Prognostic Implications of PET-Derived Tumor Volume and Uptake in Patients with Neuroendocrine Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Methodology of Literature Research
3. Role of SSTR-PET-Derived Biomarkers for the Management of NET Patients
3.1. Tumor Volume Measurement Techniques
- (i)
- One fixed global threshold for all tumor lesions across patients.
- (ii)
- Patient-specific thresholds based on uptake in reference organs, such as blood pool, liver, or spleen.
- (iii)
- Lesion-specific local thresholds (isocontour), based on the SUVmax of the respective lesion.
- (iv)
- Manual measurements without thresholding. These approaches may limit interobserver agreement based on the high degree of freedom that each observer has for lesion delineation.
- (v)
- Combinations of i/ii and iii, in which a global or patient-specific threshold is used to preselect lesions, which are thereafter segmented with a local isocontour.
3.2. Tumor Volume in the Context of SSTR-Targeted Imaging
3.3. Association of SSTR-PET-Derived Tumor Volume with Clinical Outcome
3.4. Association of PET-Derived SSTR Expression with Clinical Outcomes
3.5. Association of SSTR-PET-Derived Parameters and Outcomes after SSA or PRRT
3.6. Association of FDG-PET-Derived Features with Clinical Outcomes
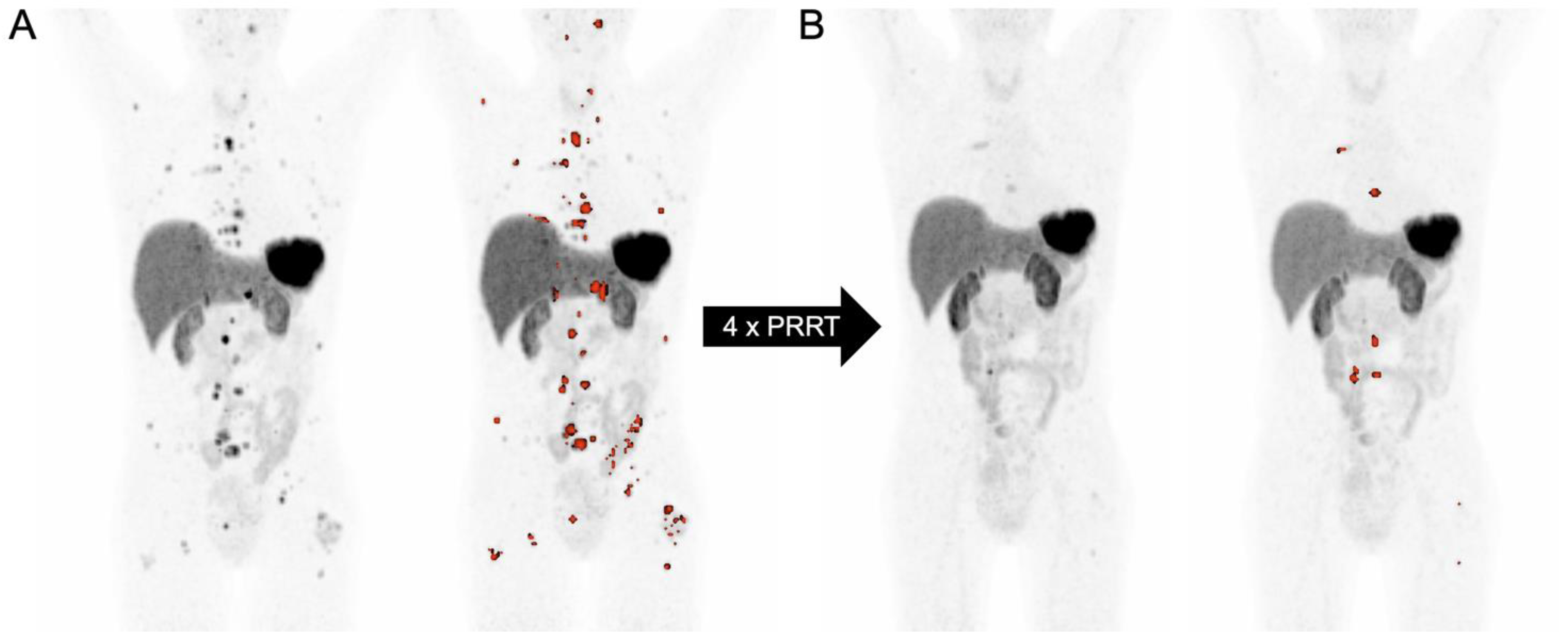
3.7. SSTR-PET-Derived Tumor Volume in the Context of PRRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SSTR | Somatostatin receptor |
| NET | Neuroendocrine tumor |
| SRS | Somatostatin receptor scintigraphy |
| SSTR-PET | Somatostatin receptor positron-emission tomography |
| PET | Positron-emission tomography |
| SUV | Standardized uptake value |
| MRI | Magnetic resonance imaging |
| CT | Computed tomography |
| PRRT | Peptide radionuclide receptor therapy |
| PSMA | Prostate-specific membrane antigen |
| SRE-TV | Somatostatin receptor-expressing tumor volume |
| PFS | Progression-free survival |
| OS | Overall survival |
| SSA | Somatostatin analogs |
| FDG | Fluorodeoxyglucose |
| MTV | Metabolic tumor volume |
| PERCIST | PET response criteria in solid tumors |
| SD | Standard deviation |
| SUVmax | Maximum standardized uptake value |
| SUVmean | Mean standardized uptake value |
| pNET | Pancreatic neuroendocrine tumor |
| TLR | Tumor-to-liver ratio |
| IBI | Inflammation-based index |
| G1/2/3 | Grade 1/2/3 |
| CA9 | Cancer antigen 9 |
| GLUT1 | Glucose transporter 1 |
| Gy | Gray |
| 5-FU | 5-fluorouracil |
| PROMISE | Prostate cancer molecular imaging standardized evaluation |
| TNM | Tumor node metastasis |
| PPP | PSMA PET progression criteria |
| RECIP | Response evaluation using PSMA PET/CT in patients with metastatic castration-resistant prostate cancer |
References
- Kulke, M.H.; Benson, A.B., 3rd; Bergsland, E.; Berlin, J.D.; Blaszkowsky, L.S.; Choti, M.A.; Clark, O.H.; Doherty, G.M.; Eason, J.; Emerson, L. Neuroendocrine tumors. J. Natl. Compr. Cancer Netw. 2012, 10, 724–764. [Google Scholar] [CrossRef]
- Dias, A.R.; Azevedo, B.C.; Alban, L.B.V.; Yagi, O.K.; Ramos, M.F.K.P.; Jacob, C.E.; Barchi, L.C.; Cecconello, I.; Ribeiro, U., Jr.; Zilberstein, B. Gastric Neuroendocrine Tumor: Review and Update. Arq. Bras. Cir. Dig. 2017, 30, 150–154. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Hrabe, J. Neuroendocrine Tumors of the Appendix, Colon, and Rectum. Surg. Oncol. Clin. N. Am. 2020, 29, 267–279. [Google Scholar] [CrossRef]
- Kasajima, A.; Kloppel, G. Neuroendocrine tumor G3 of bronchopulmonary origin and its classification. Pathol. Int. 2022, 72, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Iyoda, A.; Azuma, Y.; Sano, A. Neuroendocrine tumors of the lung: Clinicopathological and molecular features. Surg. Today 2020, 50, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.T.; Howe, J.R. Evaluation and Management of Neuroendocrine Tumors of the Pancreas. Surg. Clin. N. Am. 2019, 99, 793–814. [Google Scholar] [CrossRef]
- Patel, N.; Barbieri, A.; Gibson, J. Neuroendocrine Tumors of the Gastrointestinal Tract and Pancreas. Surg. Pathol. Clin. 2019, 12, 1021–1044. [Google Scholar] [CrossRef]
- Corey, B.; Chen, H. Neuroendocrine Tumors of the Stomach. Surg. Clin. N. Am. 2017, 97, 333–343. [Google Scholar] [CrossRef]
