Sensory Processing in Children and Adolescents with Neurofibromatosis Type 1
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.3. Parent/Caregiver Questionnaire Measures
2.3.1. Sensory Profile 2
2.3.2. Autistic Behaviors and ADHD Symptoms
2.3.3. Functional and Mental Health Measures
2.4. Data Analysis
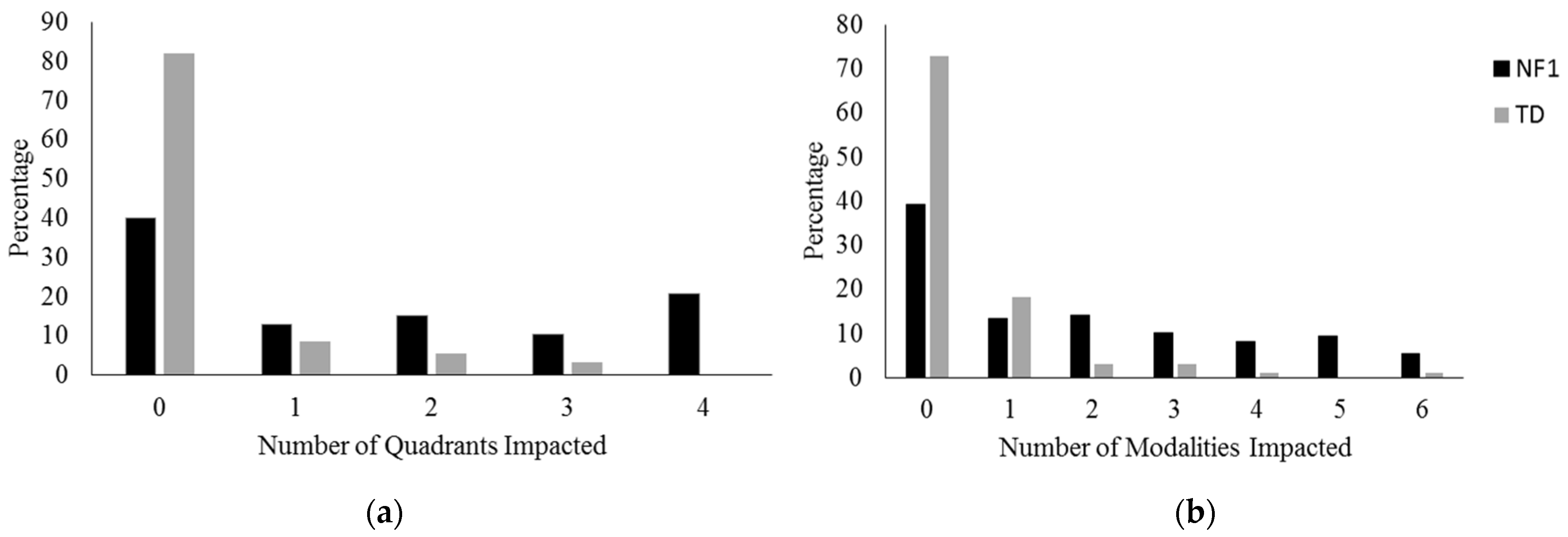
3. Results
3.1. Sensory Processing Clinical Cut-Offs
3.2. Sensory Processing in NF1 and TD Controls
3.3. Comparison of SP2 in NF1 to Published ADHD and Autism Data
3.4. Associations between Sensory Processing and Other Patient Characteristics and Functioning in Children with NF1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, W. The impact of sensory processing abilities on the daily lives of young children and their families: A conceptual model. Infants Young-Child. 1997, 9, 23–35. [Google Scholar] [CrossRef]
- Dunn, W. The Sensory Profile 2 User’s Manual: Psychological Corporation; Psych Corporation: San Antonio, TX, USA, 2014. [Google Scholar]
- Baranek, G.T.; David, F.J.; Poe, M.D.; Stone, W.L.; Watson, L.R. Sensory experiences questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. J. Child Psychol. Psychiatry 2006, 47, 591–601. [Google Scholar] [CrossRef]
- Buyuktaskin, D.; Iseri, E.; Guney, E.; Gunendi, Z.; Cengiz, B. Somatosensory Temporal Discrimination in Autism Spectrum Disorder. Autism Res. 2021, 14, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Hilton, C.L.; Harper, J.D.; Kueker, R.H.; Lang, A.R.; Abbacchi, A.M.; Todorov, A.; LaVesser, P.D. Sensory Responsiveness as a Predictor of Social Severity in Children with High Functioning Autism Spectrum Disorders. J. Autism Dev. Disord. 2010, 40, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.E.; Simmons, D.R. The Relationship between Sensory Sensitivity and Autistic Traits in the General Population. J. Autism Dev. Disord. 2013, 43, 775–784. [Google Scholar] [CrossRef]
- Watson, L.R.; Patten, E.; Baranek, G.T.; Poe, M.; Boyd, B.A.; Freuler, A.; Lorenzi, J. Differential Associations Between Sensory Response Patterns and Language, Social, and Communication Measures in Children With Autism or Other Developmental Disabilities. J. Speech, Lang. Hear. Res. 2011, 54, 1562–1576. [Google Scholar] [CrossRef]
- Lane, A.E.; Young, R.L.; Baker, A.E.Z.; Angley, M.T. Sensory Processing Subtypes in Autism: Association with Adaptive Behavior. J. Autism Dev. Disord. 2010, 40, 112–122. [Google Scholar] [CrossRef]
- Dunn, W.; Little, L.; Dean, E.; Robertson, S.S.; Evans, B. The state of the science on sensory factors and their impact on daily life for children: A scoping review. Occup. Particip. Health 2016, 36, 3s–26s. [Google Scholar]
- Ahn, R.; Miller, L.; Milberger, S.; McIntosh, D. Prevalence of parents’ perceptions of sensory processing disorders among kinder-garten children. Am. J. Occup. Ther. 2004, 58, 287–293. [Google Scholar] [CrossRef]
- Mallory, C.; Keehn, B. Implications of Sensory Processing and Attentional Differences Associated With Autism in Academic Settings: An Integrative Review. Front. Psychiatry 2021, 12, 695825. [Google Scholar] [CrossRef]
