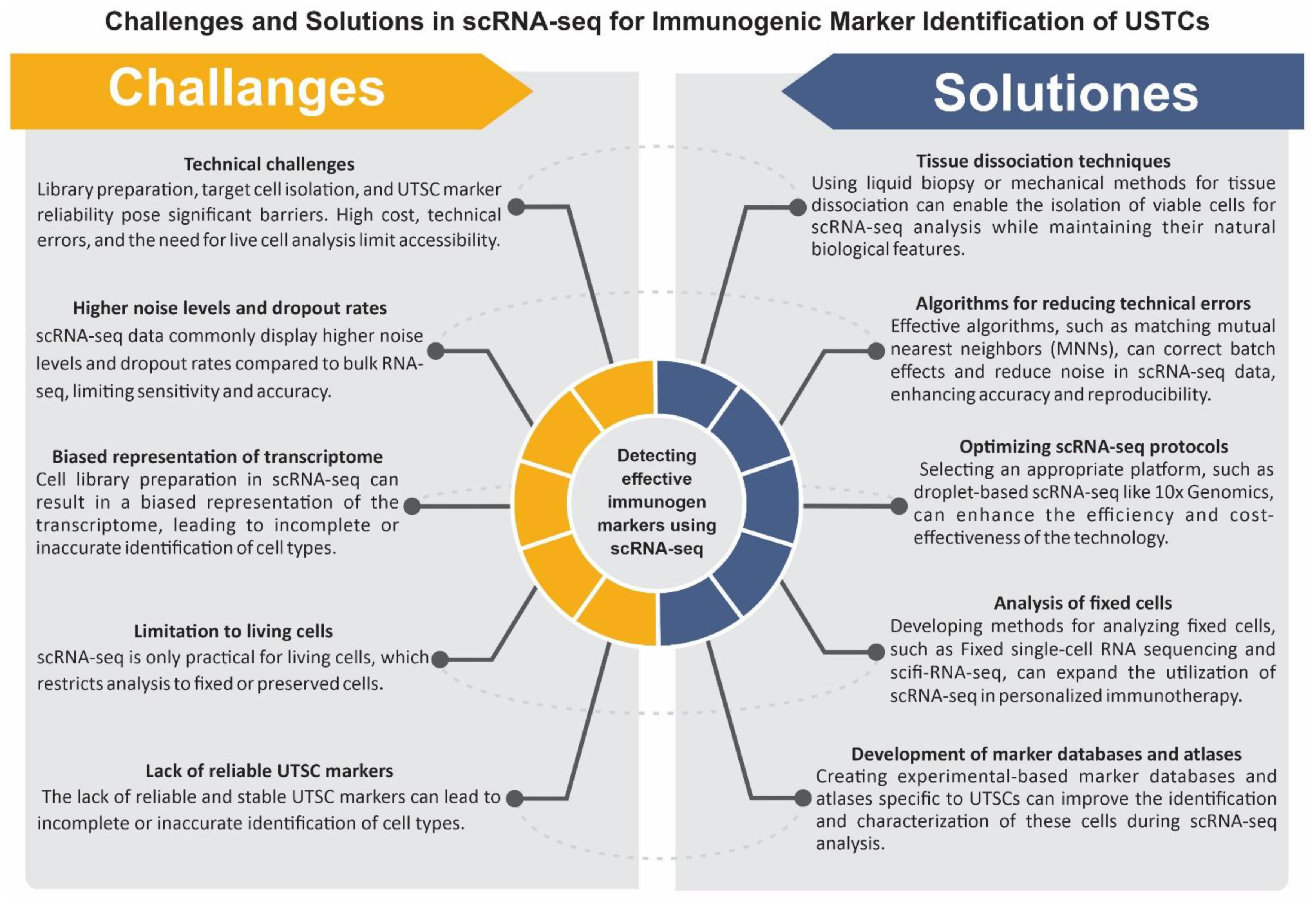
4. Single-Cell Transcriptomics for Detecting and Targeting the Immunogenicity of OTD
5. Future Steps
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Khodayari, H.; Khodayari, S.; Ebrahimi, E.; Hadjilooei, F.; Vesovic, M.; Mahmoodzadeh, H.; Saric, T.; Stücker, W.; Van Gool, S.; Hescheler, J. Stem cells-derived natural killer cells for cancer immunotherapy: Current protocols, feasibility, and benefits of ex vivo generated natural killer cells in treatment of advanced solid tumors. Cancer Immunol. Immunother. 2021, 70, 3369–3395. [Google Scholar] [PubMed]
- Vafaei, S.; Zekiy, A.O.; Khanamir, R.A.; Zaman, B.A.; Ghayourvahdat, A.; Azimizonuzi, H.; Zamani, M. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022, 22, 2. [Google Scholar] [PubMed]
- Lee, J.Y.; Kannan, B.; Lim, B.Y.; Li, Z.; Lim, A.H.; Loh, J.W.; Ko, T.K.; Ng, C.C.-Y.; Chan, J.Y. The multi-dimensional biomarker landscape in cancer immunotherapy. Int. J. Mol. Sci. 2022, 23, 7839. [Google Scholar] [PubMed]
- Shiri, S.; Alizadeh, A.M.; Baradaran, B.; Farhanghi, B.; Shanehbandi, D.; Khodayari, S.; Khodayari, H.; Tavassoli, A. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac. J. Cancer Prev. 2015, 16, 3917–3922. [Google Scholar] [CrossRef] [PubMed]
- Kalhori, M.R.; Khodayari, H.; Khodayari, S.; Vesovic, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. Regulation of long non-coding RNAs by plant secondary metabolites: A novel anticancer therapeutic approach. Cancers 2021, 13, 1274. [Google Scholar] [CrossRef]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2010, 1805, 105–117. [Google Scholar] [CrossRef]
- Arrieta, V.A.; Dmello, C.; McGrail, D.J.; Brat, D.J.; Lee-Chang, C.; Heimberger, A.B.; Chand, D.; Stupp, R.; Sonabend, A.M. Immune checkpoint blockade in glioblastoma: From tumor heterogeneity to personalized treatment. J. Clin. Investig. 2023, 133, e163447. [Google Scholar] [CrossRef]
- Suvà, M.L.; Tirosh, I. Single-cell RNA sequencing in cancer: Lessons learned and emerging challenges. Mol. Cell 2019, 75, 7–12. [Google Scholar] [CrossRef]
- Erfanian, N.; Derakhshani, A.; Nasseri, S.; Fereidouni, M.; Baradaran, B.; Tabrizi, N.J.; Brunetti, O.; Bernardini, R.; Silvestris, N.; Safarpour, H. Immunotherapy of cancer in single-cell RNA sequencing era: A precision medicine perspective. Biomed. Pharmacother. 2022, 146, 112558. [Google Scholar]
- Al-Hajj, M.; Clarke, M.F. Self-renewal and solid tumor stem cells. Oncogene 2004, 23, 7274–7282. [Google Scholar] [CrossRef] [PubMed]
- Marigoudar, J.B.; Sarkar, D.; Yuguda, Y.M.; Abutayeh, R.F.; Kaur, A.; Pati, A.; Mitra, D.; Ghosh, A.; Banerjee, D.; Borah, S. Role of vitamin D in targeting cancer and cancer stem cell populations and its therapeutic implications. Med. Oncol. 2022, 40, 2. [Google Scholar] [PubMed]
- Khalighfard, S.; Khori, V.; Esmati, E.; Ahmadi, F.; Amiriani, T.; Poorkhani, A.; Sadani, S.; Khodayari, S.; Khodayari, H.; Kalhori, M.R. Breast tumor metastasis following filgrastim administration due to the SDF-1/CXCR4 pathway. Med. Oncol. 2023, 40, 74. [Google Scholar] [CrossRef]