- Herrmann, K.; Czernin, J.; Wolin, E.M.; Gupta, P.; Barrio, M.; Gutierrez, A.; Schiepers, C.; Mosessian, S.; Phelps, M.E.; Allen-Auerbach, M.S. Impact of 68Ga-DOTATATE PET/CT on the management of neuroendocrine tumors: The referring physician’s perspective. J. Nucl. Med. 2015, 56, 70–75. [Google Scholar] [CrossRef]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K.; Eriksson, B. Endocrine tumours of the pancreas. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 753–781. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Sotiropoulos, G.C.; Li, J.; Kornasiewicz, O.; Plockinger, U. Multimodal management of neuroendocrine liver metastases. HPB 2010, 12, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Hacki, W.H.; Lamberts, S.W. Hormone-producing gastrointestinal tumors contain a high density of somatostatin receptors. J. Clin. Endocrinol. Metab. 1987, 65, 1127–1134. [Google Scholar] [CrossRef]
- Antunes, P.; Ginj, M.; Zhang, H.; Waser, B.; Baum, R.P.; Reubi, J.C.; Maecke, H. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Schär, J.-C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Krenning, E.P.; Bakker, W.H.; Kooij, P.P.M.; Breeman, W.A.P.; Oei, H.Y.; De Jong, M.; Reubi, J.C.; Visser, T.J.; Bruns, C.; Kwekkeboom, D.J.; et al. Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: Metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J. Nucl. Med. 1992, 33, 652–658. [Google Scholar]
- Walker, R.C.; Smith, G.T.; Liu, E.; Moore, B.; Clanton, J.; Stabin, M. Measured human dosimetry of 68Ga-DOTATATE. J. Nucl. Med. 2013, 54, 855–860. [Google Scholar] [CrossRef]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef]
- Putzer, D.; Gabriel, M.; Henninger, B.; Kendler, D.; Uprimny, C.; Dobrozemsky, G.; Decristoforo, C.; Bale, R.J.; Jaschke, W.; Virgolini, I.J. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J. Nucl. Med. 2009, 50, 1214–1221. [Google Scholar] [CrossRef]
- Versari, A.; Camellini, L.; Carlinfante, G.; Frasoldati, A.; Nicoli, F.; Grassi, E.; Gallo, C.; Giunta, F.P.; Fraternali, A.; Salvo, D.; et al. Ga-68 DOTATOC PET, endoscopic ultrasonography, and multidetector CT in the diagnosis of duodenopancreatic neuroendocrine tumors: A single-centre retrospective study. Clin. Nucl. Med. 2010, 35, 321–328. [Google Scholar] [CrossRef]
- Ruf, J.; Heuck, F.; Schiefer, J.; Denecke, T.; Elgeti, F.; Pascher, A.; Pavel, M.; Stelter, L.; Kropf, S.; Wiedenmann, B.; et al. Impact of Multiphase 68Ga-DOTATOC-PET/CT on therapy management in patients with neuroendocrine tumors. Neuroendocrinology 2010, 91, 101–109. [Google Scholar] [CrossRef]
- Putzer, D.; Gabriel, M.; Kendler, D.; Henninger, B.; Knoflach, M.; Kroiss, A.; Vonguggenberg, E.; Warwitz, B.; Virgolini, I.J. Comparison of 68Ga-DOTA-Tyr3-octreotide and 18F-fluoro-L-dihydroxyphenylalanine positron emission tomography in neuroendocrine tumor patients. Q. J. Nucl. Med. Mol. Imaging 2010, 54, 68–75. [Google Scholar]
- Ruf, J.; Schiefer, J.; Furth, C.; Kosiek, O.; Kropf, S.; Heuck, F.; Denecke, T.; Pavel, M.; Pascher, A.; Wiedenmann, B.; et al. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: Spotlight on the CT phases of a triple-phase protocol. J. Nucl. Med. 2011, 52, 697–704. [Google Scholar] [CrossRef]
- Kayani, I.; Bomanji, J.B.; Groves, A.; Conway, G.; Gacinovic, S.; Win, T.; Dickson, J.; Caplin, M.; Ell, P.J. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 2008, 112, 2447–2455. [Google Scholar] [CrossRef]
- Kayani, I.; Conry, B.G.; Groves, A.M.; Win, T.; Dickson, J.; Caplin, M.; Bomanji, J.B. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J. Nucl. Med. 2009, 50, 1927–1932. [Google Scholar] [CrossRef]
- Haug, A.; Auernhammer, C.J.; Wängler, B.; Tiling, R.; Schmidt, G.; Göke, B.; Bartenstein, P.; Pöpperl, G. Intraindividual comparison of 68Ga-DOTA-TATE and 18F-DOPA PET in patients with well-differentiated metastatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 765–770. [Google Scholar] [CrossRef]
- Srirajaskanthan, R.; Kayani, I.; Quigley, A.M.; Soh, J.; Caplin, M.E.; Bomanji, J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J. Nucl. Med. 2010, 51, 875–882. [Google Scholar] [CrossRef]
- Nakamoto, Y.; Sano, K.; Ishimori, T.; Ueda, M.; Temma, T.; Saji, H.; Togashi, K. Additional information gained by positron emission tomography with 68Ga-DOTATOC for suspected unknown primary or recurrent neuroendocrine tumors. Ann. Nucl. Med. 2015, 29, 512–518. [Google Scholar] [CrossRef]
- Venkitaraman, B.; Karunanithi, S.; Kumar, A.; Khilnani, G.C.; Kumar, R. Role of 68Ga-DOTATOC PET/CT in initial evaluation of patients with suspected bronchopulmonary carcinoid. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 856–864. [Google Scholar] [CrossRef]