- Baranek, G.; Boyd, B.; Poe, D.; Stone, W.L.; Watson, L.R. Hyperresponsive sensory patterns in young children with autism, devel-opmental delay, and typical development. Am. J. Ment. Retard. 2007, 112, 233–245. [Google Scholar] [CrossRef]
- Tomcheck, S.D.; Dunn, W. Sensory processing in children with and without autism: A comparative study using the short sensory profile. Am. J. Occup. Ther. 2007, 61, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Elsabbagh, M.; Fernandes, J.; Webb, S.J.; Dawson, G.; Charman, T.; Johnson, M.H. Disengagement of Visual Attention in Infancy is Associated with Emerging Autism in Toddlerhood. Biol. Psychiatry 2013, 74, 189–194. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [CrossRef]
- Hadders-Algra, M. Emerging signs of autism spectrum disorder in infancy: Putative neural substrate. Dev. Med. Child Neurol. 2022, 64, 1344–1350. [Google Scholar] [CrossRef]
- Sacrey, L.-A.R.; Zwaigenbaum, L.; Bryson, S.; Brian, J.; Smith, I.M.; Roberts, W.; Szatmari, P.; Roncadin, C.; Garon, N.; Novak, C.; et al. Can Parents’ Concerns Predict Autism Spectrum Disorder? A Prospective Study of High-Risk Siblings From 6 to 36 Months of Age. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 470–478. [Google Scholar] [CrossRef]
- Gutmann, D.; Ferner, R.E.; Listernick, R.; Korf, B.R.; Wolters, P.; Johnson, K.J. Neurofibromatosis type 1. Nat. Rev. Dis. Primers 2017, 3, 17004. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.L.; Shores, A.; North, K.N. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 2005, 65, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, A.; Howie, E.; Trump, D.; Huson, S.M. Behaviour in children with neurofibromatosis type 1: Cognition, executive function, attention, emotion, and social competence. Dev. Med. Child Neurol. 2013, 55, 111–125. [Google Scholar] [CrossRef]
- Pride, N.A.; North, K.N. The cognitive profile of NF1 children: Therapeutic implications. In Neurofibromatosis Type 1: Molecular and Cellular Biology; Upadhyaya, M., Cooper, D.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 55–70. [Google Scholar]
- Payne, J.M.; Hearps, S.J.C.; Walsh, K.; Paltin, I.; Barton, B.; Ullrich, N.; Haebich, K.M.; Coghill, D.; Gioia, G.A.; Cantor, A.; et al. Reproducibility of cognitive endpoints in clinical trials: Lessons from neurofibromatosis type 1. Ann. Clin. Transl. Neurol. 2019, 6, 2555–2565. [Google Scholar] [CrossRef]
- Payne, J.M.; Walsh, K.S.; A Pride, N.; Haebich, K.M.; Maier, A.; Chisholm, A.; Glad, D.M.; Casnar, C.L.; Rouel, M.; Lorenzo, J.; et al. Social skills and autism spectrum disorder symptoms in children with neurofibromatosis type 1: Evidence for clinical trial outcomes. Dev. Med. Child Neurol. 2020, 62, 813–819. [Google Scholar] [CrossRef]
- Pride, N.A.; Payne, J.M.; North, K.N. The impact of adhd on the cognitive and academic functioning of children with nf1. Dev. Neuropsychol. 2012, 37, 590–600. [Google Scholar] [CrossRef]
