Diagnosis, Management and Theragnostic Approach of Gastro-Entero-Pancreatic Neuroendocrine Neoplasms
Abstract
Simple Summary
Abstract
1. Introduction
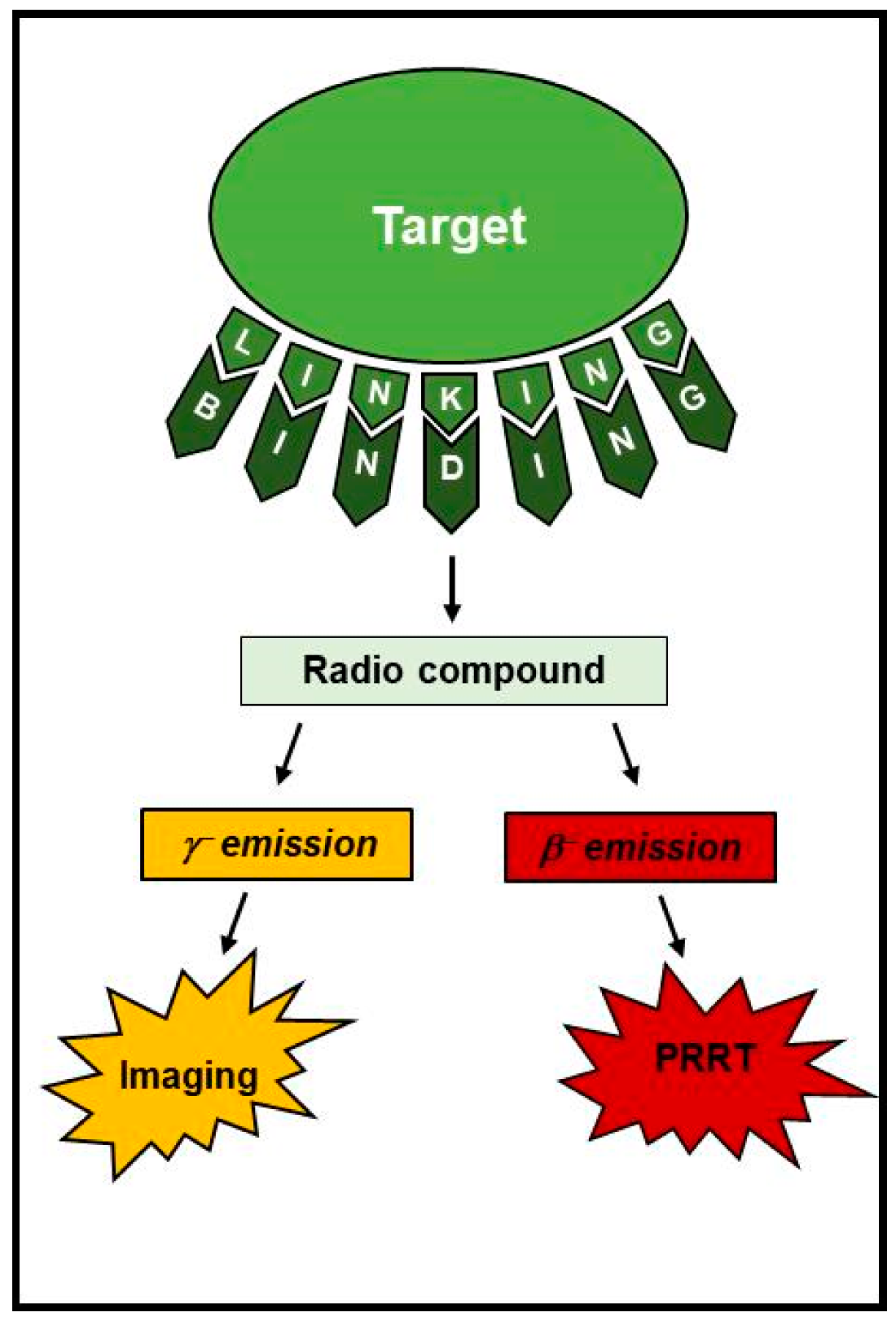
2. Nuclear Theragnostic
3. The Somatostatin Signaling
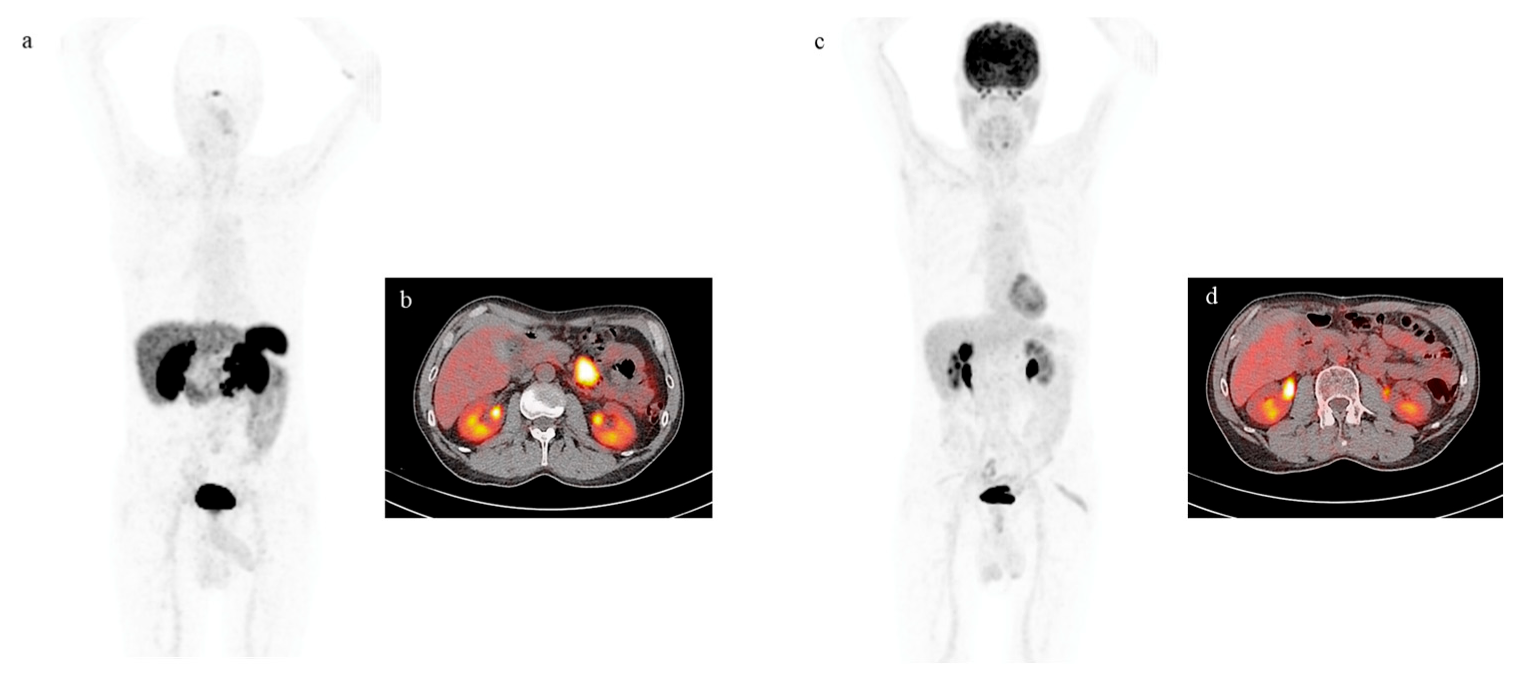
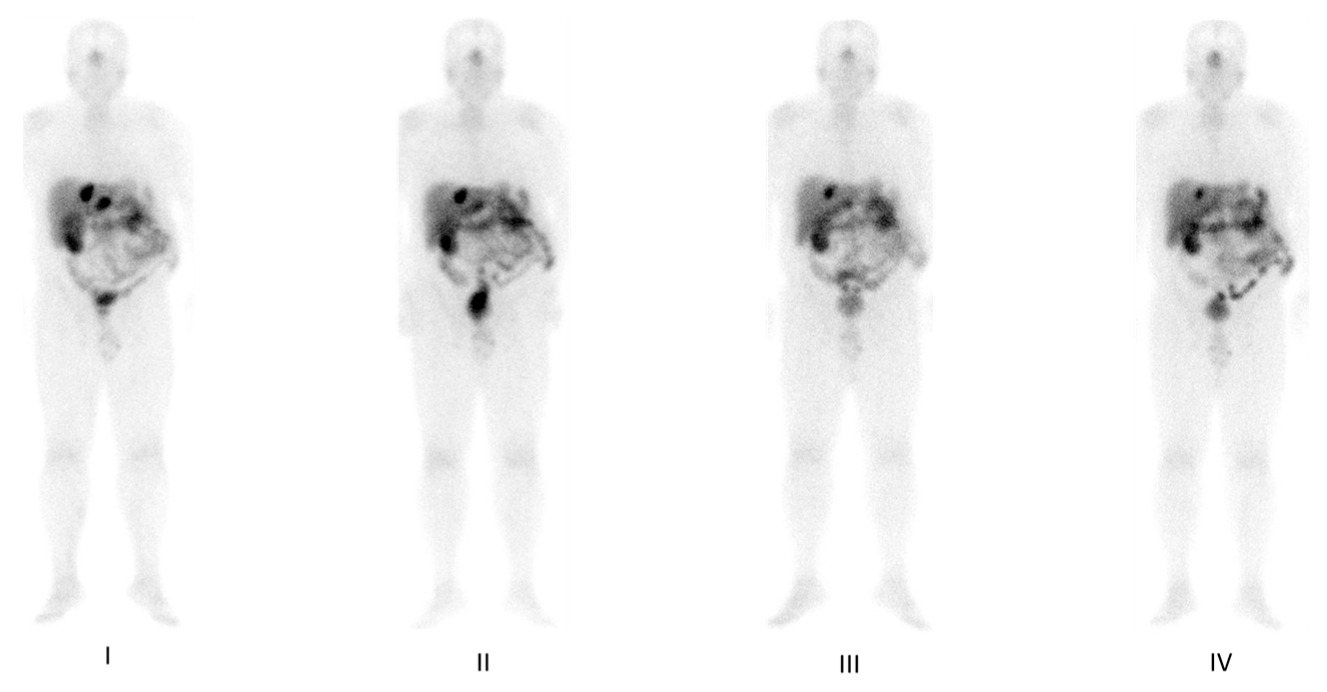
4. Functional Imaging by SST Analogs
5. Imaging Analysis
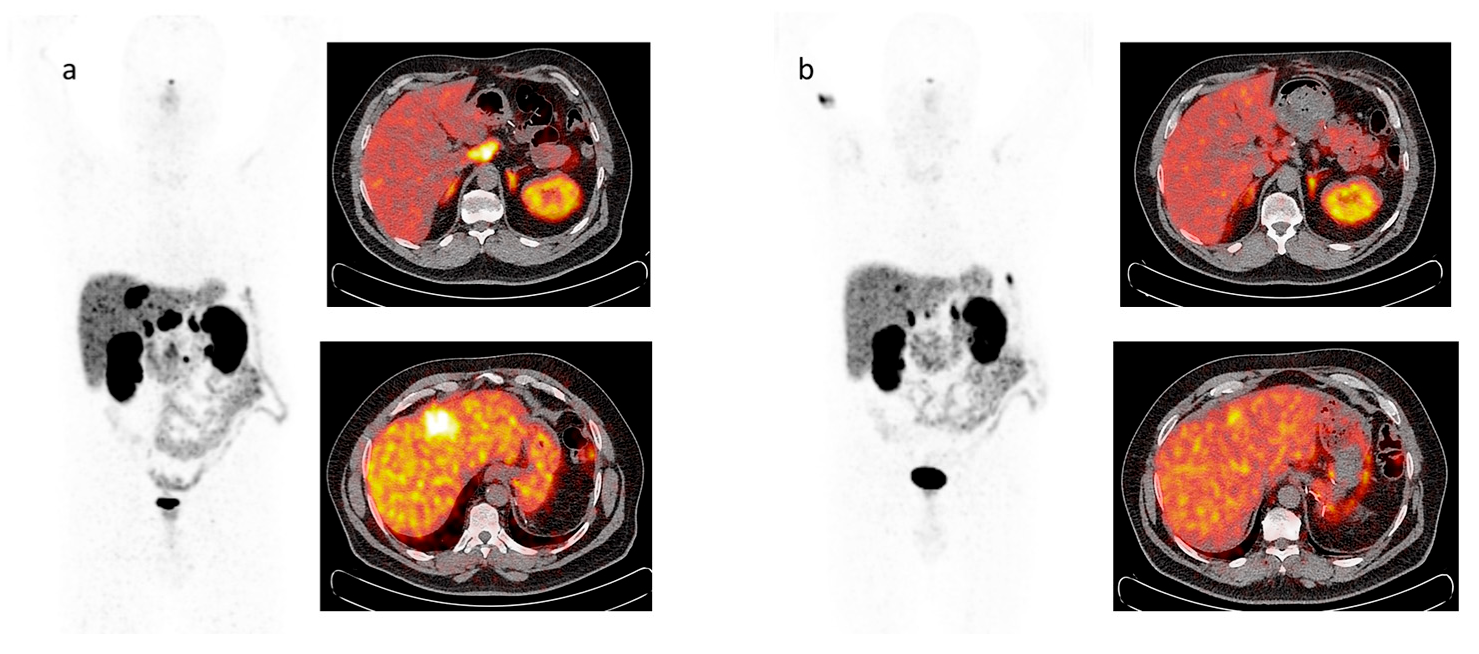
6. Functional Imaging by 18F-FDG PET/CT
7. PRRT by Radiolabeled Somatostatin Analogs
8. Response to Therapy: Which Criteria?
9. New Advances and Future Prospectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allìa, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.L.; Bussolati, G. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. Int. J. Pathol. 2002, 440, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Somatostatin and other Peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology 2004, 80 (Suppl. S1), 51–56. [Google Scholar] [CrossRef]
- Liberini, V.; Huellner, M.W.; Grimaldi, S.; Finessi, M.; Thuillier, P.; Muni, A.; Pellerito, R.E.; Papotti, M.G.; Piovesan, A.; Arvat, E.; et al. The Challenge of Evaluating Response to Peptide Receptor Radionuclide Therapy in Gastroenteropancreatic Neuroendocrine Tumors: The Present and the Future. Diagnostics 2020, 10, 1083. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Rodrigues, M.; Svirydenka, H.; Virgolini, I. Theragnostics in Neuroendocrine Tumors. PET Clin. 2021, 16, 365–373. [Google Scholar] [CrossRef]
- Hevesy, G.C. Radioactive tracers in radiobiological studies; the thirty-sixth Silvanus Thompson Memorial lecture. Br. J. Radiol. 1956, 29, 465–477. [Google Scholar] [CrossRef]
- Jadvar, H.; Chen, X.; Cai, W.; Mahmood, U. Radiotheranostics in Cancer Diagnosis and Management. Radiology 2018, 286, 388–400. [Google Scholar] [CrossRef]
- Vahidfar, N.; Eppard, E.; Farzanehfar, S.; Yordanova, A.; Fallahpoor, M.; Ahmadzadehfar, H. An Impressive Approach in Nuclear Medicine: Theranostics. PET Clin. 2021, 16, 327–340. [Google Scholar] [CrossRef]
- Klain, M.; Ricard, M.; Leboulleux, S.; Baudin, E.; Schlumberger, M. Radioiodine therapy for papillary and follicular thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2002, 29 (Suppl. S2), S479–S485. [Google Scholar] [CrossRef]
- Schlumberger, M.; Garcia, C.; Hadoux, J.; Klain, M.; Lamartina, L. Functional imaging in thyroid cancer patients with metastases and therapeutic implications. Presse Med. 2022, 51, 104113. [Google Scholar] [CrossRef]
