Divergent Oxidative Stress in Normal Tissues and Inflammatory Cells in Hodgkin and Non-Hodgkin Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. PBMCs Isolation and Flow Cytometry Analysis
2.3. Seahorse Analysis
2.4. Enzymatic Assays
2.5. FDG Imaging
2.6. Statistics
3. Results
3.1. Demographic and Hematologic Data
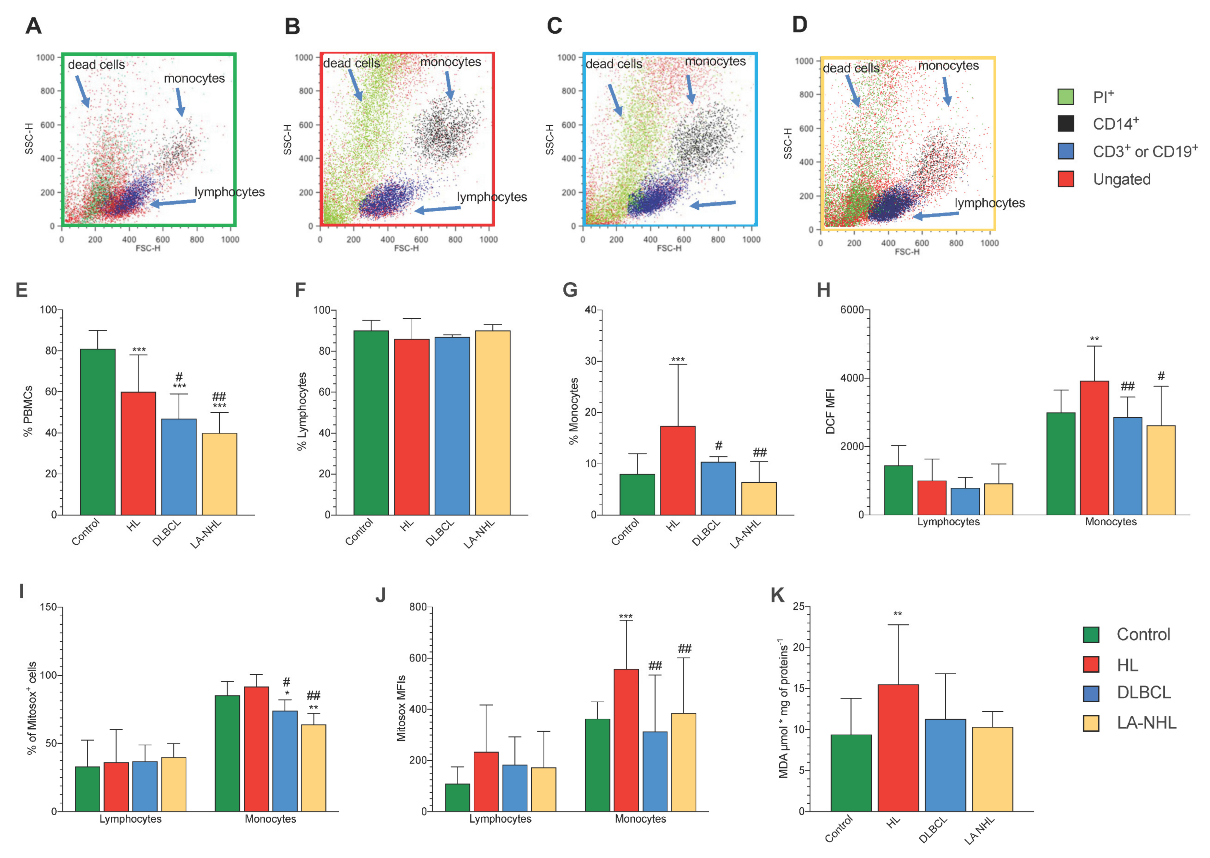
3.2. Redox Stress in PBMCs
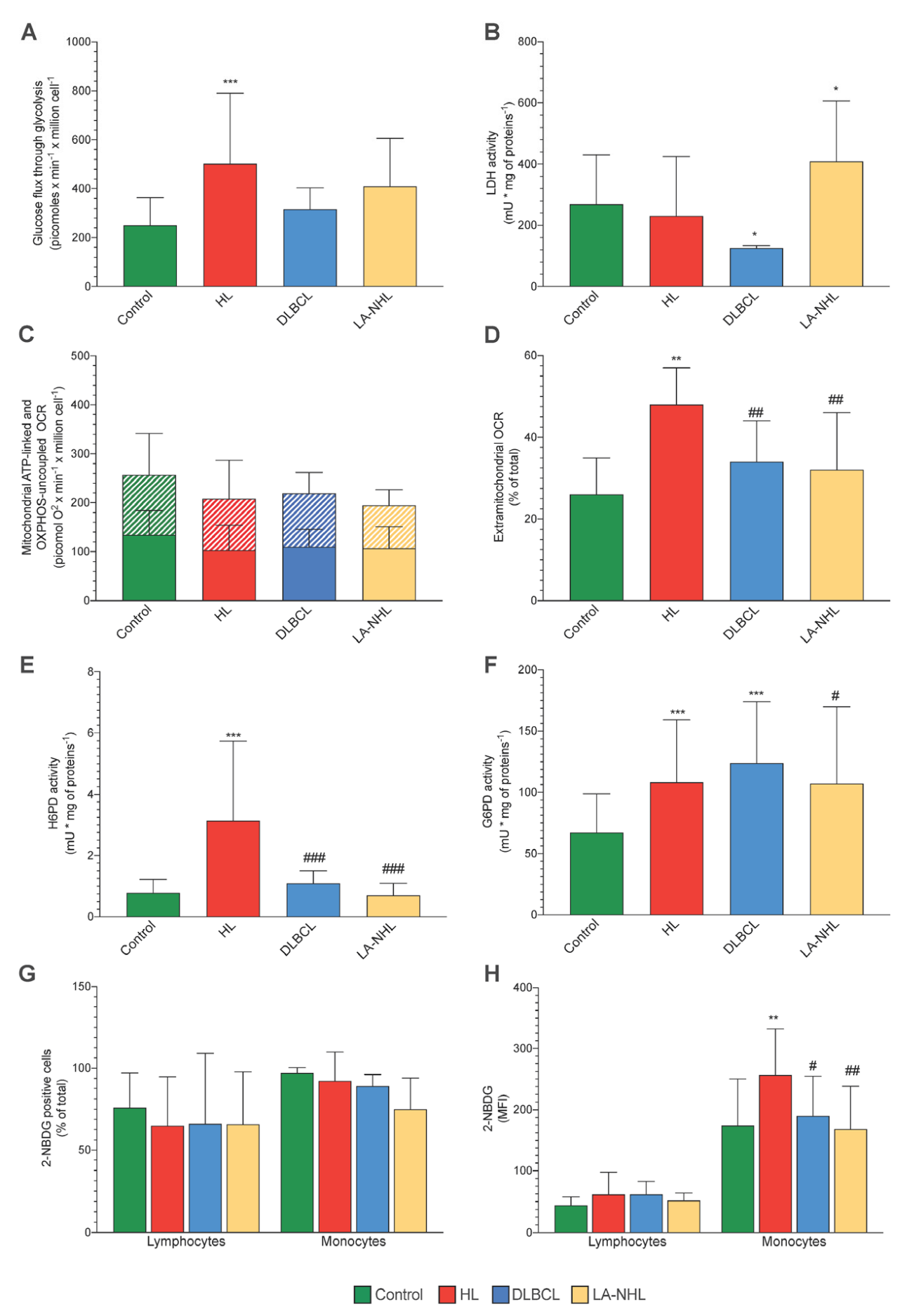
3.3. Energy Metabolism
3.4. ER-PPP Activity and H6PD Abundance in Naïve HL and NHL Patients
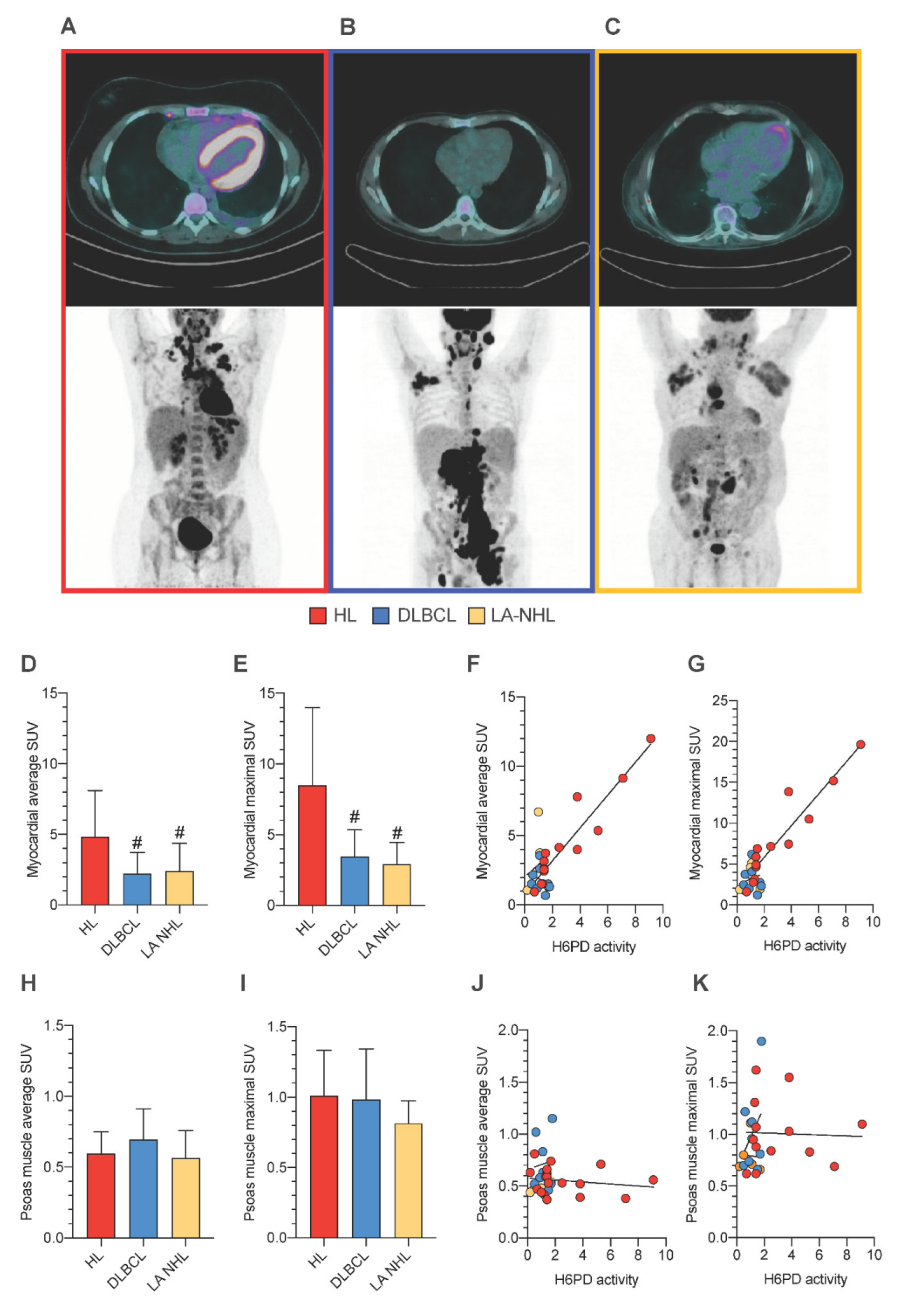
3.5. PBMCs Redox Stress and Tissue FDG Uptake
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marini, C.; Cossu, V.; Bauckneht, M.; Carta, S.; Lanfranchi, F.; D’Amico, F.; Ravera, S.; Orengo, A.M.; Ghiggi, C.; Ballerini, F.; et al. Mitochondrial Generated Redox Stress Differently Affects the Endoplasmic Reticulum of Circulating Lymphocytes and Monocytes in Treatment-Naïve Hodgkin’s Lymphoma. Antioxidants 2022, 11, 762. [Google Scholar] [CrossRef]
- Tullgren, O.; Giscombe, R.; Holm, G.; Johansson, B.; Mellstedt, H.; Björkholm, M. Increased luminol-enhanced chemiluminescence of blood monocytes and granulocytes in Hodgkin’s disease. Clin. Exp. Immunol. 1991, 85, 436–440. [Google Scholar] [CrossRef]
- Senesi, S.; Csala, M.; Marcolongo, P.; Fulceri, R.; Mandl, J.; Banhegyi, G.; Benedetti, A. Hexose-6-phosphate dehydrogenase in the endoplasmic reticulum. Biol. Chem. 2010, 391, 1–8. [Google Scholar]