- Yang, W.; Han, B.; Chen, Y.; Geng, F. SAAL1, a novel oncogene, is associated with prognosis and immunotherapy in multiple types of cancer. Aging 2022, 14, 6316. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor mutational burden as a predictor of immunotherapy response: Is more always better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef]
- Gromeier, M.; Brown, M.C.; Zhang, G.; Lin, X.; Chen, Y.; Wei, Z.; Beaubier, N.; Yan, H.; He, Y.; Desjardins, A. Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat. Commun. 2021, 12, 352. [Google Scholar] [CrossRef]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar]
- Sylvester, R.J.; van der MEIJDEN, A.P.; Lamm, D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J. Urol. 2002, 168, 1964–1970. [Google Scholar]
- Zhou, C.; Wei, Z.; Zhang, Z.; Zhang, B.; Zhu, C.; Chen, K.; Chuai, G.; Qu, S.; Xie, L.; Gao, Y. pTuneos: Prioritizing tumor neoantigens from next-generation sequencing data. Genome Med. 2019, 11, 67. [Google Scholar] [CrossRef]
- Cappell, K.M.; Kochenderfer, J.N. Long-term outcomes following CAR T cell therapy: What we know so far. Nat. Rev. Clin. Oncol. 2023, 20, 359–371. [Google Scholar]
- Mehravi, B.; Alizadeh, A.M.; Khodayari, S.; Khodayari, H.; Ashtari, K.; Mohseni, M.; Anaraki, N.I.; Dana, E.A.; Safari, S.; Amanlou, M. Acute toxicity evaluation of glycosylated Gd 3+-based silica nanoprobe. Mol. Imaging Biol. 2017, 19, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Santomasso, B.D.; Nastoupil, L.J.; Adkins, S.; Lacchetti, C.; Schneider, B.J.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J. Clin. Oncol. 2021, 39, 3978–3992. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.I.; Sheppard, N.C.; Riley, J.L. Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet. 2021, 22, 427–447. [Google Scholar] [PubMed]
- Tam, S.Y.; Wu, V.W.; Law, H.K. Hypoxia-induced epithelial-mesenchymal transition in cancers: HIF-1α and beyond. Front. Oncol. 2020, 10, 486. [Google Scholar]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial–mesenchymal transition and its transcription factors. Biosci. Rep. 2022, 42, BSR20211754. [Google Scholar]
- Friedmann-Morvinski, D.; Verma, I.M. Dedifferentiation and reprogramming: Origins of cancer stem cells. EMBO Rep. 2014, 15, 244–253. [Google Scholar] [CrossRef]
- Li, M.; Luan, F.; Zhao, Y.; Hao, H.; Zhou, Y.; Han, W.; Fu, X. Epithelial-mesenchymal transition: An emerging target in tissue fibrosis. Exp. Biol. Med. 2016, 241, 1–13. [Google Scholar] [CrossRef]
- Schito, L.; Semenza, G.L. Hypoxia-inducible factors: Master regulators of cancer progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Wang, F.; Luo, R.; Xin, H.; Zhang, Y.; Wong, B.J.C.; Wang, W.; Lei, J. Hypoxia-stimulated tumor therapy associated with the inhibition of cancer cell stemness. Biomaterials 2020, 263, 120330. [Google Scholar] [CrossRef]
- Iwadate, Y. Plasticity in glioma stem cell phenotype and its therapeutic implication. Neurol. Med. Chir. 2018, 58, 61–70. [Google Scholar] [CrossRef]
