Methadone Potentiates the Cytotoxicity of Temozolomide by Impairing Calcium Homeostasis and Dysregulation of PARP in Glioblastoma Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Cell Viability Assay
2.3. Western Blotting
2.4. Flow Cytometry
2.5. Determination of Oxidative Stress, Mitochondrial Potential, Mitochondrial Volume and Intracellular Ca2+ Concentration
2.6. TUNEL Assay
2.7. Statistics
3. Results
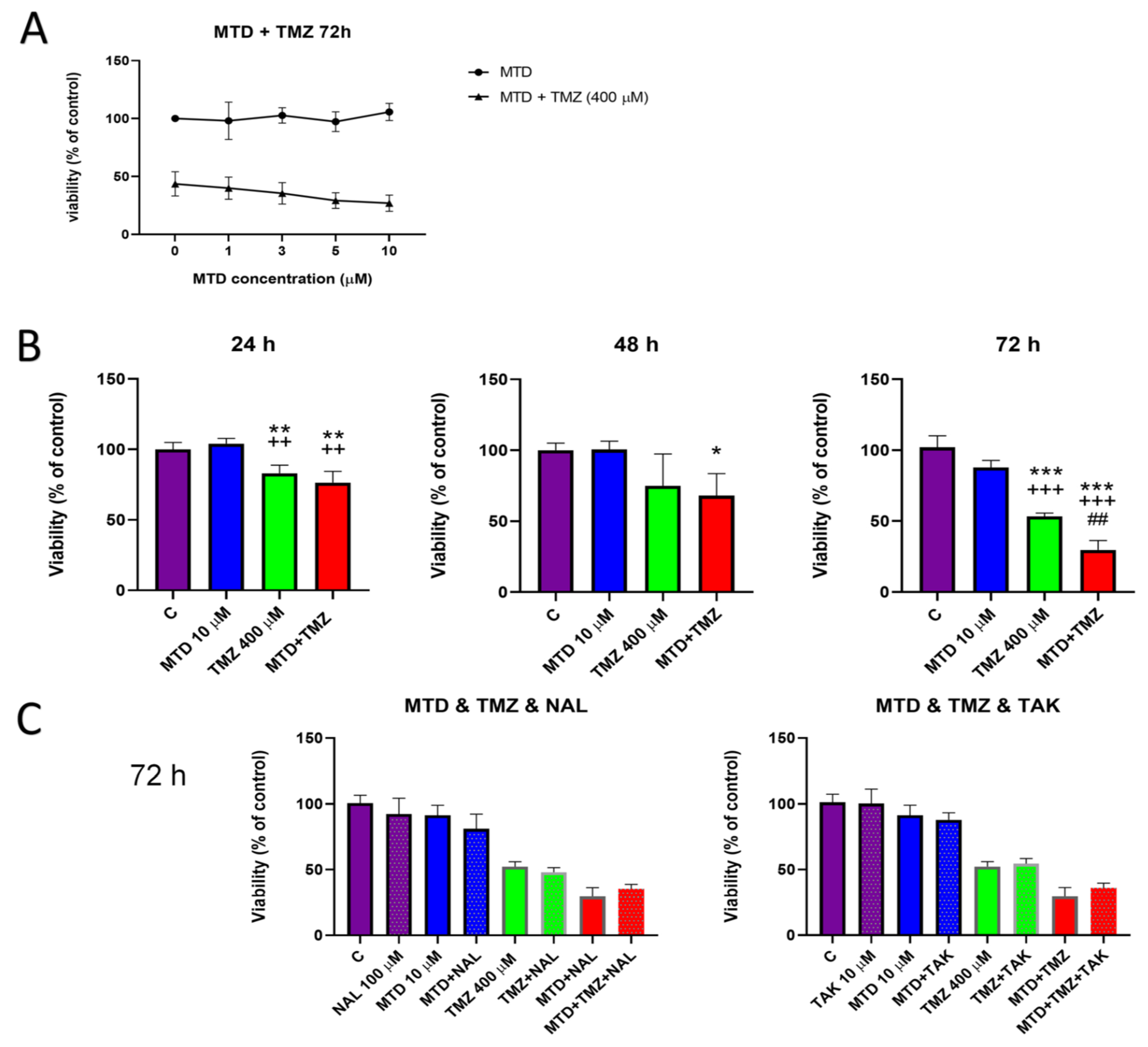
3.1. Effect of MTD on TMZ Efficacy in C6 Glioblastoma Cells
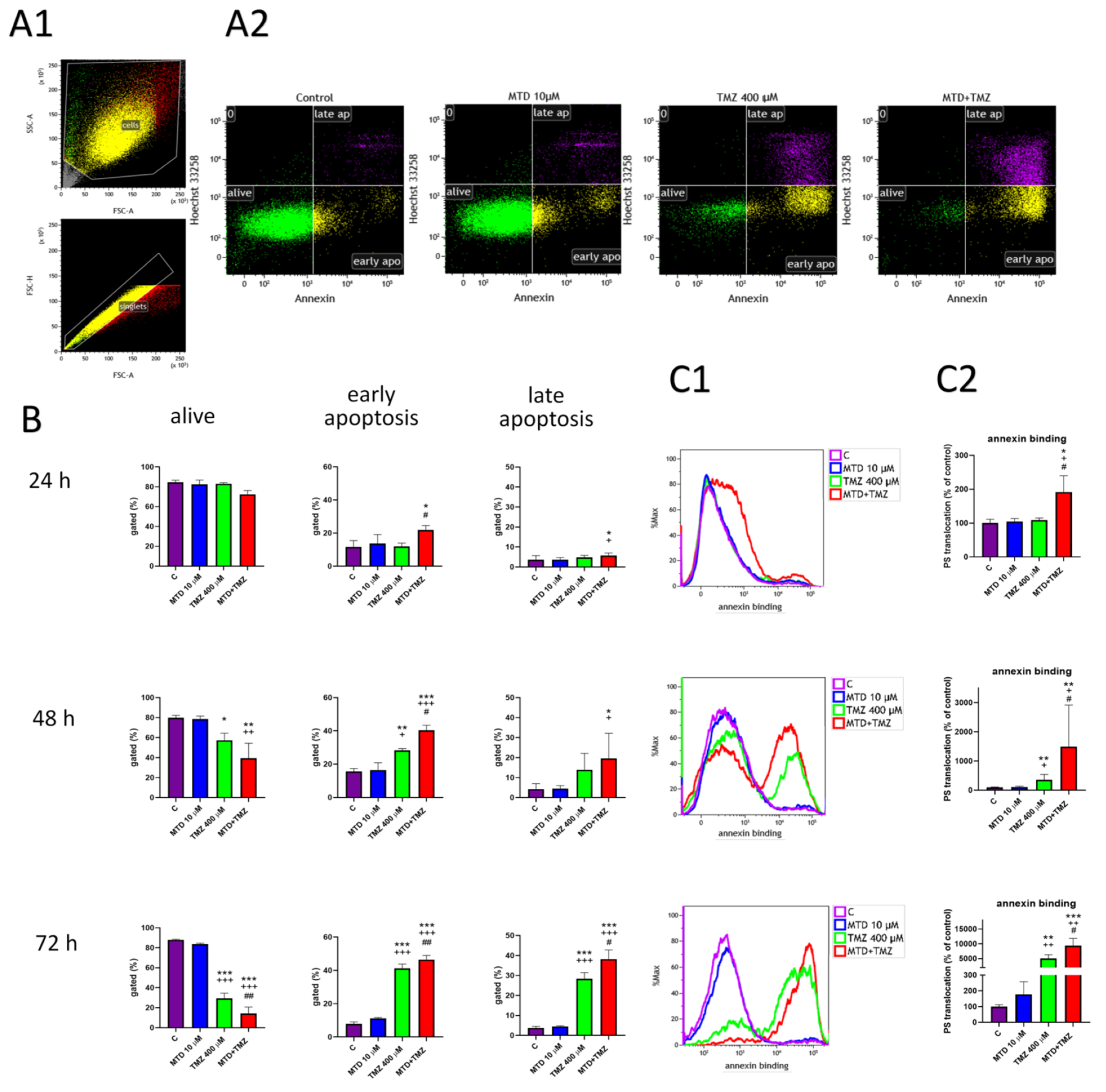
3.2. Effect of MDT and TMZ on Phosphatidylserine Localization in the Plasma Membrane
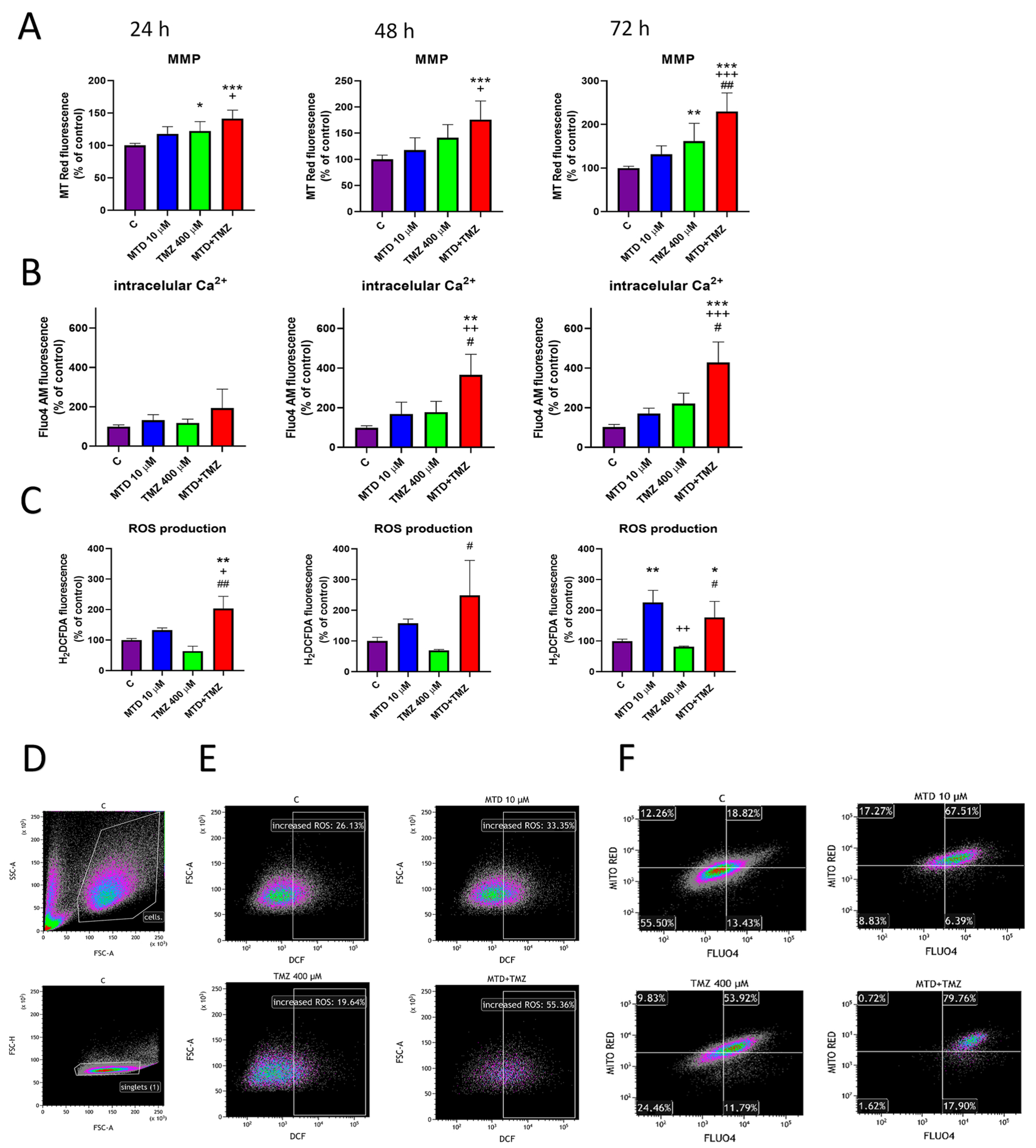
3.3. Effect of MTD and TMZ on Mitochondrial Membrane Potential, Cytosolic Ca2+ Level and ROS Production
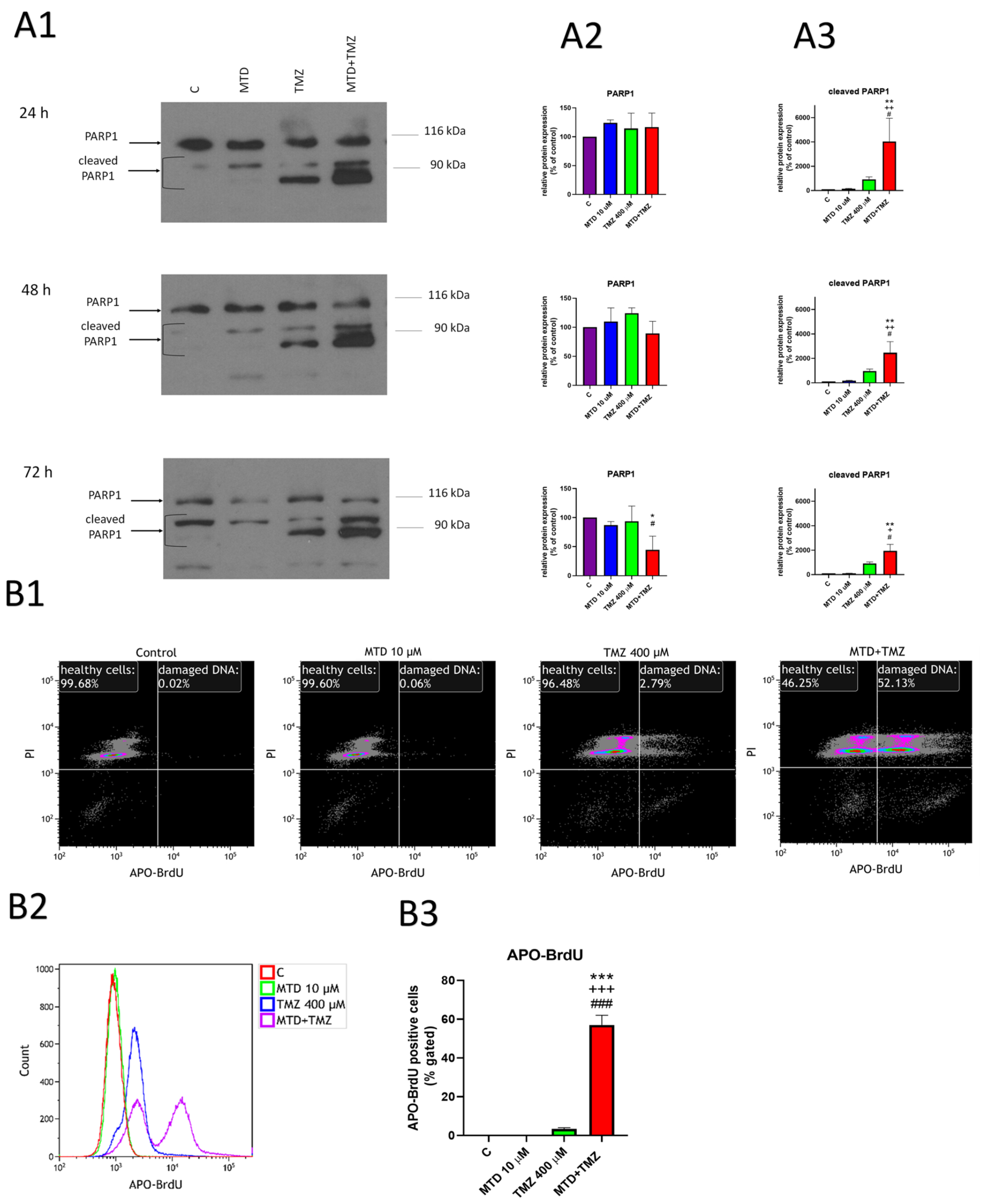
3.4. Effect of MTD and TMZ on PARP-1 Cleavage and DNA Fragmentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marrero, L.; Wyczechowska, D.; Musto, A.E.; Wilk, A.; Vashistha, H.; Zapata, A.; Walker, C.; Velasco-Gonzalez, C.; Parsons, C.; Wieland, S.; et al. Therapeutic Efficacy of Aldoxorubicin in an Intracranial Xenograft Mouse Model of Human Glioblastoma. Neoplasia 2014, 16, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Kočevar, N.; Komel, R. Glioma and Glioblastoma-How Much Do We (Not) Know? Mol. Clin. Oncol. 2013, 1, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Stavrovskaya, A.A.; Shushanov, S.S.; Rybalkina, E.Y. Problems of Glioblastoma Multiforme Drug Resistance. Biochemistry 2016, 81, 91–100. [Google Scholar] [CrossRef]