- Marini, C.; Cossu, V.; Kumar, M.; Milanese, M.; Cortese, K.; Bruno, S.; Bellese, G.; Carta, S.; Zerbo, R.A.; Torazza, C.; et al. The Role of Endoplasmic Reticulum in the Differential Endurance against Redox Stress in Cortical and Spinal Astrocytes from the Newborn SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis. Antioxidants 2021, 10, 1392. [Google Scholar]
- Bauckneht, M.; Cossu, V.; Castellani, P.; Piccioli, P.; Orengo, A.M.; Emionite, L.; Di Giulio, F.; Donegani, M.I.; Miceli, A.; Raffa, S.; et al. FDG uptake tracks the oxidative damage in diabetic skeletal muscle: An experimental study. Mol. Metab. 2020, 31, 98–108. [Google Scholar]
- Cossu, V.; Bauckneht, M.; Bruno, S.; Orengo, A.M.; Emionite, L.; Balza, E.; Castellani, P.; Piccioli, P.; Miceli, A.; Raffa, S.; et al. The Elusive Link Between Cancer FDG Uptake and Glycolytic Flux Explains the Preserved Diagnostic Accuracy of PET/CT in Diabetes. Transl. Oncol. 2020, 13, 100752. [Google Scholar] [PubMed]
- Cossu, V.; Marini, C.; Piccioli, P.; Rocchi, A.; Bruno, S.; Orengo, A.M.; Emionite, L.; Bauckneht, M.; Grillo, F.; Capitanio, S.; et al. Obligatory role of endoplasmic reticulum in brain FDG uptake. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1184–1196. [Google Scholar] [PubMed]
- Marini, C.; Ravera, S.; Buschiazzo, A.; Bianchi, G.; Orengo, A.M.; Bruno, S.; Bottoni, G.; Emionite, L.; Pastorino, F.; Monteverde, E.; et al. Discovery of a novel glucose metabolism in cancer: The role of endoplasmic reticulum beyond glycolysis and pentose phosphate shunt. Sci. Rep. 2016, 6, 25092. [Google Scholar] [CrossRef]