- Khalighfard, S.; Khori, V.; Alizadeh, A.M.; Vahabzadeh, G.; Tajaldini, M.; Sedighi, S.; Nozarian, Z.; Khodayari, H.; Khodayari, S.; Ganji, F. Dual effects of atorvastatin on angiogenesis pathways in the differentiation of mesenchymal stem cells. Eur. J. Pharmacol. 2021, 907, 174281. [Google Scholar] [CrossRef]
- López de Andrés, J.; Griñán-Lisón, C.; Jiménez, G.; Marchal, J.A. Cancer stem cell secretome in the tumor microenvironment: A key point for an effective personalized cancer treatment. J. Hematol. Oncol. 2020, 13, 136. [Google Scholar] [PubMed]
- Khodayari, H.; Khodayari, S.; Khalighfard, S.; Tahmasebifar, A.; Tajaldini, M.; Poorkhani, A.; Nikoueinejad, H.; Hamidi, G.A.; Nosrati, H.; Kalhori, M.R. Gamma-radiated immunosuppressed tumor xenograft mice can be a new ideal model in cancer research. Sci. Rep. 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.S.; Ciccarelli, F.D.; Malanchi, I. Reflected stemness as a potential driver of the tumour microenvironment. Trends Cell Biol. 2022, 32, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Čokić, V.P.; Mitrović-Ajtić, O.; Beleslin-Čokić, B.B.; Marković, D.; Buač, M.; Diklić, M.; Kraguljac-Kurtović, N.; Damjanović, S.; Milenković, P.; Gotić, M. Proinflammatory cytokine IL-6 and JAK-STAT signaling pathway in myeloproliferative neoplasms. Mediat. Inflamm. 2015, 2015, 453020. [Google Scholar] [CrossRef]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar]
- Ning, Y.; Manegold, P.C.; Hong, Y.K.; Zhang, W.; Pohl, A.; Lurje, G.; Winder, T.; Yang, D.; LaBonte, M.J.; Wilson, P.M. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int. J. Cancer 2011, 128, 2038–2049. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chang, T.M.; Huang, H.C. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Attenuate Mast Cell Activation. Antioxidants 2022, 11, 2279. [Google Scholar] [CrossRef]
- Schlessinger, J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science 2004, 306, 1506–1507. [Google Scholar] [CrossRef]
- Zhou, S.; Abdouh, M.; Arena, V.; Arena, M.; Arena, G.O. Reprogramming malignant cancer cells toward a benign phenotype following exposure to human embryonic stem cell microenvironment. PLoS ONE 2017, 12, e0169899. [Google Scholar]
- Postovit, L.-M.; Margaryan, N.V.; Seftor, E.A.; Kirschmann, D.A.; Lipavsky, A.; Wheaton, W.W.; Abbott, D.E.; Seftor, R.E.; Hendrix, M.J. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc. Natl. Acad. Sci. USA 2008, 105, 4329–4334. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Liu, J.; Huang, Z.; Li, C.; Liu, Y.; Sang, X.; Yang, L.; Wang, S.; Su, Y. Embryonic stem cell microenvironment suppresses the malignancy of cutaneous melanoma cells by down-regulating PI3K/AKT pathway. Cancer Med. 2019, 8, 4265–4277. [Google Scholar] [CrossRef] [PubMed]
- Luecken, M.D.; Theis, F.J. Current best practices in single-cell RNA-seq analysis: A tutorial. Mol. Syst. Biol. 2019, 15, e8746. [Google Scholar] [CrossRef] [PubMed]