- Yi, G.Z.; Liu, Y.W.; Xiang, W.; Wang, H.; Chen, Z.Y.; Xie, S.D.; Qi, S.T. Akt and β-Catenin Contribute to TMZ Resistance and EMT of MGMT Negative Malignant Glioma Cell Line. J. Neurol. Sci. 2016, 367, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.R.; Nozell, S.E.; Diers, A.; McClugage, S.G.; Sarkaria, J.N.; Markert, J.M.; Darley-Usmar, V.M.; Bailey, S.M.; Gillespie, G.Y.; Landar, A.; et al. Acquisition of Temozolomide Chemoresistance in Gliomas Leads to Remodeling of Mitochondrial Electron Transport Chain. J. Biol. Chem. 2010, 285, 39759–39767. [Google Scholar] [CrossRef]
- Krantz, M.J.; Mehler, P.S. Treating Opioid Dependence: Growing Implications for Primary Care. Arch. Intern. Med. 2004, 164, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S. Opioid Titration in Cancer Pain: A Critical Review. Eur. J. Pain 2007, 11, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Brawanski, K.; Brockhoff, G.; Hau, P.; Vollmann-Zwerenz, A.; Freyschlag, C.; Lohmeier, A.; Riemenschneider, M.J.; Thomé, C.; Brawanski, A.; Proescholdt, M.A. Efficacy of D,L-Methadone in the Treatment of Glioblastoma in Vitro. CNS Oncol. 2018, 7, CNS18. [Google Scholar] [CrossRef]
- Friesen, C.; Hormann, I.; Roscher, M.; Fichtner, I.; Alt, A.; Hilger, R.; Debatin, K.M.; Miltner, E. Opioid Receptor Activation Triggering Downregulation of CAMP Improves Effectiveness of Anti-Cancer Drugs in Treatment of Glioblastoma. Cell Cycle 2014, 13, 1560–1570. [Google Scholar] [CrossRef]
