In-Vivo Somatostatin-Receptor Expression in Small Cell Lung Cancer as a Prognostic Image Biomarker and Therapeutic Target
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and SSTR-Directed Molecular Imaging
2.2. Scan Interpretation
2.3. PRRT
2.4. Response Assessment
2.5. Statistical Analysis
3. Results
3.1. Patients
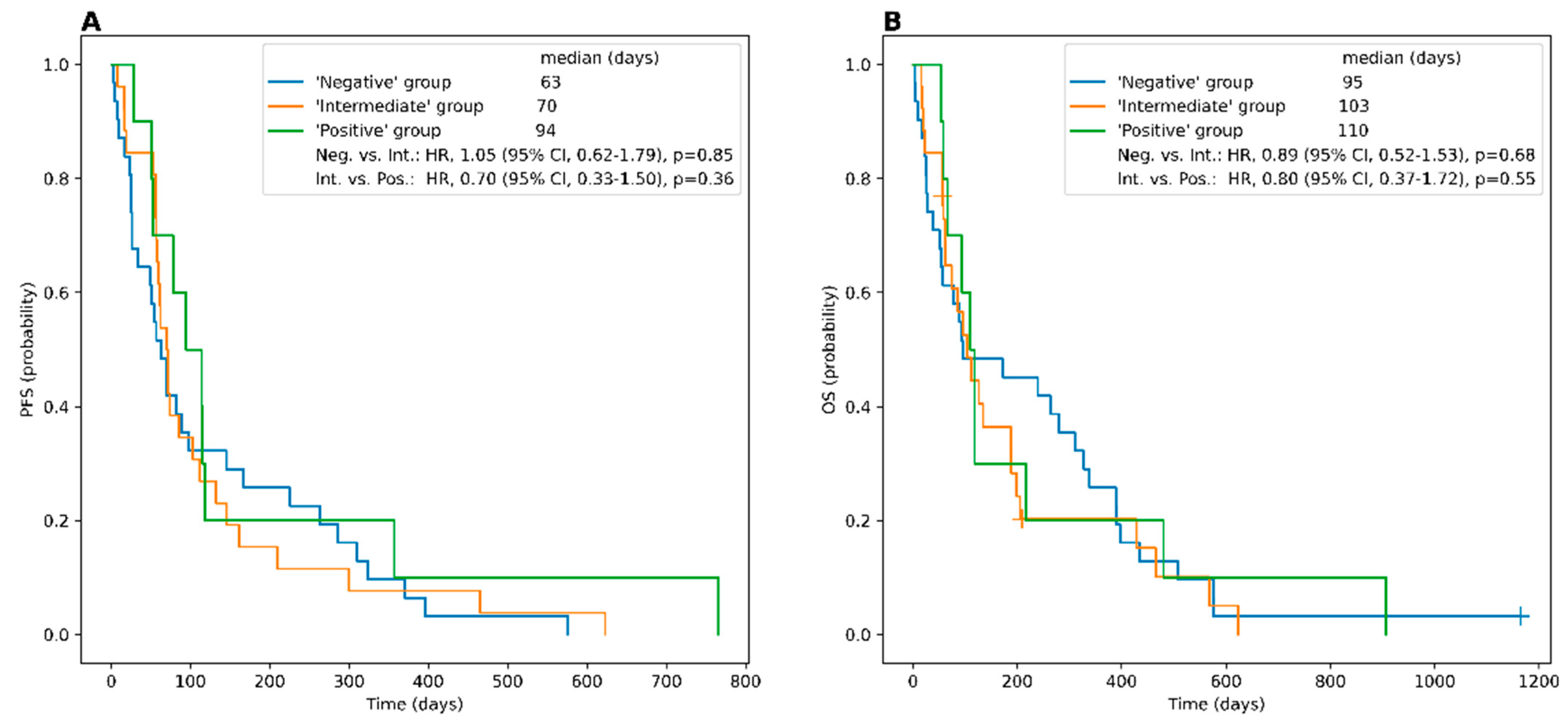
3.2. Somatostatin Receptor Imaging (STR-PET/CT)
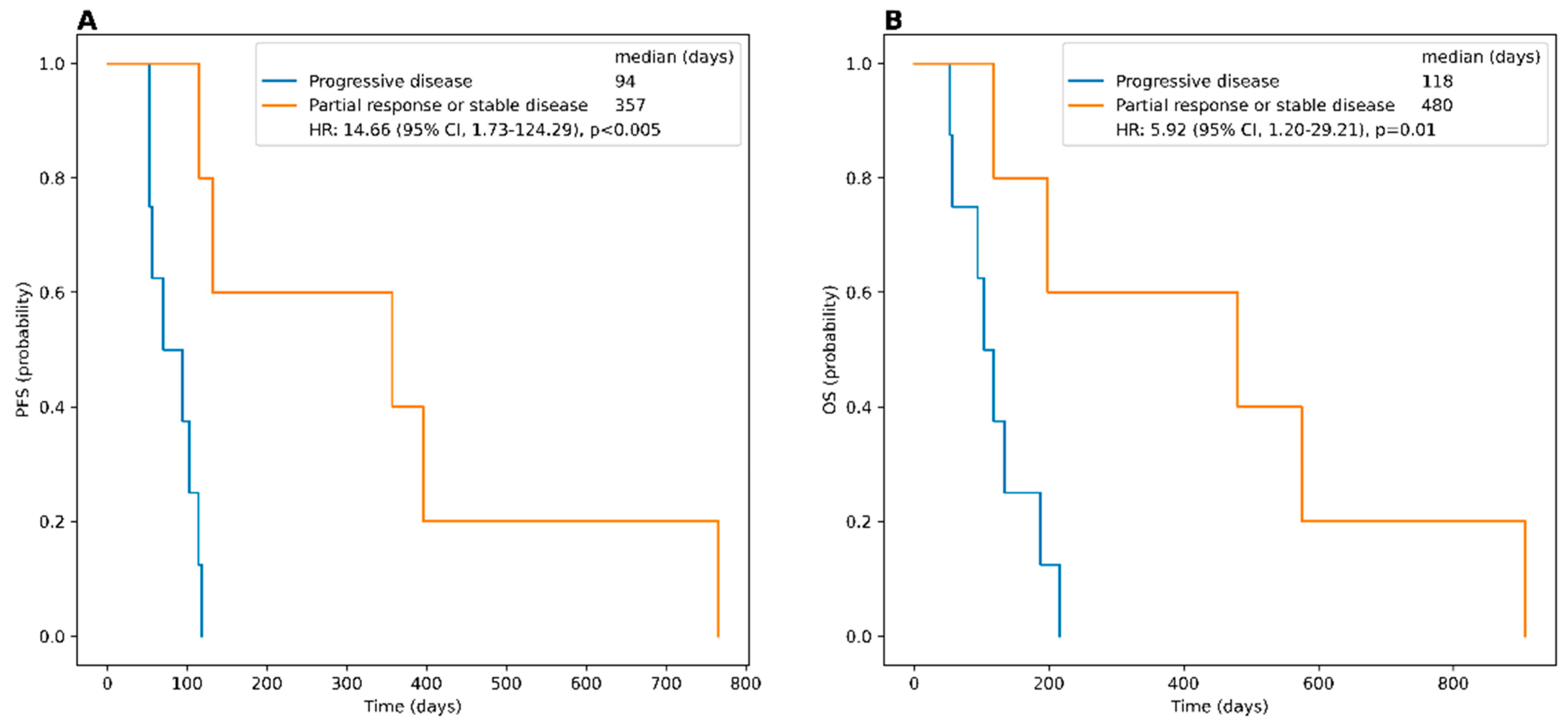
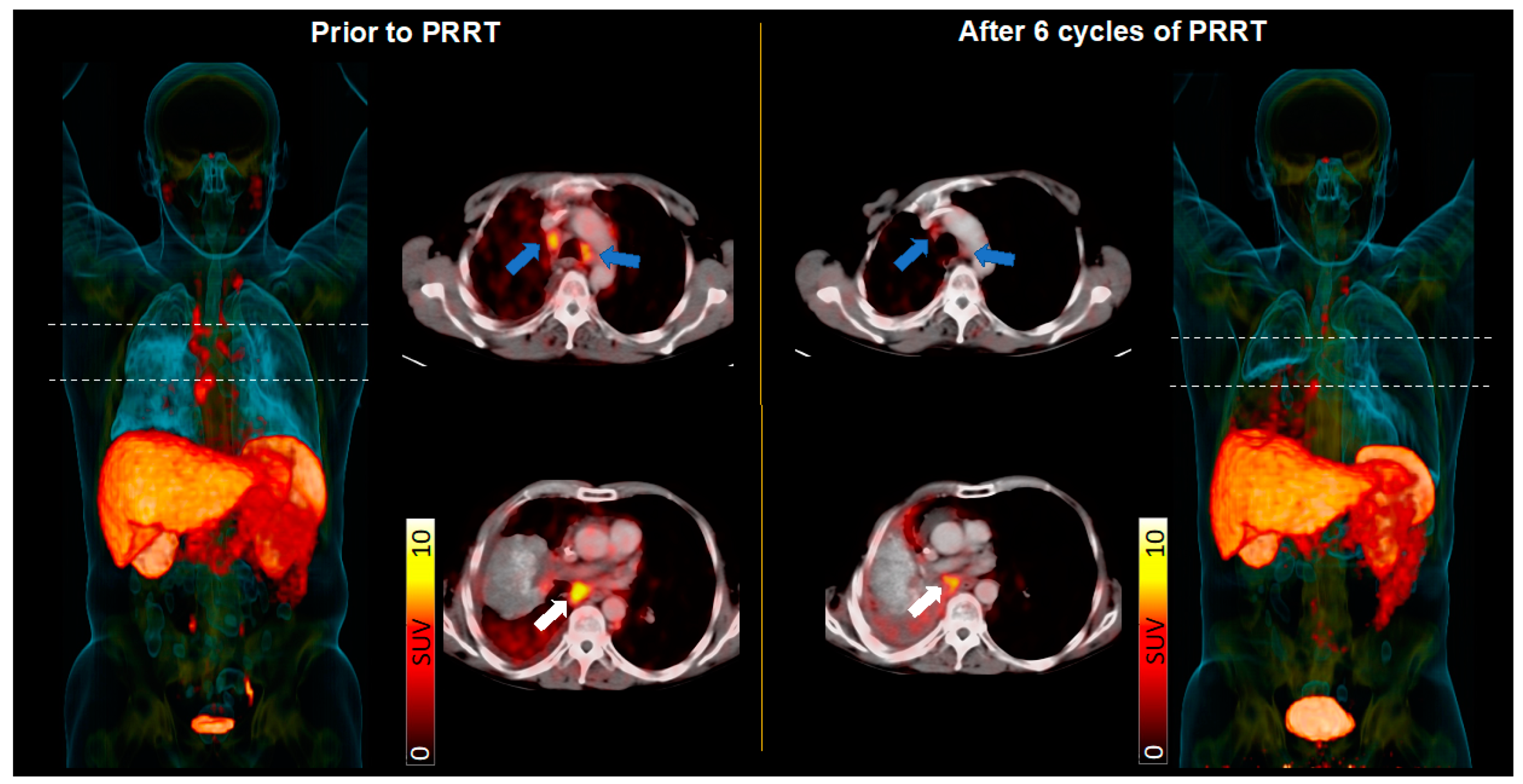
3.3. PRRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.C.; Fruh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(✩). Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Lattuca-Truc, M.; Timsit, J.F.; Levra, M.G.; Ruckly, S.; Villa, J.; Dumas, I.; Pinsolle, J.; Ferrer, L.; Guillem, P.; Moro-Sibilot, D.; et al. Trends in response rate and survival in small-cell lung cancer patients between 1997 and 2017. Lung Cancer 2019, 131, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wu, Q.; Wu, S.; Xie, X. Comparing strategy of immune checkpoint inhibitors plus chemotherapy with chemotherapy alone for small cell lung cancer: A meta-analysis based on six RCTs incorporating 2800 participants. J. Cancer Res. Clin. Oncol. 2022, 148, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, R.A.; Habacha, B.; Lutje, S.; Essler, M. Therapy of Patients with Neuroendocrine Neoplasia-Evidence-Based Approaches and New Horizons. J. Clin. Med. 2019, 8, 1474. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With (177)Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Kho, G.S.; Lamberts, S.W.; Reubi, J.C.; Laissue, J.A.; Krenning, E.P. The value of octreotide scintigraphy in patients with lung cancer. Eur. J. Nucl. Med. 1994, 21, 1106–1113. [Google Scholar] [CrossRef]