- Klain, M.; Zampella, E.; Piscopo, L.; Volpe, F.; Manganelli, M.; Masone, S.; Pace, L.; Salvatore, D.; Schlumberger, M.; Cuocolo, A. Long-Term Prognostic Value of the Response to Therapy Assessed by Laboratory and Imaging Findings in Patients with Differentiated Thyroid Cancer. Cancers 2021, 13, 4338. [Google Scholar] [CrossRef]
- Silberstein, E.B. Radioiodine: The classic theranostic agent. Semin. Nucl. Med. 2012, 42, 164–170. [Google Scholar] [CrossRef]
- Gatto, F.; Hofland, L.J. The role of somatostatin and dopamine D2 receptors in endocrine tumors. Endocr. Relat. Cancer 2011, 18, R233–R251. [Google Scholar] [CrossRef]
- Ampofo, E.; Nalbach, L.; Menger, M.D.; Laschke, M.W. Regulatory Mechanisms of Somatostatin Expression. Int. J. Mol. Sci. 2020, 21, 4170. [Google Scholar] [CrossRef]
- Rorsman, P.; Huising, M.O. The somatostatin-secreting pancreatic δ-cell in health and disease. Nat. Rev. Endocrinol. 2018, 14, 404–414. [Google Scholar] [CrossRef]
- Hadjidakis, D.J.; Raptis, S.A.; Souvatzoglou, A.; Karaiskos, C.; Diamantopoulos, E.J.; Moulopoulos, S.D. Differences between somatostatin-28 and somatostatin-14 with respect to their biological effects in healthy humans and acromegalics. Clin. Physiol. Biochem. 1986, 4, 372–383. [Google Scholar]
- Cakir, M.; Dworakowska, D.; Grossman, A. Somatostatin receptor biology in neuroendocrine and pituitary tumours: Part 1—molecular pathways. J. Cell. Mol. Med. 2010, 14, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Latorraca, N.R.; Masureel, M.; Hollingsworth, S.A.; Heydenreich, F.M.; Suomivuori, C.M.; Brinton, C.; Townshend, R.J.L.; Bouvier, M.; Kobilka, B.K.; Dror, R.O. How GPCR Phosphorylation Patterns Orchestrate Arrestin-Mediated Signaling. Cell 2020, 183, 1813–1825.e18. [Google Scholar] [CrossRef]
- Stueven, A.K.; Kayser, A.; Wetz, C.; Amthauer, H.; Wree, A.; Tacke, F.; Wiedenmann, B.; Roderburg, C.; Jann, H. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int. J. Mol. Sci. 2019, 20, 3049. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J.; Vasiljevic, A.; Lapoirie, M.; Chinezu, L.; Jouanneau, E.; Raverot, G. Pathological markers of somatotroph pituitary neuroendocrine tumors predicting the response to medical treatment. Minerva Endocrinol. 2019, 44, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hankus, J.; Tomaszewska, R. Neuroendocrine neoplasms and somatostatin receptor subtypes expression. Nucl. Med. Rev. Cent. East. Eur. 2016, 19, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhuang, C.; Xu, J.; Wang, M.; Zhang, Z.Z.; Tu, L.; Wang, C.J.; Ling, T.L.; Cao, H.; Zhang, Z.G. Somatostatin receptors in gastrointestinal stromal tumors: New prognostic biomarker and potential therapeutic strategy. Am. J. Transl. Res. 2014, 6, 831–840. [Google Scholar]