- Vasighizaker, A.; Danda, S.; Rueda, L. Discovering cell types using manifold learning and enhanced visualization of single-cell RNA-Seq data. Sci. Rep. 2022, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Heumos, L.; Schaar, A.C.; Lance, C.; Litinetskaya, A.; Drost, F.; Zappia, L.; Lücken, M.D.; Strobl, D.C.; Henao, J.; Curion, F. Best practices for single-cell analysis across modalities. Nat. Rev. Genet. 2023, 4667, 1–23. [Google Scholar]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [PubMed]
- Blankenstein, T.; Coulie, P.G.; Gilboa, E.; Jaffee, E.M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 2012, 12, 307–313. [Google Scholar] [CrossRef]
- Corchete, L.A.; Rojas, E.A.; Alonso-López, D.; De Las Rivas, J.; Gutiérrez, N.C.; Burguillo, F.J. Systematic comparison and assessment of RNA-seq procedures for gene expression quantitative analysis. Sci. Rep. 2020, 10, 19737. [Google Scholar] [CrossRef]
- Pan, X.-W.; Zhang, H.; Xu, D.; Chen, J.-X.; Chen, W.-J.; Gan, S.-S.; Qu, F.-J.; Chu, C.-M.; Cao, J.-W.; Fan, Y.-H. Identification of a novel cancer stem cell subpopulation that promotes progression of human fatal renal cell carcinoma by single-cell RNA-seq analysis. Int. J. Biol. Sci. 2020, 16, 3149. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Peng, M.; Tang, L.; Ouyang, J.; Xiong, F.; Guo, C.; Tang, Y.; Zhou, Y.; Liao, Q. Single-cell RNA sequencing in cancer research. J. Exp. Clin. Cancer Res. 2021, 40, 81. [Google Scholar]
- Dzobo, K.; Ganz, C.; Thomford, N.E.; Senthebane, D.A. Cancer stem cell markers in relation to patient survival outcomes: Lessons for integrative diagnostics and next-generation anticancer drug development. Omics A J. Integr. Biol. 2021, 25, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.-X.; Chen, Y.-X.; Wu, H.-X.; Yin, L.; Zhao, Q.; Luo, H.-Y.; Zeng, Z.-L.; Qiu, M.-Z.; Xu, R.-H. Integrated analysis of single-cell and bulk RNA sequencing data reveals a pan-cancer stemness signature predicting immunotherapy response. Genome Med. 2022, 14, 45. [Google Scholar] [PubMed]
- Larson, M.H.; Pan, W.; Kim, H.J.; Mauntz, R.E.; Stuart, S.M.; Pimentel, M.; Zhou, Y.; Knudsgaard, P.; Demas, V.; Aravanis, A.M. A comprehensive characterization of the cell-free transcriptome reveals tissue-and subtype-specific biomarkers for cancer detection. Nat. Commun. 2021, 12, 2357. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Watts, J.A.; Jamshidi-Parsian, A.; Nadeem, U.; Siegel, E.R.; Zharov, V.P.; Galanzha, E.I. Lymph liquid biopsy for detection of cancer stem cells. Cytom. Part A 2021, 99, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F. Circulating cancer stem cells: An interesting niche to explore. Explor. Target. Anti-Tumor Ther. 2020, 1, 253. [Google Scholar] [CrossRef]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: The potentiality of blood samples. J. Exp. Clin. Cancer Res. 2020, 39, 95. [Google Scholar]
- Dong, Y.; Wang, Z.; Shi, Q. Liquid Biopsy Based Single-Cell Transcriptome Profiling Characterizes Heterogeneity of Disseminated Tumor Cells from Lung Adenocarcinoma. Proteomics 2020, 20, 1900224. [Google Scholar] [CrossRef]