- Tsuta, K.; Wistuba, I.I.; Moran, C.A. Differential expression of somatostatin receptors 1-5 in neuroendocrine carcinoma of the lung. Pathol. Res. Pract. 2012, 208, 470–474. [Google Scholar] [CrossRef]
- Lapa, C.; Hanscheid, H.; Wild, V.; Pelzer, T.; Schirbel, A.; Werner, R.A.; Droll, S.; Herrmann, K.; Buck, A.K.; Luckerath, K. Somatostatin receptor expression in small cell lung cancer as a prognostic marker and a target for peptide receptor radionuclide therapy. Oncotarget 2016, 7, 20033–20040. [Google Scholar] [CrossRef]
- Sollini, M.; Farioli, D.; Froio, A.; Chella, A.; Asti, M.; Boni, R.; Grassi, E.; Roncali, M.; Versari, A.; Erba, P.A. Brief report on the use of radiolabeled somatostatin analogs for the diagnosis and treatment of metastatic small-cell lung cancer patients. J. Thorac. Oncol. 2013, 8, 1095–1101. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Horsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczesna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Peters, S.; Pujol, J.L.; Dafni, U.; Domine, M.; Popat, S.; Reck, M.; Andrade, J.; Becker, A.; Moro-Sibilot, D.; Curioni-Fontecedro, A.; et al. Consolidation nivolumab and ipilimumab versus observation in limited-disease small-cell lung cancer after chemo-radiotherapy—Results from the randomised phase II ETOP/IFCT 4-12 STIMULI trial. Ann. Oncol. 2022, 33, 67–79. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.; Ciuleanu, T.E.; Tsekov, H.; Shparyk, Y.; Cucevia, B.; Juhasz, G.; Thatcher, N.; Ross, G.A.; Dane, G.C.; Crofts, T. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J. Clin. Oncol. 2006, 24, 5441–5447. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Ohkuni, Y.; Kaneko, N.; Yamaguchi, E.; Kubo, A. Relapsed small cell lung cancer: Treatment options and latest developments. Ther. Adv. Med. Oncol. 