- Friesen, C.; Roscher, M.; Hormann, I.; Fichtner, I.; Alt, A.; Hilger, R.A.; Debatin, K.M.; Miltner, E. Cell Death Sensitization of Leukemia Cells by Opioid Receptor Activation. Oncotarget 2013, 4, 677–690. [Google Scholar] [CrossRef]
- Landgraf, V.; Griessmann, M.; Roller, J.; Polednik, C.; Schmidt, M. DL-Methadone as an Enhancer of Chemotherapeutic Drugs in Head and Neck Cancer Cell Lines. Anticancer Res. 2019, 39, 3633–3639. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B.; Beltzig, L.; Piee-Staffa, A.; Haas, B. Cytotoxic and Senolytic Effects of Methadone in Combination with Temozolomide in Glioblastoma Cells. Int. J. Mol. Sci. 2020, 21, 7006. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.; Roscher, M.; Alt, A.; Miltner, E. Methadone, Commonly Used as Maintenance Medication for Outpatient Treatment of Opioid Dependence, Kills Leukemia Cells and Overcomes Chemoresistance. Cancer Res. 2008, 68, 6059–6064. [Google Scholar] [CrossRef] [PubMed]
- Tognoli, E.; Proto, P.L.; Motta, G.; Galeone, C.; Mariani, L.; Valenza, F. Methadone for Postoperative Analgesia: Contribution of N-Methyl-D-Aspartate Receptor Antagonism: A Randomised Controlled Trial. Eur. J. Anaesthesiol. 2020, 37, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Pakkanen, J.S.; Nousiainen, H.; Yli-Kauhaluoma, J.; Kylänlahti, I.; Möykkynen, T.; Korpi, E.R.; Peng, J.H.; Lukas, R.J.; Ahtee, L.; Tuominen, R.K. Methadone Increases Intracellular Calcium in SH-SY5Y and SH-EP1-Hα7 Cells by Activating Neuronal Nicotinic Acetylcholine Receptors. J. Neurochem. 2005, 94, 1329–1341. [Google Scholar] [CrossRef]