- Caracó, C.; Aloj, L.; Chen, L.Y.; Chou, J.Y.; Eckelman, W.C. Cellular release of [18F]2-fluoro-2-deoxyglucose as a function of the glucose-6-phosphatase enzyme system. J. Biol. Chem. 2000, 275, 18489–18494. [Google Scholar] [CrossRef]
- Bauckneht, M.; Ferrarazzo, G.; Fiz, F.; Morbelli, S.; Sarocchi, M.; Pastorino, F.; Ghidella, A.; Pomposelli, E.; Miglino, M.; Ameri, P.; et al. Doxorubicin Effect on Myocardial Metabolism as a Prerequisite for Subsequent Development of Cardiac Toxicity: A Translational 18F-FDG PET/CT Observation. J. Nucl. Med. 2017, 58, 1638–1645. [Google Scholar] [PubMed]
- Bauckneht, M.; Pastorino, F.; Castellani, P.; Cossu, V.; Orengo, A.M.; Piccioli, P.; Emionite, L.; Capitanio, S.; Yosifov, N.; Bruno, S.; et al. Increased myocardial 18F-FDG uptake as a marker of Doxorubicin-induced oxidative stress. J. Nucl. Cardiol. 2020, 27, 2183–2194. [Google Scholar]
- Rodríguez-García, A.; García-Vicente, R.; Morales, M.L.; Ortiz-Ruiz, A.; Martínez-López, J.; Linares, M. Protein Carbonylation and, Lipid Peroxidation in Hematological Malignancies. Antioxidants 2020, 9, 1212. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T.; Ingravallo, G.; Specchia, G. Inflammatory microenvironment in classical Hodgkin’s lymphoma with special stress on mast cells. Front. Oncol. 2022, 12, 964573. [Google Scholar] [CrossRef]
- Imbesi, S.; Musolino, C.; Allegra, A.; Saija, A.; Morabito, F.; Calapai, G.; Gangemi, S. Oxidative stress in oncohematologic diseases: An update. Expert. Rev. Hematol. 2013, 6, 317–325. [Google Scholar] [PubMed]
- Vendrame, E.; Martínez-Maza, O. Assessment of pre-diagnosis biomarkers of immune activation and inflammation: Insights on the etiology of lymphoma. J. Proteome Res. 2011, 10, 113–119. [Google Scholar]