- Krenning, E.P.; Bakker, W.H.; Breeman, W.A.; Koper, J.W.; Kooij, P.P.; Ausema, L.; Lameris, J.S.; Reubi, J.C.; Lamberts, S.W. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989, 1, 242–244. [Google Scholar] [CrossRef]
- Krenning, E.P.; Kwekkeboom, D.J.; Reubi, J.C.; van Hagen, P.M.; van Eijck, C.H.; Oei, H.Y.; Lamberts, S.W. 111In-octreotide scintigraphy in oncology. Digestion 1993, 54 (Suppl. S1), 84–87. [Google Scholar] [CrossRef]
- Krenning, E.P.; Kwekkeboom, D.J.; Bakker, W.H.; Breeman, W.A.; Kooij, P.P.; Oei, H.Y.; van Hagen, M.; Postema, P.T.; de Jong, M.; Reubi, J.C.; et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: The Rotterdam experience with more than 1000 patients. Eur. J. Nucl. Med. 1993, 20, 716–731. [Google Scholar] [CrossRef]
- Virgolini, I.; Traub-Weidinger, T.; Decristoforo, C. Nuclear medicine in the detection and management of pancreatic islet-cell tumours. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 213–227. [Google Scholar] [CrossRef]
- Lastoria, S.; Maurea, S.; Vergara, E.; Acampa, W.; Varrella, P.; Klain, M.; Muto, P.; Bernardy, J.D.; Salvatore, M. Comparison of labeled MIBG and somatostatin analogs in imaging neuroendocrine tumors. Q. J. Nucl. Med. 1995, 39, 145–149. [Google Scholar]
- De Herder, W.W.; Niederle, B.; Scoazec, J.-Y.; Pauwels, S.; Klöppel, G.; Falconi, M.; Kwekkeboom, D.J.; Öberg, K.; Eriksson, B.; Wiedenmann, B.; et al. Frascati Consensus Conference; European Neuroendocrine Tumor Society. Well-differentiated pancreatic tumor/carcinoma: Insulinoma. Neuroendocrinology 2006, 84, 183–188. [Google Scholar] [CrossRef]
- Bombardieri, E.; Coliva, A.; Maccauro, M.; Seregni, E.; Orunesu, E.; Chiti, A.; Lucignani, G. Imaging of neuroendocrine tumours with gamma-emitting radiopharmaceuticals. Q. J. Nucl. Med. Mol. Imaging 2010, 54, 3–15. [Google Scholar]
- Pepe, G.; Moncayo, R.; Bombardieri, E.; Chiti, A. Somatostatin receptor SPECT. Eur. J. Nucl. Med. Mol. Imaging 2012, 39 (Suppl. S1), S41–S51. [Google Scholar] [CrossRef]
- Geijer, H.; Breimer, L.H. Somatostatin receptor PET/CT in neuroendocrine tumours: Update on systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1770–1780. [Google Scholar] [CrossRef]
- Herrmann, K.; Czernin, J.; Wolin, E.M.; Gupta, P.; Barrio, M.; Gutierrez, A.; Schiepers, C.; Mosessian, S.; Phelps, M.E.; Allen-Auerbach, M.S. Impact of 68Ga-DOTATATE PET/CT on the management of neuroendocrine tumors: The referring physician’s perspective. J. Nucl. Med. 2015, 56, 70–75. [Google Scholar] [CrossRef]