2014, 6, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Hanscheid, H.; Lapa, C.; Buck, A.K.; Lassmann, M.; Werner, R.A. Dose Mapping After Endoradiotherapy with (177)Lu-DOTATATE/DOTATOC by a Single Measurement After 4 Days. J. Nucl. Med. 2018, 59, 75–81. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Nunez, R. Targeted alpha-Emitter Therapy with (212)Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef]
- Fani, M.; Nicolas, G.P.; Wild, D. Somatostatin Receptor Antagonists for Imaging and Therapy. J. Nucl. Med. 2017, 58, 61S–66S. [Google Scholar] [CrossRef]
- Poirier, J.T.; George, J.; Owonikoko, T.K.; Berns, A.; Brambilla, E.; Byers, L.A.; Carbone, D.; Chen, H.J.; Christensen, C.L.; Dive, C.; et al. New Approaches to SCLC Therapy: From the Laboratory to the Clinic. J. Thorac. Oncol. 2020, 15, 520–540. [Google Scholar] [CrossRef]
- Kim, C.; Liu, S.V.; Subramaniam, D.S.; Torres, T.; Loda, M.; Esposito, G.; Giaccone, G. Phase I study of the (177)Lu-DOTA(0)-Tyr(3)-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J. Immunother. Cancer 2020, 8, e000980. [Google Scholar] [CrossRef]
- Cetin, K.; Christiansen, C.F.; Jacobsen, J.B.; Norgaard, M.; Sorensen, H.T. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: A Danish population-based cohort study. Lung Cancer 2014, 86, 247–254. [Google Scholar] [CrossRef] [PubMed]
| Total Cohort (n = 67) | Treatment Cohort (n = 14) | |
|---|---|---|
| Demographic data | ||
| Age (years, mean, range) | 61.2 (41–80) | 65.3 (48–79) |
| Female | 27 (40.3%) | 3 (21.4%) |
| Previous therapies | ||
| Surgery | 7 (10.4%) | 2 (14.2%) |
| Radiation treatment | 48 (71.6%) | 8 (57.1%) |
| 1st line CTx (cis-/carboplatin, etoposide) | 66 (98.5%) | 14 (100.0%) |
| 2nd line CTx (topotecan, gemcitabine or ixoten) | 30 (46.3%) | 11 (78.6%) |
| Other systemic treatment * | 2 (3.0%) | 0 |
| Metastases | ||
| Lymph node | 61 (91.0%) | 14 (100.0%) |
| Bone | 35 (52.2%) | 8 (57.1%) |