- Park, T.S.; Groh, E.M.; Patel, K.; Kerkar, S.P.; Lee, C.-C.R.; Rosenberg, S.A. Expression of MAGE-A and NY-ESO-1 in Primary and Metastatic Cancers. J. Immunother. 2016, 39, 1. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Lian, Y.; Zhou, X.; Shan, B. MAGE-A family: Attractive targets for cancer immunotherapy. Vaccine 2011, 29, 8496–8500. [Google Scholar] [CrossRef]
- Gordeeva, O. Cancer-testis antigens: Unique cancer stem cell biomarkers and targets for cancer therapy. In Seminars in Cancer Biology; Elsevier: London, England, 2018; pp. 75–89. [Google Scholar]
- Dianatpour, M.; Mehdipour, P.; Nayernia, K.; Mobasheri, M.-B.; Ghafouri-Fard, S.; Savad, S.; Modarressi, M.H. Expression of testis specific genes TSGA10, TEX101 and ODF3 in breast cancer. Iran. Red Crescent Med. J. 2012, 14, 722. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, C.; Javadian-Elyaderani, P.; Schweyer, S.; Schütte, D.; Shoukier, M.; Karimi-Busheri, F.; Weinfeld, M.; Rasouli-Nia, A.; Hengstler, J.G. Pathways of proliferation and antiapoptosis driven in breast cancer stem cells by stem cell protein piwil2. Cancer Res. 2010, 70, 4569–4579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, X.; Lin, J.; Lin, Q.; Wong, K.-C. Review of single-cell RNA-seq data clustering for cell-type identification and characterization. RNA 2023, 29, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, T.; Liu, F.; Chen, Y.; Yao, J.; Li, Z.; Huang, Y.; Wang, J. Comparative analysis of droplet-based ultra-high-throughput single-cell RNA-seq systems. Mol. Cell 2019, 73, 130–142.e135. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.B.; Raveane, A.; Nathan, A.; Soranzo, N.; Raychaudhuri, S. Methods and Insights from Single-Cell Expression Quantitative Trait Loci. Annu. Rev. Genom. Hum. Genet. 2023, 24. [Google Scholar] [CrossRef]
- Yu, X.; Abbas-Aghababazadeh, F.; Chen, Y.A.; Fridley, B.L. Statistical and bioinformatics analysis of data from bulk and single-cell RNA sequencing experiments. Transl. Bioinform. Ther. Dev. 2021, 2194, 143–175. [Google Scholar]
- Qiu, P. Embracing the dropouts in single-cell RNA-seq analysis. Nat. Commun. 2020, 11, 1169. [Google Scholar] [CrossRef]
- Hwang, B.; Lee, J.H.; Bang, D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar]
- Nguyen, A.; Khoo, W.H.; Moran, I.; Croucher, P.I.; Phan, T.G. Single cell RNA sequencing of rare immune cell populations. Front. Immunol. 2018, 9, 1553. [Google Scholar] [CrossRef]
- Dumitrascu, B.; Villar, S.; Mixon, D.G.; Engelhardt, B.E. Optimal marker gene selection for cell type discrimination in single cell analyses. Nat. Commun. 2021, 12, 1186. [Google Scholar] [CrossRef]
- Liu, X.; Gosline, S.J.; Pflieger, L.T.; Wallet, P.; Iyer, A.; Guinney, J.; Bild, A.H.; Chang, J.T. Knowledge-based classification of fine-grained immune cell types in single-cell RNA-Seq data. Brief. Bioinform. 2021, 22, bbab039. [Google Scholar] [CrossRef]
- Montanari, M.; Burattini, S.; Ciacci, C.; Ambrogini, P.; Carloni, S.; Balduini, W.; Lopez, D.; Panza, G.; Papa, S.; Canonico, B. Automated—Mechanical Procedure Compared to Gentle Enzymatic Tissue Dissociation in Cell Function Studies. Biomolecules 2022, 12, 701. [Google Scholar] [CrossRef]