- Menda, Y.; O’dorisio, T.M.; Howe, J.R.; Schultz, M.; Dillon, J.S.; Dick, D.; Watkins, G.L.; Ginader, T.; Bushnell, D.L.; Sunderland, J.J.; et al. Localization of Unknown Primary Site with 68Ga-DOTATOC PET/CT in Patients with Metastatic Neuroendocrine Tumor. J. Nucl. Med. 2017, 58, 1054–1057. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Garg, P.; Karunanithi, S.; Naswa, N.; Sharma, R.; Thulkar, S.; Lata, S.; Malhotra, A. Role of 68Ga-DOTATOC PET-CT in the diagnosis and staging of pancreatic neuroendocrine tumours. Eur. Radiol. 2011, 21, 2408–2416. [Google Scholar] [CrossRef]
- Beiderwellen, K.J.; Poeppel, T.D.; Hartung-Knemeyer, V.; Buchbender, C.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Simultaneous 68Ga-DOTATOC PET/MRI in patients with gastroenteropancreatic neuroendocrine tumors: Initial results. Investig. Radiol. 2013, 48, 273–279. [Google Scholar] [CrossRef]
- Schraml, C.; Schwenzer, N.F.; Sperling, O.; Aschoff, P.; Lichy, M.P.; Muller, M.; Brendle, C.; Werner, M.K.; Claussen, C.D.; Pfannenberg, C. Staging of neuroendocrine tumours: Comparison of [68Ga]DOTATOC multiphase PET/CT and whole-body MRI. Cancer Imaging 2013, 13, 63–72. [Google Scholar] [CrossRef]
- Hope, T.A.; Pampaloni, M.H.; Nakakura, E.; VanBrocklin, H.; Slater, J.; Jivan, S.; Aparici, C.M.; Yee, J.; Bergsland, E. Simultaneous 68Ga-DOTA-TOC PET/MRI with gadoxetate disodium in patients with neuroendocrine tumor. Abdom. Imaging 2015, 40, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, L.M.; Deuschl, C.; Beiderwellen, K.; Ruhlmann, V.; Poeppel, T.D.; Heusch, P.; Lahner, H.; Führer, D.; Bockisch, A.; Herrmann, K.; et al. Evaluation of 68Ga-DOTATOC PET/MRI for whole-body staging of neuroendocrine tumours in comparison with 68Ga-DOTATOC PET/CT. Eur. Radiol. 2017, 27, 4091–4099. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, S.M.; Neychev, V.; Millo, C.; Shih, J.; Nilubol, N.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Kebebew, E. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J. Clin. Oncol. 2016, 34, 588–596. [Google Scholar] [CrossRef]
- Sanli, Y.; Garg, I.; Kandathil, A.; Kendi, T.; Zanetti, M.J.B.; Kuyumcu, S.; Subramaniam, R.M. Neuroendocrine Tumor Diagnosis and Management: 68Ga-DOTATATE PET/CT. AJR Am. J. Roentgenol. 2018, 211, 267–277. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Jensen, R.T. Imaging of pancreatic neuroendocrine tumors: Recent advances, current status, and controversies. Expert Rev. Anticancer Ther. 2018, 18, 837–860. [Google Scholar] [CrossRef] [PubMed]
- Koukouraki, S.; Strauss, L.G.; Georgoulias, V.; Eisenhut, M.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Comparison of the pharmacokinetics of 68Ga-DOTATOC and [18F]FDG in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1115–1122. [Google Scholar] [CrossRef]
- Kumar, A.; Jindal, T.; Dutta, R.; Kumar, R. Functional imaging in differentiating bronchial masses: An initial experience with a combination of 18F-FDG PET-CT scan and 68Ga DOTA-TOC PET-CT scan. Ann. Nucl. Med. 2009, 23, 745–751. [Google Scholar] [CrossRef]
- Nilica, B.; Waitz, D.; Stevanovic, V.; Uprimny, C.; Kendler, D.; Buxbaum, S.; Warwitz, B.; Gerardo, L.; Henninger, B.; Virgolini, I.; et al. Direct comparison of 68Ga-DOTA-TOC and 18F-FDG PET/CT in the follow-up of patients with neuroendocrine tumour treated with the first full peptide receptor radionuclide therapy cycle. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1585–1592. [Google Scholar] [CrossRef]
- Lococo, F.; Rapicetta, C.; Mengoli, M.C.; Filice, A.; Paci, M.; Di Stefano, T.; Coruzzi, C.; Versari, A. Diagnostic performances of 68Ga-DOTATOC versus 18Fluorodeoxyglucose positron emission tomography in pulmonary carcinoid tumours and interrelationship with histological features. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 957–960. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chang, Y.-C.; Hwang, T.-L.; Chen, J.-S.; Chou, W.-C.; Hsieh, C.-H.; Yeh, T.-S.; Hsu, J.-T.; Yeh, C.-N.; Tseng, J.-H.; et al. 68Ga-DOTATOC and 18F-FDG PET/CT for identifying the primary lesions of suspected and metastatic neuroendocrine tumors: A prospective study in Taiwan. J. Formos. Med. Assoc. 2018, 117, 480–487. [Google Scholar] [CrossRef]