- Kumar, K. The Current Status of the Production and Supply of Gallium-68. Cancer Biother. Radiopharm. 2020, 35, 163–166. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schär, J.C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Liu, Q.; Laissue, J.A.; Schonbrunn, A. Subcellular distribution of somatostatin sst2A receptors in human tumors of the nervous and neuroendocrine systems: Membranous versus intracellular location. J. Clin. Endocrinol. Metab. 2000, 85, 3882–3891. [Google Scholar] [CrossRef]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef]
- Frilling, A.; Sotiropoulos, G.C.; Radtke, A.; Malago, M.; Bockisch, A.; Kuehl, H.; Li, J.; Broelsch, C.E. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann. Surg. 2010, 252, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Ambrosini, V.; Hommann, M.; Hoersch, D.; Fanti, S.; Baum, R.P. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68)Ga-DOTA-NOC receptor PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 67–77. [Google Scholar] [CrossRef]
- Ambrosini, V.; Nanni, C.; Zompatori, M.; Campana, D.; Tomassetti, P.; Castellucci, P.; Allegri, V.; Rubello, D.; Montini, G.; Franchi, R.; et al. (68)Ga-DOTA-NOC PET/CT in comparison with CT for the detection of bone metastasis in patients with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Putzer, D.; Gabriel, M.; Henninger, B.; Kendler, D.; Uprimny, C.; Dobrozemsky, G.; Decristoforo, C.; Bale, R.J.; Jaschke, W.; Virgolini, I.J. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J. Nucl. Med. 2009, 50, 1214–1221. [Google Scholar] [CrossRef]
- Hope, T.A.; Allen-Auerbach, M.; Bodei, L.; Calais, J.; Dahlbom, M.; Dunnwald, L.K.; Graham, M.M.; Jacene, H.A.; Heath, C.L.; Mittra, E.S.; et al. SNMMI Procedure Standard/EANM Practice Guideline for SSTR PET: Imaging Neuroendocrine Tumors. J. Nucl. Med. 2023, 64, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Opalińska, M.; Morawiec-Sławek, K.; Kania-Kuc, A.; Al Maraih, I.; Sowa-Staszczak, A.; Hubalewska-Dydejczyk, A. Potential value of pre- and post-therapy [68Ga]Ga-DOTA-TATE PET/CT in the prognosis of response to PRRT in disseminated neuroendocrine tumors. Front. Endocrinol. 2022, 13, 929391. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of Atherosclerotic Inflammation by 68Ga-DOTATATE PET Compared to [18F]FDG PET Imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef]
- Kuyumcu, S.; Özkan, Z.G.; Sanli, Y.; Yilmaz, E.; Mudun, A.; Adalet, I.; Unal, S. Physiological and tumoral uptake of (68)Ga-DOTATATE: Standardized uptake values and challenges in interpretation. Ann. Nucl. Med. 2013, 27, 538–545. [Google Scholar] [CrossRef]
- Bombardieri, E.; Ambrosini, V.; Aktolun, C.; Baum, R.P.; Bishof-Delaloye, A.; Del Vecchio, S.; Maffioli, L.; Mortelmans, L.; Oyen, W.; Pepe, G.; et al. 111In-pentetreotide scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1441–1448. [Google Scholar] [CrossRef]