- Mautner, V.-F.; Kluwe, L.; Thakker, S.D.; A Leark, R. Treatment of ADHD in neurofibromatosis type 1. Dev. Med. Child Neurol. 2002, 44, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Lehtonen, A.; Huson, S.M.; Emsley, R.; Trump, D.; Evans, D.G.; Green, J. Autism and other psychiatric comorbidity in neurofi-bromatosis type 1: Evidence from a population-based study. Dev. Med. Child Neurol. 2013, 55, 139–145. [Google Scholar] [CrossRef]
- Morris, S.M.; Acosta, M.T.; Garg, S.; Green, J.; Legius, E.; North, K.; Payne, J.M.; A Weiss, L.; Constantino, J.N.; Gutmann, D.H. Autism in neurofibromatosis type 1: Misuse of covariance to dismiss autistic trait burden. Dev. Med. Child Neurol. 2021, 63, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, A.K.; Lami, F.; Haebich, K.M.; Ure, A.; Brignell, A.; Maloof, T.; Pride, N.A.; Walsh, K.S.; Maier, A.; Rouel, M.; et al. Sex- and age-related differences in autistic behaviours in children with neurofibromatosis type 1. J. Autism Dev. Disord. 2023, 53, 2835–2850. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Green, J.; Leadbitter, K.; Emsley, R.; Lehtonen, A.; Evans, D.G.; Huson, S.M. Neurofibromatosis type 1 and autism spectrum disorder. Pediatrics 2013, 132, e1642–e1648. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, A.; Haebich, K.; Pride, N.A.; Walsh, K.; Lami, F.; Ure, A.; Maloof, T.; Brignell, A.; Rouel, M.; Granader, Y.; et al. Delineating the autistic phenotype in children with neuro-fibromatosis type 1. Mol. Autism 2022, 13, 3. [Google Scholar] [CrossRef]
- Dunn, W.; Bennett, D. Patterns of Sensory Processing in Children with Attention Deficit Hyperactivity Disorder. OTJR: Occup. Particip. Health 2002, 22, 4–15. [Google Scholar] [CrossRef]
- Mangeot, S.; Miller, L.J.; McIntosh, D.; McGrath-Clarke, J.; Simon, J.; Hagerman, R.; Goldson, E. Sensory modulation dysfunction in children with attention-deficit-hyperactivity-disorder. Dev. Med. Child Neurol. 2001, 43, 399–406. [Google Scholar] [CrossRef]
- Marco, E.J.; Hinkley, L.B.N.; Hill, S.S.; Nagarajan, S.S. Sensory processing in autism: A review of neurophysiologic findings. Pediatr. Res. 2011, 69, 48R–54R. [Google Scholar] [CrossRef]
- Begum-Ali, J.; Kolesnik-Taylor, A.; Quiroz, I.; Mason, L.; Garg, S.; Green, J.; Johnson, M.H.; Jones, E.J.H.; The STAARS and EDEN Teams. Early differences in auditory processing relate to Autism spectrum disorder traits in infants with Neurofibromatosis Type 1. J. Neurodev. Disord. 2021, 13, 22. [Google Scholar] [PubMed]
- Rance, G.; Zanin, J.; Maier, A.; Chisari, D.; Haebich, K.; North, K.; Dabscheck, G.; Seal, M.L.; Delatycki, M.B.; Payne, J.M. Auditory dysfunction among individuals with neurofibro-matosis type 1. JAMA Netw. Open 2021, 4, e2136842. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.; Lemos, S.; Rodrigues, C.; de Rezende, N. Auditory temporal processing deficits and language disorders in patients with neurofibromatosis type 1. J. Commun. Disorders 2014, 48, 18–26. [Google Scholar] [CrossRef]
- Norrix, L.W.; Plante, E.; Vance, R.; Boliek, C.A. Auditory-visual integration for speech by children with and without specific lan-guage impairment. J. Speech Lang. Hear. Res. 2007, 50, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Marton, K. Imitation of body postures and hand movements in children with specific language impairment. J. Exp. Child Psychol. 2009, 102, 1–13. [Google Scholar] [CrossRef]