- Cingarlini, S.; Ortolani, S.; Salgarello, M.; Butturini, G.; Malpaga, A.; Malfatti, V.; D’Onofrio, M.; Davì, M.V.; Vallerio, P.; Ruzzenente, A.; et al. Role of Combined 68Ga-DOTATOC and 18F-FDG Positron Emission Tomography/Computed Tomography in the Diagnostic Workup of Pancreas Neuroendocrine Tumors: Implications for Managing Surgical Decisions. Pancreas 2017, 46, 42–47. [Google Scholar] [CrossRef]
- Rodrigues, M.; Svirydenka, H.; Virgolini, I. Theragnostics in Neuroendocrine Tumors. PET Clin. 2021, 16, 365–373. [Google Scholar] [CrossRef]
- Desai, H.; Borges-Neto, S.; Wong, T.Z. Molecular Imaging and Therapy for Neuroendocrine Tumors. Curr. Treat. Options Oncol. 2019, 20, 78. [Google Scholar] [CrossRef]
- Pollard, J.; McNeely, P.; Menda, Y. Nuclear Imaging of Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2020, 29, 209–221. [Google Scholar] [CrossRef]
- Gotthardt, M.; Dijkgraaf, I.; Boerman, O.C.; Oyen, W.J. Nuclear medicine imaging and therapy of neuroendocrine tumours. Cancer Imaging 2006, 6, S178–S184. [Google Scholar] [CrossRef]
- Kaltsas, G.; Rockall, A.; Papadogias, D.; Reznek, R.; Grossman, A.B. Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours. Eur. J. Endocrinol. 2004, 151, 15–27. [Google Scholar] [CrossRef]
- Koopmans, K.P.; Neels, O.N.; Kema, I.P.; Elsinga, P.H.; Links, T.P.; de Vries, E.G.; Jager, P.L. Molecular imaging in neuroendocrine tumors: Molecular uptake mechanisms and clinical results. Crit. Rev. Oncol. Hematol. 2009, 71, 199–213. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Krenning, E.P.; Lebtahi, R.; Komminoth, P.; Kos-Kudła, B.; de Herder, W.W.; Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology 2009, 90, 220–226. [Google Scholar] [CrossRef]
- Ramage, J.K.; Ahmed, A.; Ardill, J.; Bax, N.; Breen, D.J.; Caplin, M.E.; Corrie, P.; Davar, J.; Davies, A.H.; Lewington, V.; et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 2012, 61, 6–32. [Google Scholar] [CrossRef]
- Geijer, H.; Breimer, L.H. Somatostatin receptor PET/CT in neuroendocrine tumours: Update on systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1770–1780. [Google Scholar] [CrossRef]
- Frilling, A.; Sotiropoulos, G.C.; Radtke, A.; Malago, M.; Bockisch, A.; Kuehl, H.; Li, J.; Broelsch, C.E. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann. Surg. 2010, 252, 850–856. [Google Scholar] [CrossRef]
- Ambrosini, V.; Campana, D.; Bodei, L.; Nanni, C.; Castellucci, P.; Allegri, V.; Montini, G.C.; Tomassetti, P.; Paganelli, G.; Fanti, S. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J. Nucl. Med. 2010, 51, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Naswa, N.; Sharma, P.; Kumar, A.; Nazar, A.H.; Kumar, R.; Chumber, S.; Bal, C. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: A prospective single-center study. AJR Am. J. Roentgenol. 2011, 197, 1221–1228. [Google Scholar] [CrossRef]
- Krausz, Y.; Freedman, N.; Rubinstein, R.; Lavie, E.; Orevi, M.; Tshori, S.; Salmon, A.; Glaser, B.; Chisin, R.; Mishani, E.; et al. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: Comparison with 111In-DTPA-octreotide (OctreoScan(R)). Mol. Imaging Biol. 2011, 13, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Froeling, V.; Elgeti, F.; Maurer, M.H.; Scheurig-Muenkler, C.; Beck, A.; Kroencke, T.J.; Pape, U.-F.; Hamm, B.; Brenner, W.; Schreiter, N.F. Impact of Ga-68 DOTATOC PET/CT on the diagnosis and treatment of patients with multiple endocrine neoplasia. Ann. Nucl. Med. 2012, 26, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Kong, G.; Neels, O.C.; Eu, P.; Hong, E.; Hicks, R.J. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J. Med. Imaging Radiat. Oncol. 2012, 56, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Simsek, D.H.; Kuyumcu, S.; Turkmen, C.; Sanlı, Y.; Aykan, F.; Unal, S.; Adalet, I. Can complementary 68Ga-DOTATATE and 18F-FDG PET/CT establish the missing link between histopathology and therapeutic approach in gastroenteropancreatic neuroendocrine tumors? J. Nucl. Med. 2014, 55, 1811–1817. [Google Scholar] [CrossRef]
- Ilhan, H.; Fendler, W.P.; Cyran, C.C.; Spitzweg, C.; Auernhammer, C.J.; Gildehaus, F.-J.; Bartenstein, P.; Angele, M.K.; Haug, A.R. Impact of 68Ga-DOTATATE PET/CT on the surgical management of primary neuroendocrine tumors of the pancreas or ileum. Ann. Surg. Oncol. 2015, 22, 164–171. [Google Scholar] [CrossRef]
- Skoura, E.; Michopoulou, S.; Mohmaduvesh, M.; Panagiotidis, E.; Al Harbi, M.; Toumpanakis, C.; Almukhailed, O.; Kayani, I.; Syed, R.; Navalkissoor, S.; et al. The Impact of 68Ga-DOTATATE PET/CT Imaging on Management of Patients with Neuroendocrine Tumors: Experience from a National Referral Center in the United Kingdom. J. Nucl. Med. 2016, 57, 34–40. [Google Scholar] [CrossRef]