- Menon, B.K.; Kalshetty, A.; Bhattacharjee, A.; Basu, S. Standardized uptake values and ratios on 68Ga-DOTATATE PET-computed tomography for normal organs and malignant lesions and their correlation with Krenning score in patients with metastatic neuroendocrine tumors. Nucl. Med. Commun. 2020, 41, 1095–1099. [Google Scholar] [CrossRef]
- Hope, T.A.; Calais, J.; Zhang, L.; Dieckmann, W.; Millo, C. 111In-Pentetreotide Scintigraphy Versus 68Ga-DOTATATE PET: Impact on Krenning Scores and Effect of Tumor Burden. J. Nucl. Med. 2019, 60, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Solnes, L.B.; Javadi, M.S.; Weich, A.; Gorin, M.A.; Pienta, K.J.; Higuchi, T.; Buck, A.K.; Pomper, M.G.; Rowe, S.P.; et al. SSTR-RADS Version 1.0 as a Reporting System for SSTR PET Imaging and Selection of Potential PRRT Candidates: A Proposed Standardization Framework. J. Nucl. Med. 2018, 59, 1085–1091. [Google Scholar] [CrossRef]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. PSMA-RADS Version 1.0: A Step Towards Standardizing the Interpretation and Reporting of PSMA-targeted PET Imaging Studies. Eur. Urol. 2018, 73, 485–487. [Google Scholar] [CrossRef]
- Abdulrezzak, U.; Kurt, Y.K.; Kula, M.; Tutus, A. Combined imaging with 68Ga-DOTA-TATE and 18F-FDG PET/CT on the basis of volumetric parameters in neuroendocrine tumors. Nucl. Med. Commun. 2016, 37, 874–881. [Google Scholar] [CrossRef]
- Becker, J.; Schwarzenböck, S.M.; Krause, B.J. FDG PET Hybrid Imaging. Recent. Results Cancer Res. 2020, 216, 625–667. [Google Scholar] [CrossRef] [PubMed]
- Kletter, K.; Becherer, A. FDG-PET in der Onkologie. Methodische Grundlagen und klinische Anwendung [FDG-PET in oncology. Methodological principles and clinical applications]. Radiologe 1999, 39, 600–609. [Google Scholar] [CrossRef]
- Pinilla, I.; Rodríguez-Vigil, B.; Gómez-León, N. Integrated FDG PET/CT: Utility and Applications in Clinical Oncology. Clin. Med. Oncol. 2008, 2, 181–198. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Bahri, H.; Laurence, L.; Edeline, J.; Leghzali, H.; Devillers, A.; Raoul, J.L.; Cuggia, M.; Mesbah, H.; Clement, B.; Boucher, E.; et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: A long-term evaluation. J. Nucl. Med. 2014, 55, 1786–1790. [Google Scholar] [CrossRef]
- Garin, E.; Le Jeune, F.; Devillers, A.; Cuggia, M.; de Lajarte-Thirouard, A.S.; Bouriel, C.; Boucher, E.; Raoul, J.L. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J. Nucl. Med. 2009, 50, 858–864. [Google Scholar] [CrossRef]