- Poppema, S.; Potters, M.; Emmens, R.; Visser, L.; van den Berg, A. Immune reactions in classical Hodgkin’s lymphoma. Semin. Hematol. 1999, 36, 253–259. [Google Scholar] [PubMed]
- Carta, S.; Penco, F.; Lavieri, R.; Martini, A.; Dinarello, C.A.; Gattorno, M.; Rubartelli, A. Cell stress increases ATP release in NLRP3 inflammasome-mediated autoinflammatory diseases, resulting in cytokine imbalance. Proc. Natl. Acad. Sci. USA 2015, 112, 2835–2840. [Google Scholar]
- Riedhammer, C.; Halbritter, D.; Weissert, R. Peripheral Blood Mononuclear Cells: Isolation, Freezing, Thawing, and Culture. Methods Mol. Biol. 2016, 1304, 53–61. [Google Scholar] [PubMed]
- Cappelli, E.; Bertola, N.; Bruno, S.; Degan, P.; Regis, S.; Corsolini, F.; Banelli, B.; Dufour, C.; Ravera, S. A Multidrug Approach to Modulate the Mitochondrial Metabolism Impairment and Relative Oxidative Stress in Fanconi Anemia Complementation Group A. Metabolites 2021, 12, 6. [Google Scholar] [PubMed]
- Jones, N.; Piasecka, J.; Bryant, A.H.; Jones, R.H.; Skibinski, D.O.; Francis, N.J.; Thornton, C.A. Bioenergetic analysis of human peripheral blood mononuclear cells. Clin. Exp. Immunol. 2015, 182, 69–80. [Google Scholar] [PubMed]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [PubMed]
- Marini, C.; Cossu, V.; Bonifacino, T.; Bauckneht, M.; Torazza, C.; Bruno, S.; Castellani, P.; Ravera, S.; Milanese, M.; Venturi, C.; et al. Mechanisms underlying the predictive power of high skeletal muscle uptake of FDG in amyotrophic lateral sclerosis. EJNMMI Res. 2020, 10, 76. [Google Scholar] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [PubMed]