- Welch, E.C.; Yu, H.; Barabino, G.; Tapinos, N.; Tripathi, A. Electric-field facilitated rapid and efficient dissociation of tissues Into viable single cells. Sci. Rep. 2022, 12, 10728. [Google Scholar] [CrossRef]
- Welch, E.C.; Tripathi, A. Preparation of tissues and heterogeneous cellular samples for single-cell analysis. Sample Prep. Tech. Chem. Anal. 2021, 49. [Google Scholar] [CrossRef]
- Datlinger, P.; Rendeiro, A.F.; Boenke, T.; Senekowitsch, M.; Krausgruber, T.; Barreca, D.; Bock, C. Ultra-high-throughput single-cell RNA sequencing and perturbation screening with combinatorial fluidic indexing. Nat. Methods 2021, 18, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sheng, Y.; Qian, W.; Pan, M.; Zhao, X.; Ge, Q. scRNA-seq data analysis method to improve analysis performance. IET Nanobiotechnol. 2023, 17, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, Y.; Li, L. cKBET: Assessing goodness of batch effect correction for single-cell RNA-seq. Front. Comput. Sci. 2024, 18, 181901. [Google Scholar]
- Lindeboom, R.G.; Regev, A.; Teichmann, S.A. Towards a human cell atlas: Taking notes from the past. Trends Genet. 2021, 37, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.; Taylor, D.; Linnarsson, S.; Aronow, B.J.; Bader, G.D.; Barker, R.A.; Camara, P.G.; Camp, J.G.; Chédotal, A.; Copp, A. A roadmap for the human developmental cell atlas. Nature 2021, 597, 196–205. [Google Scholar]
- Van Phan, H.; van Gent, M.; Drayman, N.; Basu, A.; Gack, M.U.; Tay, S. Fixed single-cell RNA sequencing for understanding virus infection and host response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Phan, H.V.; van Gent, M.; Drayman, N.; Basu, A.; Gack, M.U.; Tay, S. High-throughput RNA sequencing of paraformaldehyde-fixed single cells. Nat. Commun. 2021, 12, 5636. [Google Scholar] [CrossRef]
| Method | Technology Name | Minimum Cells | Developer Company | Advantages | Disadvantages | Cost | Library Preparation Time | Sequencing Depth | Applications | Platforms for Analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Droplet-based | Drop-seq | 1000 | Macosko Lab | High throughput, low cost per cell, UMI-based quantification | Low coverage, limited information on isoforms, SNPs and VDJ rearrangements, cell doublets may occur | USD 0.06–0.2 per cell | 1–2 days | 0.1–0.5 million reads per cell | Cell type identification, gene expression profiling, trajectory inference | Seurat, Monocle, Scanpy |
| inDrop | 1000 | Klein Lab and Shalek Lab | High throughput, low cost per cell, UMI-based quantification, flexible barcode design | Low coverage, limited information on isoforms, SNPs and VDJ rearrangements, cell doublets may occur | USD 0.06–0.2 per cell | 1–2 days | 0.1–0.5 million reads per cell | Cell type identification, gene expression profiling, trajectory inference | Seurat, Monocle, Scanpy | |