- Perez-Alvarez, S.; Solesio, M.E.; Cuenca-Lopez, M.D.; Melero-Fernndez De Mera, R.M.; Villalobos, C.; Kmita, H.; Galindo, M.F.; Jordán, J. Pharmacological Characterization of the Mechanisms Involved in Delayed Calcium Deregulation in SH-SY5Y Cells Challenged with Methadone. Int. J. Cell Biol. 2012, 2012, 642482. [Google Scholar] [CrossRef]
- Theile, D.; Mikus, G. Methadone against Cancer: Lost in Translation. Int. J. Cancer 2018, 143, 1840–1848. [Google Scholar] [CrossRef]
- Voelker, D.R. Phosphatidylserine Translocation to the Mitochondrion Is an ATP-Dependent Process in Permeabilized Animal Cells. Proc. Natl. Acad. Sci. USA 1989, 86, 9921–9925. [Google Scholar] [CrossRef]
- Monteiro, L.D.B.; Davanzo, G.G.; de Aguiar, C.F.; Moraes-Vieira, P.M.M. Using Flow Cytometry for Mitochondrial Assays. MethodsX 2020, 7, 100938. [Google Scholar] [CrossRef]
- Ledur, P.F.; Onzi, G.R.; Zong, H.; Lenz, G. Culture Conditions Defining Glioblastoma Cells Behavior: What Is the Impact for Novel Discoveries? Oncotarget 2017, 8, 69185. [Google Scholar] [CrossRef]
- Perez-Alvarez, S.; Iglesias-Guimarais, V.; Solesio, M.E.; Melero-Fernandez De Mera, R.M.; Yuste, V.J.; Galindo, M.F.; Jordán, J. Methadone Induces CAD Degradation and AIF-Mediated Necrotic-like Cell Death in Neuroblastoma Cells. Pharmacol. Res. 2011, 63, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Budd, S.L. Mitochondria and Neuronal Survival. Physiol. Rev. 2000, 80, 315–360. [Google Scholar] [CrossRef] [PubMed]