- Inglese, E.; Leva, L.; Matheoud, R.; Sacchetti, G.; Secco, C.; Gandolfo, P.; Brambilla, M.; Sambuceti, G. Spatial and temporal heterogeneity of regional myocardial uptake in patients without heart disease under fasting conditions on repeated whole-body 18F-FDG PET/CT. J. Nucl. Med. 2007, 48, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Marini, C.; Bauckneht, M.; Borra, A.; Lai, R.; Donegani, M.I.; Miceli, A.; Campi, C.; Cossu, V.; Schenone, D.; Morbelli, S.; et al. Myocardial Metabolic Response Predicts Chemotherapy Curative Potential on Hodgkin Lymphoma: A Proof-of-Concept Study. Biomedicines 2021, 9, 971. [Google Scholar] [PubMed]
| Control Subjects | HL | DLBCL | LA-NHL | |
|---|---|---|---|---|
| Gender (M/F) | 24/21 | (17/11) | (6/3) | (4/3) |
| Age | 53 ± 12 | 54 ± 19 | 69 ± 16 | 65 ± 11 |
| Isolated PBMC (×106) | 20.2 ± 10.3 | 26.1 ± 19.5 | 20.2 ± 1.1 | 21.1 ± 11.7 |
| RBC (×106) | 4.6 ± 2.2 | 4.8 ± 0.6 | 4.5 ± 0.3 | 4.2 ± 0.7 |
| HB (g/L) | 141.2 ± 16.2 | 129.2 ± 15.7 | 126.2 ± 15.5 | 117 ± 22 |
| HCT (%) | 38.45 ± 6.2 | 39.2 ± 4.6 | 37.9 ± 4.8 | 34.8 ± 5.8 |
| PLT (×103) | 287.5 ± 116.2 | 295.2 ± 121.1 | 270.1 ± 144.9 | 214.5 ± 138 |
| ± | ||||
| WBC (×103) | 9.8 ± 4.2 | 9.4 ± 3.5 | 7.6 ± 2.8 | 9.6 ± 5.7 |
| Granulocytes (×103) | 4.2 ± 2.1 | 6.9 ± 3.4 | 5.6 ± 2.1 | 4.5 ± 2.7 |
| Lymphocytes (×103) | 1.5 ± 0.4 | 1.6 ± 0.7 | 1.2 ± 0.8 | 3.1 ± 2.4 |
| Monocytes (×103) | 0.6 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.5 ± 0.2 |