| Chromium 10× | 500–10,000 | 10× Genomics | High throughput, low cost per cell, UMI-based quantification, multiple applications (e.g., immune profiling, spatial transcriptomics) | Low coverage, limited information on isoforms, SNPs and VDJ rearrangements, cell doublets may occur | USD 0.55–1.1 per cell | 1–2 days | 0.5–2 million reads per cell | Cell type identification, gene expression profiling, trajectory inference, immune repertoire analysis, spatial transcriptomics | Cell Ranger, Seurat, Monocle, Scanpy | |
| Full-length | Smart-seq2 (SS2) | 1–96 | Picelli Lab and Sandberg Lab | High coverage, detection of isoforms, SNPs and VDJ rearrangements, low technical noise | Low throughput, high cost per cell, no UMI-based quantification | USD 35–70 per cell | 2–3 days | 5–20 million reads per cell | Isoform detection and quantification, SNP calling and phasing, VDJ rearrangement analysis | Cufflinks, DESeq2, edgeR |
| Smart-seq3 (SS3) | 1–96 | Sandberg Lab and Linnarsson Lab | High coverage, detection of isoforms, SNPs and VDJ rearrangements, low technical noise, UMI-based quantification | Low throughput, high cost per cell, requires fine-tuning to balance internal and UMI-containing reads | USD 35–70 per cell (estimated) | 2–3 days | 5–20 million reads per cell | Isoform detection and quantification, SNP calling and phasing, VDJ rearrangement analysis | Cufflinks, DESeq2, edgeR | |
| FLASH-seq (FS) | 1–96 | Picelli Lab | High coverage, detection of isoforms, SNPs and VDJ rearrangements, low technical noise, UMI-based quantification with reduced strand-invasion artifacts, fast and simple protocol | Low throughput, high cost per cell | USD 35–70 per cell (estimated) | <4.5 h | 5–20 million reads per cell | Isoform detection and quantification, SNP calling and phasing, VDJ rearrangement analysis | Cufflinks, DESeq2, edgeR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodayari, S.; Khodayari, H.; Saeedi, E.; Mahmoodzadeh, H.; Sadrkhah, A.; Nayernia, K. Single-Cell Transcriptomics for Unlocking Personalized Cancer Immunotherapy: Toward Targeting the Origin of Tumor Development Immunogenicity. Cancers 2023, 15, 3615. https://doi.org/10.3390/cancers15143615
Khodayari S, Khodayari H, Saeedi E, Mahmoodzadeh H, Sadrkhah A, Nayernia K. Single-Cell Transcriptomics for Unlocking Personalized Cancer Immunotherapy: Toward Targeting the Origin of Tumor Development Immunogenicity. Cancers. 2023; 15(14):3615. https://doi.org/10.3390/cancers15143615
Chicago/Turabian StyleKhodayari, Saeed, Hamid Khodayari, Elnaz Saeedi, Habibollah Mahmoodzadeh, Alireza Sadrkhah, and Karim Nayernia. 2023. "Single-Cell Transcriptomics for Unlocking Personalized Cancer Immunotherapy: Toward Targeting the Origin of Tumor Development Immunogenicity" Cancers 15, no. 14: 3615. https://doi.org/10.3390/cancers15143615
APA StyleKhodayari, S., Khodayari, H., Saeedi, E., Mahmoodzadeh, H., Sadrkhah, A., & Nayernia, K. (2023). Single-Cell Transcriptomics for Unlocking Personalized Cancer Immunotherapy: Toward Targeting the Origin of Tumor Development Immunogenicity. Cancers, 15(14), 3615. https://doi.org/10.3390/cancers15143615