- Haebich, K.M.; A Pride, N.; Walsh, K.S.; Chisholm, A.; Rouel, M.; Maier, A.; Anderson, V.; Barton, B.; Silk, T.; Korgaonkar, M.; et al. Understanding autism spectrum disorder and social functioning in children with neurofibromatosis type 1: Protocol for a cross-sectional multimodal study. BMJ Open 2019, 9, e030601. [Google Scholar] [CrossRef]
- Neurofibromatosis Conference Statement. National Institutes of Health Consensus Development Conference. Arch. Neurol. 1988, 45, 575–578. [Google Scholar]
- Legius, E.; Messiaen, L.; Wolkenstein, P.; Pancza, P.; Avery, R.A.; Berman, Y.; Blakeley, J.; Babovic-Vuksanovic, D.; Cunha, K.S.; Ferner, R.; et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An international consensus recommendation. Anesthesia Analg. 2021, 23, 1506–1513. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children, Fifth Edition: Australian and New Zealand Standardised Edition (WISC-V A&NZ); Pearson: Bloomington, MN, USA, 2016. [Google Scholar]
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence, 4th ed.; Pearson: San Antonio, TX, USA, 2012. [Google Scholar]
- Constantino, J.; Gruber, C.P. Social Responsiveness Scale, 2nd ed.; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Conners, C.K. Conners’ ADHD/DSM-IV Scales: Parent Version; Multi-Health Systems Inc.: New York, NY, USA, 1999. [Google Scholar]
- Conners, C.K. Conners 3rd Edition Parent Toronto; Multi-Health Systems Inc.: North Tonawanda, NY, USA, 2008. [Google Scholar]
- Harrison, P.; Oakland, T. Adaptive Behavior Assessment System, 3rd ed.; (ABAS-3); Western Psychological Services: Chicago, IL, USA, 2015. [Google Scholar]
- Elliott, S.N.; Gresham, F.M. Social skills interventions for children. Behav. Modif. 1993, 17, 287–313. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA School-Age Forms and Profiles; University of Vermont, Research Center for Children, Youth and Families: Burlington, VT, USA, 2001. [Google Scholar]
- Little, L.; Dean, E.; Tomcheck, S.D.; Dunn, W. Sensory processing patterns in autism, attention deficit hyperactivity disorder, and typical development. Phys. Occup. Ther. Pediatr. 2018, 38, 243–254. [Google Scholar] [CrossRef]
- Ermer, J.; Dunn, W. The sensory profile: A discriminant analysis of children with and without disabilities. Am. J. Occup. Ther. 1998, 52, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef]
- Cheung, P.; Siu, A.M. A comparison of patterns of sensory processing in children with and without development disabilities. Res. Dev. Disabil. 2009, 30, 1468–1480. [Google Scholar] [CrossRef]