| Mono/Lympho | 0.5 ± 0.4 | 0.5 ± 0.4 | 0.93 ± 1.3 | 0.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, C.; Cossu, V.; Lanfranchi, F.; Carta, S.; Vitale, F.; D’Amico, F.; Bauckneht, M.; Morbelli, S.; Donegani, M.I.; Chiola, S.; et al. Divergent Oxidative Stress in Normal Tissues and Inflammatory Cells in Hodgkin and Non-Hodgkin Lymphoma. Cancers 2023, 15, 3533. https://doi.org/10.3390/cancers15133533
Marini C, Cossu V, Lanfranchi F, Carta S, Vitale F, D’Amico F, Bauckneht M, Morbelli S, Donegani MI, Chiola S, et al. Divergent Oxidative Stress in Normal Tissues and Inflammatory Cells in Hodgkin and Non-Hodgkin Lymphoma. Cancers. 2023; 15(13):3533. https://doi.org/10.3390/cancers15133533
Chicago/Turabian StyleMarini, Cecilia, Vanessa Cossu, Francesco Lanfranchi, Sonia Carta, Francesca Vitale, Francesca D’Amico, Matteo Bauckneht, Silvia Morbelli, Maria Isabella Donegani, Silvia Chiola, and et al. 2023. "Divergent Oxidative Stress in Normal Tissues and Inflammatory Cells in Hodgkin and Non-Hodgkin Lymphoma" Cancers 15, no. 13: 3533. https://doi.org/10.3390/cancers15133533
APA StyleMarini, C., Cossu, V., Lanfranchi, F., Carta, S., Vitale, F., D’Amico, F., Bauckneht, M., Morbelli, S., Donegani, M. I., Chiola, S., Raffa, S., Sofia, L., Di Raimondo, T., Ballerini, F., Ghiggi, C., Durando, P., Ravera, S., Riondato, M., Orengo, A. M., ... Sambuceti, G. (2023). Divergent Oxidative Stress in Normal Tissues and Inflammatory Cells in Hodgkin and Non-Hodgkin Lymphoma. Cancers, 15(13), 3533. https://doi.org/10.3390/cancers15133533