- Panagiotidis, E.; Bomanji, J. Role of 18F-fluorodeoxyglucose PET in the study of neuroendocrine tumors. PET Clin. 2014, 9, 43–55. [Google Scholar] [CrossRef]
- Calabrò, D.; Argalia, G.; Ambrosini, V. Role of PET/CT and Therapy Management of Pancreatic Neuroendocrine Tumors. Diagnostics 2020, 10, 1059. [Google Scholar] [CrossRef]
- Tchou, J.; Sonnad, S.S.; Bergey, M.R.; Basu, S.; Tomaszewski, J.; Alavi, A.; Schnall, M. Degree of tumor FDG uptake correlates with proliferation index in triple negative breast cancer. Mol. Imaging Biol. 2010, 12, 657–662. [Google Scholar] [CrossRef]
- Sundin, A. Novel Functional Imaging of Neuroendocrine Tumors. Endocrinol. Metab. Clin. N. Am. 2018, 47, 505–523. [Google Scholar] [CrossRef]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M.; et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Johnbeck, C.B.; Loft, A.; Berthelsen, A.K.; Oturai, P.; Mortensen, J.; Federspiel, B.; Langer, S.W.; Kjaer, A. 18F-FDG PET is Superior to WHO Grading as a Prognostic Tool in Neuroendocrine Neoplasms and Useful in Guiding PRRT: A Prospective 10-Year Follow-up Study. J. Nucl. Med. 2021, 62, 808–815. [Google Scholar] [CrossRef]
- Cingarlini, S.; Ortolani, S.; Salgarello, M.; Butturini, G.; Malpaga, A.; Malfatti, V.; D’Onofrio, M.; Davì, M.V.; Vallerio, P.; Ruzzenente, A.; et al. Role of Combined 68Ga-DOTATOC and 18F-FDG Positron Emission Tomography/Computed Tomography in the Diagnostic Workup of Pancreas Neuroendocrine Tumors: Implications for Managing Surgical Decisions. Pancreas 2017, 46, 42–47. [Google Scholar] [CrossRef]
- Sansovini, M.; Severi, S.; Ianniello, A.; Nicolini, S.; Fantini, L.; Mezzenga, E.; Ferroni, F.; Scarpi, E.; Monti, M.; Bongiovanni, A.; et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 490–499. [Google Scholar] [CrossRef]
- Severi, S.; Nanni, O.; Bodei, L.; Sansovini, M.; Ianniello, A.; Nicoletti, S.; Scarpi, E.; Matteucci, F.; Gilardi, L.; Paganelli, G. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 881–888. [Google Scholar] [CrossRef]
- Nilica, B.; Waitz, D.; Stevanovic, V.; Uprimny, C.; Kendler, D.; Buxbaum, S.; Warwitz, B.; Gerardo, L.; Henninger, B.; Virgolini, I.; et al. Direct comparison of (68)Ga-DOTA-TOC and (18)F-FDG PET/CT in the follow-up of patients with neuroendocrine tumour treated with the first full peptide receptor radionuclide therapy cycle. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1585–1592. [Google Scholar] [CrossRef]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Kaewput, C.; Vinjamuri, S. Role of Combined 68Ga DOTA-Peptides and 18F FDG PET/CT in the Evaluation of Gastroenteropancreatic Neuroendocrine Neoplasms. Diagnostics 2022, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, D.L., Jr.; O’Dorisio, T.M.; O’Dorisio, M.S.; Menda, Y.; Hicks, R.J.; Van Cutsem, E.; Baulieu, J.