- Baranek, G.T.; Watson, L.R.; Boyd, B.A.; Poe, M.D.; David, F.J.; McGuire, L. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Dev. Psychopathol. 2013, 25, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Zwaigenbaum, L.; Bryson, S.E.; Rogers, T.; Toberts, W.; Brian, J.; Szatmari, P. Faculty opinions recommendation of behavioral manifestations of autism in the first year of life. Int. J. Dev. Neurosci. 2005, 23, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.S.; Courchesne, E.; Townsend, J.; A Carper, R.; Lord, C. Neuroanatomic contributions to slowed orienting of attention in children with autism. Cogn. Brain Res. 1999, 8, 61–71. [Google Scholar] [CrossRef]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and ventral attention systems. Neuroscientist 2014, 20, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Mundy, P.; Newell, L. Attention, joint attention and social cognition. Curr. Dir. Psychol. Sci. 2007, 16, 269–274. [Google Scholar] [CrossRef]
- Mundy, P.; Jarrold, W. Infant joint attention, neural networks and social cognition. Neural Netw. 2010, 23, 985–997. [Google Scholar] [CrossRef]
- Pride, N.A.; Korgaonkar, M.S.; North, K.N.; Payne, J.M. Impaired engagement of the ventral attention system in neurofibromatosis type 1. Brain Imaging Behav. 2018, 12, 499–508. [Google Scholar] [CrossRef]
- Itahashi, T.; Fujino, J.; Sato, T.; Ohta, H.; Nakamura, M.; Kato, N.; Hashimoto, R.-I.; Di Martino, A.; Aoki, Y.Y. Neural correlates of shared sensory symptoms in autism and attention-deficit/hyperactivity disorder. Brain Commun. 2020, 2, fcaa186. [Google Scholar] [CrossRef] [PubMed]
- Loitfelder, M.; Huijbregts, S.C.; Veer, I.M.; Swaab, H.; Van Buchem, V.; Schmidt, R.; Rombouts, S.A. Functional connectivity changes and exec-utive social problems in neurofibromatosis type 1. Brain Connect. 2015, 5, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.J.; d’Almeida, O.C.; Ramos, F.; Saraiva, J.; Silva, E.D.; Castelo-Branco, M. Abnormal late visual responses and alpha oscil-lations in neurofibromatosis type 1: A link to visual and attention deficits. J. Neurodev. Disord. 2014, 6, 4. [Google Scholar] [CrossRef]
- Moscato, E.H.; Dubowy, C.; Walker, J.A.; Kayser, M.S. Social Behavioral Deficits with Loss of Neurofibromin Emerge from Peripheral Chemosensory Neuron Dysfunction. Cell Rep. 2020, 32, 107856. [Google Scholar] [CrossRef]
- Dyson, A.; Ryan, M.; Garg, S.; Evans, G.; Baines, R.A. Loss of NF1 in drosophila larvae causes tactile hypersensitivity and impaired synaptic transmission at the neuromuscular junction. J. Neurosci. 2022, 42, 9450–9572. [Google Scholar] [CrossRef]
- Walsh, K.S.; I Vélez, J.; Kardel, P.G.; Imas, D.M.; Muenke, M.; Packer, R.J.; Castellanos, F.X.; Acosta, M.T. Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Dev. Med. Child Neurol. 2013, 55, 131–138. [Google Scholar] [CrossRef]
- Johnson, N.S.; Saal, H.M.; Lovell, A.M.; Schorry, E.K. Social and emotional problems in children with neurofibromatosis type 1: Evidence and proposed interventions. J. Pediatr. 1999, 134, 767–772. [Google Scholar] [CrossRef]
- Sandbank, M.; Bottema-Beutel, K.; Crowley, S.; Cassidy, M.; Dunham, K.; Feldman, J.L. Project AIM: Autism intervention me-ta-analysis for studies of young children. Psychol. Bull. 2020, 146, 1–29. [Google Scholar] [CrossRef]