- Bagkos, G.; Koufopoulos, K.; Piperi, C. A New Model for Mitochondrial Membrane Potential Production and Storage. Med. Hypotheses 2014, 83, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A Mitochondrial Love-Hate Triangle. Am. J. Physiol. Cell Physiol. 2004, 287, 817–833. [Google Scholar] [CrossRef]
- Danson, S.J.; Middleton, M.R. Temozolomide: A Novel Oral Alkylating Agent. Expert Rev. Anticancer Ther. 2001, 1, 13–19. [Google Scholar] [CrossRef]
- Lomeli, N.; Di, K.; Pearre, D.C.; Chung, T.F.; Bota, D.A. Mitochondrial-Associated Impairments of Temozolomide on Neural Stem/Progenitor Cells and Hippocampal Neurons. Mitochondrion 2020, 52, 56–66. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.G.; Bradshaw, T.D. Temozolomide: Mechanisms of Action, Repair and Resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Gao, F.; de Groot, J.F.; Koul, D.; Yung, W.K.A. PARP-Mediated PARylation of MGMT Is Critical to Promote Repair of Temozolomide-Induced O6-Methylguanine DNA Damage in Glioblastoma. Neuro-Oncology 2021, 23, 920–931. [Google Scholar] [CrossRef]
- Zampieri, L.X.; Sboarina, M.; Cacace, A.; Grasso, D.; Thabault, L.; Hamelin, L.; Vazeille, T.; Dumon, E.; Rossignol, R.; Frédérick, R.; et al. Olaparib Is a Mitochondrial Complex i Inhibitor That Kills Temozolomide-Resistant Human Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 11938. [Google Scholar] [CrossRef]