| Liver | 33 (49.3%) | 6 (42.9%) |
| CNS | 23 (34.3%) | 5 (35.7%) |
| Adrenal | 13 (19.4%) | 3 (21.4%) |
| Lung | 13 (19.4%) | 3 (21.4%) |
| Pleura | 11 (16.4%) | 5 (35.7%) |
| Pancreas | 4 (6.0%) | 1 (7.1%) |
| Other Locations | 9 (13.4%) | 1 (7.1%) |
| Sex | Age | Previous Therapies | Sites of Disease | SSTR Positivity | Number of PRRT Cycles | Cumulative Activity (GBq) | Best Response | PFS | OS |
|---|---|---|---|---|---|---|---|---|---|
| F | 74 | Surgery (primary), adjuvant RCTx (1st line), CTx (1st/ 2nd line) | Lymph nodes, pleura | all | 5 | 39.5 | PR | 766 | 907 |
| M | 69 | CTx (1st/ 2nd line) | Lymph nodes, skeletal, adrenal, pulmonary, pleura | all | 1 | 7.4 | SD | 118 | 118 |
| M | 66 | CTx (1st/ 2nd/ 3rd line) | Lymph nodes, skeletal, pleura | all | 1 | 7.6 | PD | 53 | 53 |
| M | 48 | CTx (1st/ 2nd line) | Lymph nodes, cerebral, pulmonary | majority | 6 | 44.3 | SD | 396 | 575 |
| M | 55 | RCTx (1st line), CTx (1st line), RTx (prophyl. brain, metastasis), CTx (2nd line), RCTx (metastasis) | Lymph nodes, skeletal, cerebral | all | 2 | 14.8 | PD | 94 | 94 |
| M | 69 | RCTx (1st line), Surgery (metastasis), RTx (metastasis), CTx (1st line) | Lymph nodes, skeletal, hepatic, cerebral | majority | 1 | 6.0 | PD | 53 | 187 |
| M | 68 | CTx (1st/2nd line) | Lymph nodes, hepatic, cerebral, adrenal, pleural | majority | 1 | 6.9 | PD | 70 | 134 |
| M | 79 | CTx (1st/2nd line) | Lymph nodes, skeletal, hepatic | majority | 1 | 7.21 | PD | 56 | 56 |
| M | 70 | RCTx (1st line) | Lymph nodes | all | 3 | 21.3 | SD | 357 | 480 |
| F | 67 | CTx (1st/2nd line) | Lymph nodes, skeletal, hepatic | all | 1 | 7.3 | PD | 118 | 118 |
| F | 59 | CTx (1st line), RTx (metastasis), CTx (2nd line) | Lymph nodes, pulmonary | all | 2 | 15.2 | PD | 114 | 216 |
| M | 61 | CTx (1st line), RTx (metastasis), CTx (2nd line) | Lymph nodes, adrenal, pancreatic, renal | majority | 1 | 8.0 | PD | 103 | 103 |