- Camarata, S.; Miller, L.J.; Wallace, M.T. Evaluating sensory integration/sensory processing treatment: Issues and analysis. Front. Integr. Neurosci. 2020, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.A.; Koenig, K.; Kinnealey, M.; Sheppard, M.; Henderson, L. Effectiveness of sensory integration interventions in children with autism spectrum disorders: A pilot study. Am. J. Occup. Ther. 2011, 65, 76–85. [Google Scholar] [CrossRef]
- Schaaf, R.C.; Benevides, T.; Mailoux, Z.; Faller, P.; Hunt, J.; van Hooydonk, E.; Freeman, R.; Leiby, B.; Sendecki, J.; Kelly, D. An intervention for sensory difficulties in children with autism: A randomized trial. J. Autism Dev. Disord. 2014, 44, 1493–1506. [Google Scholar]



| Variable | NF1 Mean (SD) | TD Controls Mean (SD) | F/χ2 | p |
|---|---|---|---|---|
| Age (years) | 8.1 (3.2) | 8.1 (3.1) | 0.001 | 0.982 |
| Sex (% male) | 54 | 49 | 0.59 a | 0.440 |
| FSIQ b | 89.1 (12.7) | 106.0 (17.8) | 73.83 | <0.001 |
| SRS-2 Total c | 61.9 (14.1) | 46.6 (5.8) | 100.56 | <0.001 |
| ADHD Inattentive c | 64.9 (15.6) | 52.3 (11.3) | 46.74 | <0.001 |
| ADHD Hyperactive/Impulsive c | 64.0 (16.5) | 51.4 (12.6) | 40.91 | <0.001 |
| ABAS-3 GAC b | 86.3 (13.7) | 101.7 (11.3) | 79.92 | <0.001 |
| SSIS-RS Total b | 90.03 (17.5) | 105.4 (13.7) | 51.3 | <0.001 |
| CBCL Anxiety c | 57.7 (8.8) | 53.2 (4.8) | 52.85 | <0.001 |
| CBCL Affective c | 60.8 (9.0) | 53.6 (5.2) | 52.86 | <0.001 |
| SP2 Quadrants | ||||
|---|---|---|---|---|
| Sensory Avoiding | Hypersensitivity | Registration | Sensory Seeking | |
| Age | 0.07 | 0.01 | −0.02 | 0.11 |
| Sex | −0.19 | −0.10 | −0.10 | −0.29 |
| ADHD Inattentive | 0.67 * | 0.73 * | 0.75 * | 0.75 * |
| ADHD Hyperactive/Impulsive | 0.62 * | 0.69 * | 0.68 * | 0.75 * |
| FSIQ | −0.19 | −0.22 | −0.27 * | −0.31 * |
| ABAS-3 GAC | −0.51 * | −0.60 * | −0.50 * | −0.53 * |
| SSIS-RS Total | −0.57 * | −0.62 * | −0.55 * | −0.50 * |
| SRS-2 Total | 0.70 * | 0.79 * | 0.72 * | 0.72 * |
| CBCL Anxiety | 0.61 * | 0.53 * | 0.42 * | 0.48 * |
| CBCL Affective | 0.67 * | 0.66 * | 0.56 * | 0.56 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pride, N.A.; Haebich, K.M.; Walsh, K.S.; Lami, F.; Rouel, M.; Maier, A.; Chisholm, A.K.; Lorenzo, J.; Hearps, S.J.C.; North, K.N.; et al. Sensory Processing in Children and Adolescents with Neurofibromatosis Type 1. Cancers 2023, 15, 3612. https://doi.org/10.3390/cancers15143612
Pride NA, Haebich KM, Walsh KS, Lami F, Rouel M, Maier A, Chisholm AK, Lorenzo J, Hearps SJC, North KN, et al. Sensory Processing in Children and Adolescents with Neurofibromatosis Type 1. Cancers. 2023; 15(14):3612. https://doi.org/10.3390/cancers15143612
Chicago/Turabian StylePride, Natalie A., Kristina M. Haebich, Karin S. Walsh, Francesca Lami, Melissa Rouel, Alice Maier, Anita K. Chisholm, Jennifer Lorenzo, Stephen J. C. Hearps, Kathryn N. North, and et al. 2023. "Sensory Processing in Children and Adolescents with Neurofibromatosis Type 1" Cancers 15, no. 14: 3612. https://doi.org/10.3390/cancers15143612
APA StylePride, N. A., Haebich, K. M., Walsh, K. S., Lami, F., Rouel, M., Maier, A., Chisholm, A. K., Lorenzo, J., Hearps, S. J. C., North, K. N., & Payne, J. M. (2023). Sensory Processing in Children and Adolescents with Neurofibromatosis Type 1. Cancers, 15(14), 3612. https://doi.org/10.3390/cancers15143612