- Oliva, C.R.; Moellering, D.R.; Gillespie, G.Y.; Griguer, C.E. Acquisition of Chemoresistance in Gliomas Is Associated with Increased Mitochondrial Coupling and Decreased ROS Production. PLoS ONE 2011, 6, e24665. [Google Scholar] [CrossRef]
- Mourdjeva, M.; Kyurkchiev, D.; Mandinova, A.; Altankova, I.; Kehayov, I.; Kyurkchiev, S. Dynamics of Membrane Translocation of Phosphatidylserine during Apoptosis Detected by a Monoclonal Antibody. Apoptosis 2005, 10, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Suzuki, J.; Segawa, K.; Fujii, T. Exposure of Phosphatidylserine on the Cell Surface. Cell Death Differ. 2016, 23, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.A.; Wilkison, S.; Qi, Q.; Chen, G.; Li, P.A. Mitochondrial Dysfunction Contributes to Rapamycininduced Apoptosis of Human Glioblastoma Cells-A Synergistic Effect with Temozolomide. Int. J. Med. Sci. 2020, 17, 2831–2843. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Pinton, P. The Mitochondrial Permeability Transition Pore and Cancer: Molecular Mechanisms Involved in Cell Death. Front. Oncol. 2014, 4, 302. [Google Scholar] [CrossRef]
- Yuan, Y.; Xue, X.; Guo, R.B.; Sun, X.L.; Hu, G. Resveratrol Enhances the Antitumor Effects of Temozolomide in Glioblastoma via ROS-Dependent AMPK-TSC-MTOR Signaling Pathway. CNS Neurosci. Ther. 2012, 18, 536–546. [Google Scholar] [CrossRef]