- Deppen, S.A.; Liu, E.; Blume, J.D.; Clanton, J.; Shi, C.; Jones-Jackson, L.B.; Lakhani, V.; Baum, R.P.; Berlin, J.; Smith, G.T.; et al. Safety and Efficacy of 68Ga-DOTATATE PET/CT for Diagnosis, Staging, and Treatment Management of Neuroendocrine Tumors. J. Nucl. Med. 2016, 57, 708–714. [Google Scholar] [CrossRef]
- Barrio, M.; Czernin, J.; Fanti, S.; Ambrosini, V.; Binse, I.; Du, L.; Eiber, M.; Herrmann, K.; Fendler, W.P. The Impact of Somatostatin Receptor-Directed PET/CT on the Management of Patients with Neuroendocrine Tumor: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2017, 58, 756–761. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Stefanova, M.; Mavriopoulou, E.; Holland-Letz, T.; Dimitrakopoulou-Strauss, A.; Afshar-Oromieh, A.; Mier, W.; Haberkorn, U.; Giesel, F.L. SUV of [68Ga]DOTATOC-PET/CT Predicts Response Probability of PRRT in Neuroendocrine Tumors. Mol. Imaging Biol. 2015, 17, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Mittra, E.S. Neuroendocrine Tumor Therapy: 177Lu-DOTATATE. AJR Am. J. Roentgenol. 2018, 211, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Kobayashi, N.; Takano, S.; Kato, I.; Endo, K.; Inoue, T. Neuroendocrine tumor theranostics. Cancer Sci. 2022, 113, 1930–1938. [Google Scholar] [CrossRef]
- Hicks, R.J.; Hofman, M.S. Is there still a role for SPECT-CT in oncology in the PET-CT era? Nat. Rev. Clin. Oncol. 2012, 9, 712–720. [Google Scholar] [CrossRef]
- Hope, T.A.; Calais, J.; Zhang, L.; Dieckmann, W.; Millo, C. 111In-Pentetreotide Scintigraphy Versus 68Ga-DOTATATE PET: Impact on Krenning Scores and Effect of Tumor Burden. J. Nucl. Med. 2019, 60, 1266–1269. [Google Scholar] [CrossRef]
- Weber, M.; Kessler, L.; Schaarschmidt, B.M.; Fendler, W.P.; Lahner, H.; Antoch, G.; Umutlu, L.; Herrmann, K.; Rischpler, C. Treatment-related changes in neuroendocrine tumors as assessed by textural features derived from 68Ga-DOTATOC PET/MRI with simultaneous acquisition of apparent diffusion coefficient. BMC Cancer 2020, 20, 326. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Kessler, L.; Schaarschmidt, B.; Fendler, W.P.; Lahner, H.; Antoch, G.; Umutlu, L.; Herrmann, K.; Rischpler, C. Textural analysis of hybrid DOTATOC-PET/MRI and its association with histological grading in patients with liver metastases from neuroendocrine tumors. Nucl. Med. Commun. 2020, 41, 363–369. [Google Scholar] [CrossRef]
- Gafita, A.; Calais, J.; Grogan, T.R.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R.; et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: An international, multicentre, retrospective study. Lancet Oncol. 2021, 22, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Bernard, E.J.; Schembri, G.; Roach, P.J.; Johnson, M.; Pavlakis, N.; Clarke, S.J.; Bailey, D.L. High Metabolic Tumour Volume on 18-Fluorodeoxyglucose Positron Emission Tomography Predicts Poor Survival from Neuroendocrine Neoplasms. Neuroendocrinology 2020, 110, 950–958. [Google Scholar] [CrossRef]
- Lim, S.M.; Kim, H.; Kang, B.; Kim, H.S.; Rha, S.Y.; Noh, S.H.; Hyung, W.J.; Cheong, J.-H.; Kim, H.-I.; Chung, H.C.; et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography in patients with gastric neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma. Ann. Nucl. Med. 2016, 30, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.; Wong, R.K.; Schaefferkoetter, J.; Veit-Haibach, P.; Myrehaug, S.; Juergens, R.; Laidley, D.; Anconina, R.; Liu, Z.A.; Metser, U. Quantitative 68Ga-DOTATATE PET/CT Parameters for the Prediction of Therapy Response in Patients with Progressive Metastatic Neuroendocrine Tumors Treated with 177Lu-DOTATATE. J. Nucl. Med. 2021, 62, 1406–1414. [Google Scholar] [CrossRef]