-L.; Borson-Chazot, F.; Anthony, L.; Benson, A.B.; et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J. Clin. Oncol. 2010, 28, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Weiss, G.R. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Investig. New. Drugs 1992, 10, 239–253. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.-J. Lu-177-Based Peptide Receptor Radionuclide Therapy for Advanced Neuroendocrine Tumors. Nucl. Med. Mol. Imaging 2018, 52, 208–215. [Google Scholar] [CrossRef]
- Erbas, B.; Tuncel, M. Renal Function Assessment During Peptide Receptor Radionuclide Therapy. Semin. Nucl. Med. 2016, 46, 462–478. [Google Scholar] [CrossRef]
- Sitani, K.; Parghane, R.; Talole, S.; Basu, S. The efficacy, toxicity and survival of salvage retreatment PRRT with 177Lu-DOTATATE in patients with progressive NET following initial course of PRRT. Br. J. Radiol. 2022, 95, 20210896. [Google Scholar] [CrossRef]
- Harris, P.E.; Zhernosekov, K. The evolution of PRRT for the treatment of neuroendocrine tumors; What comes next? Front. Endocrinol. 2022, 13, 941832. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. NETTER-1 investigators. 177Lu-Dotatate plus long-acting octreotide versus high dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Sansovini, M.; Severi, S.; Ambrosetti, A.; Monti, M.; Nanni, O.; Sarnelli, A.; Bodei, L.; Garaboldi, L.; Bartolomei, M.; Paganelli, G. Treatment with the radiolabelled somatostatin analog Lu-DOTATATE for advanced pancreatic neuroendocrine tumors. Neuroendocrinology 2013, 97, 347–354. [Google Scholar] [CrossRef]
- Paganelli, G.; Sansovini, M.; Nicolini, S.; Grassi, I.; Ibrahim, T.; Amadori, E.; Di Iorio, V.; Monti, M.; Scarpi, E.; Bongiovanni, A.; et al. 177Lu-PRRT in advanced gastrointestinal neuroendocrine tumors: 10-year follow-up of the IRST phase II prospective study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 152–160. [Google Scholar] [CrossRef]
- Huizing, D.M.V.; de Wit-van der Veen, B.J.; Verheij, M.; Stokkel, M.P.M. Dosimetry methods and clinical applications in peptide receptor radionuclide therapy for neuroendocrine tumours: A literature review. EJNMMI Res. 2018, 8, 89. [Google Scholar] [CrossRef]
- Stolniceanu, C.R.; Nistor, I.; Bilha, S.C.; Constantin, V.; Simona, V.; Matovic, M.; Stefanescu, C.; Covic, A. Nephrotoxicity/renal failure after therapy with 90Yttrium- and 177Lutetium-radiolabeled somatostatin analogs in different types of neuroendocrine tumors: A systematic review. Nucl. Med. Commun. 2020, 41, 601–617. [Google Scholar] [CrossRef]