- Koo, H.N.; Hong, S.H.; Kim, C.Y.; Ahn, J.W.; Lee, Y.G.; Kim, J.J.; Lyu, Y.S.; Kim, H.M. Inhibitory Effect of Apoptosis in Human Astrocytes CCF-STTG1 Cells by Lemon Oil. Pharmacol. Res. 2002, 45, 469–473. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 Cleavage Fragments: Signatures of Cell-Death Proteases in Neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef]
- Prasad, C.B.; Prasad, S.B.; Yadav, S.S.; Pandey, L.K.; Singh, S.; Pradhan, S.; Narayan, G. Olaparib Modulates DNA Repair Efficiency, Sensitizes Cervical Cancer Cells to Cisplatin and Exhibits Anti-Metastatic Property. Sci. Rep. 2017, 7, 12876. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honc, O.; Novotny, J. Methadone Potentiates the Cytotoxicity of Temozolomide by Impairing Calcium Homeostasis and Dysregulation of PARP in Glioblastoma Cells. Cancers 2023, 15, 3567. https://doi.org/10.3390/cancers15143567
Honc O, Novotny J. Methadone Potentiates the Cytotoxicity of Temozolomide by Impairing Calcium Homeostasis and Dysregulation of PARP in Glioblastoma Cells. Cancers. 2023; 15(14):3567. https://doi.org/10.3390/cancers15143567
Chicago/Turabian StyleHonc, Ondrej, and Jiri Novotny. 2023. "Methadone Potentiates the Cytotoxicity of Temozolomide by Impairing Calcium Homeostasis and Dysregulation of PARP in Glioblastoma Cells" Cancers 15, no. 14: 3567. https://doi.org/10.3390/cancers15143567
APA StyleHonc, O., & Novotny, J. (2023). Methadone Potentiates the Cytotoxicity of Temozolomide by Impairing Calcium Homeostasis and Dysregulation of PARP in Glioblastoma Cells. Cancers, 15(14), 3567. https://doi.org/10.3390/cancers15143567