| M | 70 | CTx (1st line), RTx (residual tumor) | Lymph nodes, skeletal, hepatic, cerebral, pleural | majority | 1 | 7.7 | N/A * | 17 | 17 |
| M | 59 | CTx (1st line), RTx (prophyl. brain), CTx (2nd line) | Lymph nodes, skeletal, hepatic | majority | 3 | 22.6 | SD | 132 | 198 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şen, F.; Sheikh, G.T.; von Hinten, J.; Schindele, A.; Kircher, M.; Dierks, A.; Pfob, C.H.; Serfling, S.E.; Buck, A.K.; Pelzer, T.; et al. In-Vivo Somatostatin-Receptor Expression in Small Cell Lung Cancer as a Prognostic Image Biomarker and Therapeutic Target. Cancers 2023, 15, 3595. https://doi.org/10.3390/cancers15143595
Şen F, Sheikh GT, von Hinten J, Schindele A, Kircher M, Dierks A, Pfob CH, Serfling SE, Buck AK, Pelzer T, et al. In-Vivo Somatostatin-Receptor Expression in Small Cell Lung Cancer as a Prognostic Image Biomarker and Therapeutic Target. Cancers. 2023; 15(14):3595. https://doi.org/10.3390/cancers15143595
Chicago/Turabian StyleŞen, Feyza, Gabriel T. Sheikh, Johannes von Hinten, Andreas Schindele, Malte Kircher, Alexander Dierks, Christian H. Pfob, Sebastian E. Serfling, Andreas K. Buck, Theo Pelzer, and et al. 2023. "In-Vivo Somatostatin-Receptor Expression in Small Cell Lung Cancer as a Prognostic Image Biomarker and Therapeutic Target" Cancers 15, no. 14: 3595. https://doi.org/10.3390/cancers15143595
APA StyleŞen, F., Sheikh, G. T., von Hinten, J., Schindele, A., Kircher, M., Dierks, A., Pfob, C. H., Serfling, S. E., Buck, A. K., Pelzer, T., Higuchi, T., Weich, A., Bundschuh, R. A., Werner, R. A., & Lapa, C. (2023). In-Vivo Somatostatin-Receptor Expression in Small Cell Lung Cancer as a Prognostic Image Biomarker and Therapeutic Target. Cancers, 15(14), 3595. https://doi.org/10.3390/cancers15143595