- Ebbers, S.C.; Heimgartner, M.; Barentsz, M.W.; van Leeuwaarde, R.S.; van Treijen, M.J.C.; Lam, M.M.E.G.; Braat, A.J.A.T. Gallium-68-somatostatin receptor PET/CT parameters as potential prognosticators for clinical time to progression after peptide receptor radionuclide therapy: A cohort study. Eur. J. Hybrid Imaging 2021, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Yoo, C.; Oh, S.J.; Lee, S.J.; Kang, J.; Hwang, H.-S.; Hong, S.-M.; Ryoo, B.-Y.; Ryu, J.-S. Tumour-to-liver ratio determined by [68Ga]Ga-DOTA-TOC PET/CT as a prognostic factor of lanreotide efficacy for patients with well-differentiated gastroenteropancreatic-neuroendocrine tumours. EJNMMI Res. 2020, 10, 63. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Johnbeck, C.B.; Loft, M.; Pfeifer, A.; Oturai, P.; Langer, S.W.; Knigge, U.; Ladefoged, C.N.; Kjaer, A. Semiautomatic Tumor Delineation for Evaluation of 64Cu-DOTATATE PET/CT in Patients with Neuroendocrine Neoplasms: Prognostication Based on Lowest Lesion Uptake and Total Tumor Volume. J. Nucl. Med. 2021, 62, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Stokmo, H.L.; Aly, M.; Lothe, I.M.B.; Borja, A.J.; Seraj, S.M.; Ghorpade, R.; Miao, X.; Hjortland, G.O.; Malinen, E.; Sorbye, H.; et al. Volumetric parameters from [18F]FDG PET/CT predicts survival in patients with high-grade gastroenteropancreatic neuroendocrine neoplasms. J. Neuroendocrinol. 2022, 34, e13170. [Google Scholar] [CrossRef]
- Satoh, K.; Sadowski, S.M.; Dieckmann, W.; Quezado, M.; Nilubol, N.; Kebebew, E.; Patel, D. 18F-FDG PET/CT Volumetric Parameters are Associated with Tumor Grade and Metastasis in Pancreatic Neuroendocrine Tumors in von Hippel-Lindau Disease. Ann. Surg. Oncol. 2016, 23, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Long, T.; Yang, Y.; Chen, D.; Hu, S. The Potential Prognostic Value of Dual-Imaging PET Parameters Based on 18F-FDG and 18F-OC for Neuroendocrine Neoplasms. Mol. Imaging 2022, 2022, 6511179. [Google Scholar] [CrossRef]
- Abdulrezzak, U.; Kurt, Y.K.; Kula, M.; Tutus, A. Combined imaging with 68Ga-DOTA-TATE and 18F-FDG PET/CT on the basis of volumetric parameters in neuroendocrine tumors. Nucl. Med. Commun. 2016, 37, 874–881. [Google Scholar] [CrossRef]
- Ohnona, J.; Nataf, V.; Gauthé, M.; Balogová, S.; Benesty, O.B.; Zhang-Yin, J.; Talbot, J.-N.; Montravers, F. Prognostic value of functional tumor burden on 68Ga-DOTATOC PET/CT in patients with pancreatic neuro-endocrine tumors. Neoplasma 2019, 66, 140–148. [Google Scholar] [CrossRef]
- Durmo, R.; Filice, A.; Fioroni, F.; Cervati, V.; Finocchiaro, D.; Coruzzi, C.; Besutti, G.; Fanello, S.; Frasoldati, A.; Versari, A. Predictive and Prognostic Role of Pre-Therapy and Interim 68Ga-DOTATOC PET/CT Parameters in Metastatic Advanced Neuroendocrine Tumor Patients Treated with PRRT. Cancers 2022, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Partelli, S.; Salgarello, M.; Doraku, J.; Muffatti, F.; Lena, M.S.; Pasetto, S.; Bezzi, C.; Bettinardi, V.; Andreasi, V.; et al. Dual Tracer 68Ga-DOTATOC and 18F-FDG PET Improve Preoperative Evaluation of Aggressiveness in Resectable Pancreatic Neuroendocrine Neoplasms. Diagnostics 2021, 11, 192. [Google Scholar] [CrossRef]
- Ohlsson, H.; Galne, A.; Tragardh, E.; Malmstrom, M.; Sundlov, A.; Almquist, M. Relationship between somatostatin receptor expressing tumour volume and health-related quality of life in patients with metastatic GEP-NET. J. Neuroendocrinol. 2022, 34, e13139. [Google Scholar] [CrossRef]
- Toriihara, A.; Baratto, L.; Nobashi, T.; Park, S.; Hatami, N.; Davidzon, G.; Kunz, P.L.; Iagaru, A. Prognostic value of somatostatin receptor expressing tumor volume calculated from 68Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2244–2251. [Google Scholar] [CrossRef]
- Liberini, V.; De Santi, B.; Rampado, O.; Gallio, E.; Dionisi, B.; Ceci, F.; Polverari, G.; Thuillier, P.; Molinari, F.; Deandreis, D. Impact of segmentation and discretization on radiomic features in 68Ga-DOTA-TOC PET/CT images of neuroendocrine tumor. EJNMMI Phys. 2021, 8, 21. [Google Scholar] [CrossRef]
- Reddy, R.P.; Schmidtlein, C.R.; Giancipoli, R.G.; Mauguen, A.; LaFontaine, D.; Schoder, H.; Bodei, L. The Quest for an Accurate Functional Tumor Volume with 68Ga-DOTATATE PET/CT. J. Nucl. Med. 2022, 63, 1027–1032. [Google Scholar] [CrossRef]
- Chen, L.; Jumai, N.; He, Q.; Liu, M.; Lin, Y.; Luo, Y.; Wang, Y.; Chen, M.-H.; Zeng, Z.; Zhang, X.; et al. The role of quantitative tumor burden based on [68Ga]Ga-DOTA-NOC PET/CT in well-differentiated neuroendocrine tumors: Beyond prognosis. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 525–534. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Sanli, Y.; Subramaniam, R.M. Impact of Point-Spread Function Reconstruction on 68Ga-DOTATATE PET/CT Quantitative Imaging Parameters. AJR Am. J. Roentgenol. 2019, 213, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Sigfridsson, J.; Lindström, E.; Iyer, V.; Holstensson, M.; Velikyan, I.; Sundin, A.; Lubberink, M. Prospective data-driven respiratory gating of [68Ga]Ga-DOTATOC PET/CT. EJNMMI Res. 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Papadakis, G.Z.; Millo, C.; Sadowski, S.M.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Yang, L.; Nockel, P.; Shell, J.; et al. Association between neuroendocrine tumors biomarkers and primary tumor site and disease type based on total 68Ga-DOTATATE-Avid tumor volume measurements. Eur. J. Endocrinol. 2017, 176, 575–582. [Google Scholar] [CrossRef]
- Tirosh, A.; Papadakis, G.Z.; Millo, C.; Hammoud, D.; Sadowski, S.M.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Yang, L.; Nockel, P.; et al. Prognostic Utility of Total 68Ga-DOTATATE-Avid Tumor Volume in Patients With Neuroendocrine Tumors. Gastroenterology 2018, 154, 998–1008. [Google Scholar] [CrossRef]
- Chan, H.; Moseley, C.; Zhang, L.; Bergsland, E.K.; Pampaloni, M.H.; Van Loon, K.; Hope, T.A. Correlation of DOTATOC Uptake and Pathologic Grade in Neuroendocrine Tumors. Pancreas 2019, 48, 948–952. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, Y.I. Prognostic Value of Maximum Standardized Uptake Value in 68Ga-Somatostatin Receptor Positron Emission Tomography for Neuroendocrine Tumors: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2019, 44, 777–783. [Google Scholar] [CrossRef]
- Panagiotidis, E.; Alshammari, A.; Michopoulou, S.; Skoura, E.; Naik, K.; Maragkoudakis, E.; Mohmaduvesh, M.; Al-Harbi, M.; Belda, M.; Caplin, M.E.; et al. Comparison of the Impact of 68Ga-DOTATATE and 18F-FDG PET/CT on Clinical Management in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Campana, D.; Ambrosini, V.; Pezzilli, R.; Fanti, S.; Labate, A.M.M.; Santini, D.; Ceccarelli, C.; Nori, F.; Franchi, R.; Corinaldesi, R.; et al. Standardized uptake values of 68Ga-DOTANOC PET: A promising prognostic tool in neuroendocrine tumors. J. Nucl. Med. 2010, 51, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Auernhammer, C.J.; Geisler, J.; Spitzweg, C.; Cyran, C.C.; Ilhan, H.; Bartenstein, P.; Haug, A. Treatment with octreotide in patients with well-differentiated neuroendocrine tumors of the ileum: Prognostic stratification with Ga-68-DOTA-TATE positron emission tomography. Mol. Imaging 2014, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Dekervel, J.; Verslype, C.; Clement, P.M.; Dooms, C.; Baete, K.; Goffin, K.; Jentjens, S.; Van Laere, K.; Van Cutsem, E.; et al. [68Ga]Ga-DOTATATE-avid tumor volume, uptake and inflammation-based index correlate with survival in neuroendocrine tumor patients treated with [177Lu]Lu-DOTATATE PRRT. Am. J. Nucl. Med. Mol. Imaging 2022, 12, 152–162. [Google Scholar]
- Graf, J.; Pape, U.-F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendocrine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lau, W.F.; Hicks, R.J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef]
- Speisky, D.; Duces, A.; Bièche, I.; Rebours, V.; Hammel, P.; Sauvanet, A.; Richard, S.; Bedossa, P.; Vidaud, M.; Murat, A.; et al. Molecular profiling of pancreatic neuroendocrine tumors in sporadic and Von Hippel-Lindau patients. Clin. Cancer Res. 2012, 18, 2838–2849. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Singh, A.; Schuchardt, C.; Kulkarni, H.R.; Baum, R.P. Prognostic Value of 18F-FDG PET/CT in a Large Cohort of Patients with Advanced Metastatic Neuroendocrine Neoplasms Treated with Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2020, 61, 1560–1569. [Google Scholar] [CrossRef]
- Ezziddin, S.; Adler, L.; Sabet, A.; Pöppel, T.D.; Grabellus, F.; Yüce, A.; Fischer, H.-P.; Simon, B.; Höller, T.; Biersack, H.-J.; et al. Prognostic stratification of metastatic gastroenteropancreatic neuroendocrine neoplasms by 18F-FDG PET: Feasibility of a metabolic grading system. J. Nucl. Med. 2014, 55, 1260–1266. [Google Scholar] [CrossRef]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef]
- Chan, D.L.; Ulaner, G.A.; Pattison, D.A.; Wyld, D.; Ladwa, R.; Kirchner, J.; Li, B.T.; Lai, W.V.; Pavlakis, N.; Roach, P.J.; et al. Dual PET Imaging in Bronchial Neuroendocrine Neoplasms: The NETPET Score as a Prognostic Biomarker. J. Nucl. Med. 2021, 62, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Lococo, F.; Perotti, G.; Cardillo, G.; De Waure, C.; Filice, A.; Graziano, P.; Rossi, G.; Sgarbi, G.; Stefanelli, A.; Giordano, A.; et al. Multicenter comparison of 18F-FDG and 68Ga-DOTA-peptide PET/CT for pulmonary carcinoid. Clin. Nucl. Med. 2015, 40, e183-9. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Gonzalez, R.S.; Zhao, Z.; Koyama, T.; Cornish, T.; Hande, K.R.; Walker, R.; Sandler, M.; Berlin, J.; Liu, E.H. Liver metastases of small intestine neuroendocrine tumors: Ki-67 heterogeneity and World Health Organization grade discordance with primary tumors. Am. J. Clin. Pathol. 2015, 143, 398–404. [Google Scholar] [CrossRef]
- Hou, J.; Yang, Y.; Chen, N.; Chen, D.; Hu, S. Prognostic Value of Volume-Based Parameters Measured by SSTR PET/CT in Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 771912. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.C.; Drymousis, P.; Flora, R.; Goldin, R.; Spalding, D.; Frilling, A. Role of Ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J. Surg. 2014, 38, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Bodei, L.; Kidd, M.; Modlin, I.M. Whither peptide receptor radionuclide therapy for neuroendocrine tumors: An Einsteinian view of the facts and myths. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1825–1830. [Google Scholar] [CrossRef]
- Kashyap, R.; Hofman, M.S.; Michael, M.; Kong, G.; Akhurst, T.; Eu, P.; Zannino, D.; Hicks, R.J. Favourable outcomes of 177Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 176–185. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J. Changing paradigms with molecular imaging of neuroendocrine tumors. Discov. Med. 2012, 14, 71–81. [Google Scholar]
- Cherk, M.H.; Kong, G.; Hicks, R.J.; Hofman, M.S. Changes in biodistribution on 68Ga-DOTA-Octreotate PET/CT after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging 2018, 18, 3. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Sadaghiani, M.S.; Sheikhbahaei, S.; Werner, R.A.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A.; Solnes, L.B.; Rowe, S.P. 177 Lu-PSMA radioligand therapy effectiveness in metastatic castration-resistant prostate cancer: An updated systematic review and meta-analysis. Prostate 2022, 82, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Emmett, L.; Rowe, S.P.; Herrmann, K.; Hadaschik, B.; Calais, J.; Giesel, F.L.; Reiter, R.; Maurer, T.; Heck, M.; et al. Second Version of the Prostate Cancer Molecular Imaging Standardized Evaluation Framework Including Response Evaluation for Clinical Trials (PROMISE V2). Eur. Urol. 2023, 83, 405–412. [Google Scholar] [CrossRef]
- Fanti, S.; Hadaschik, B.; Herrmann, K. Proposal for Systemic-Therapy Response-Assessment Criteria at the Time of PSMA PET/CT Imaging: The PSMA PET Progression Criteria. J. Nucl. Med. 2020, 61, 678–682. [Google Scholar] [CrossRef]
- Gafita, A.; Rauscher, I.; Weber, M.; Hadaschik, B.; Wang, H.; Armstrong, W.R.; Tauber, R.; Grogan, T.R.; Czernin, J.; Rettig, M.B.; et al. Novel Framework for Treatment Response Evaluation Using PSMA PET/CT in Patients with Metastatic Castration-Resistant Prostate Cancer (RECIP 1.0): An International Multicenter Study. J. Nucl. Med. 2022, 63, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef]
- Ilan, E.; Velikyan, I.; Sandström, M.; Sundin, A.; Lubberink, M. Tumor-to-Blood Ratio for Assessment of Somatostatin Receptor Density in Neuroendocrine Tumors Using 68Ga-DOTATOC and 68Ga-DOTATATE. J. Nucl. Med. 2020, 61, 217–221. [Google Scholar] [CrossRef]
- Velikyan, I.; Sundin, A.; Sörensen, J.; Lubberink, M.; Sandström, M.; Garske-Román, U.; Lundqvist, H.; Granberg, D.; Eriksson, B. Quantitative and Qualitative Intrapatient Comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: Net Uptake Rate for Accurate Quantification. J. Nucl. Med. 2014, 55, 204–210. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, M.; Telli, T.; Kersting, D.; Seifert, R. Prognostic Implications of PET-Derived Tumor Volume and Uptake in Patients with Neuroendocrine Tumors. Cancers 2023, 15, 3581. https://doi.org/10.3390/cancers15143581
Weber M, Telli T, Kersting D, Seifert R. Prognostic Implications of PET-Derived Tumor Volume and Uptake in Patients with Neuroendocrine Tumors. Cancers. 2023; 15(14):3581. https://doi.org/10.3390/cancers15143581
Chicago/Turabian StyleWeber, Manuel, Tugce Telli, David Kersting, and Robert Seifert. 2023. "Prognostic Implications of PET-Derived Tumor Volume and Uptake in Patients with Neuroendocrine Tumors" Cancers 15, no. 14: 3581. https://doi.org/10.3390/cancers15143581
APA StyleWeber, M., Telli, T., Kersting, D., & Seifert, R. (2023). Prognostic Implications of PET-Derived Tumor Volume and Uptake in Patients with Neuroendocrine Tumors. Cancers, 15(14), 3581. https://doi.org/10.3390/cancers15143581