- Sandström, M.; Freedman, N.; Fröss-Baron, K.; Kahn, T.; Sundin, A. Kidney dosimetry in 777 patients during 177Lu-DOTATATE therapy: Aspects on extrapolations and measurement time points. EJNMMI Phys. 2020, 7, 73. [Google Scholar] [CrossRef]
- Ilan, E.; Sandström, M.; Wassberg, C.; Sundin, A.; Garske-Román, U.; Eriksson, B.; Granberg, D.; Lubberink, M. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J. Nucl. Med. 2015, 56, 177–182. [Google Scholar] [CrossRef]
- Del Prete, M.; Arsenault, F.; Saighi, N.; Zhao, W.; Buteau, F.A.; Celler, A.; Beauregard, J.M. Accuracy and reproducibility of simplified QSPECT dosimetry for personalized 177Lu-octreotate PRRT. EJNMMI Phys. 2018, 5, 25. [Google Scholar] [CrossRef]
- Freedman, N.; Sandström, M.; Kuten, J.; Shtraus, N.; Ospovat, I.; Schlocker, A.; Even-Sapir, E. Personalized radiation dosimetry for PRRT-how many scans are really required? EJNMMI Phys. 2020, 7, 26. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Damle, N.A.; Sahoo, R.K.; Bal, C. Concomitant 177Lu-DOTATATE and Capecitabine Therapy in Patients With Advanced Neuroendocrine Tumors: A Long-term-Outcome, Toxicity, Survival, and Quality-of-Life Study. Clin. Nucl. Med. 2017, 42, e457–e466. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, M.; Grover, P.; Lam, W.S.; Claringbold, P.G.; Turner, J.H. Long-term hematologic toxicity of 177Lu-octreotate-capecitabine-temozolomide therapy of GEPNET. Endocr. Relat. Cancer 2021, 28, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Claringbold, P.G.; Price, R.A.; Turner, J.H. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother. Radiopharm. 2012, 27, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, S.; Bodei, L.; Bongiovanni, A.; Sansovini, M.; Grassi, I.; Ibrahim, T.; Monti, M.; Caroli, P.; Sarnelli, A.; Diano, D.; et al. Combined use of 177Lu-DOTATATE and metronomic capecitabine (Lu-X) in FDG-positive gastro-entero-pancreatic neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3260–3267. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Van Vliet, E.I.; Krenning, E.P.; Teunissen, J.J.; Bergsma, H.; Kam, B.L.; Kwekkeboom, D.J. Comparison of response evaluation in patients with gastroenteropancreatic and thoracic neuroendocrine tumors after treatment with [177Lu-DOTA0,Tyr3]octreotate. J. Nucl. Med. 2013, 54, 1689–1696. [Google Scholar] [CrossRef]
- Huizing, D.M.V.; Aalbersberg, E.A.; Versleijen, M.W.J.; Tesselaar, M.E.T.; Walraven, I.; Lahaye, M.J.; Veen, B.J.D.W.D.; Stokkel, M.P.M. Early response assessment and prediction of overall survival after peptide receptor radionuclide therapy. Cancer Imaging 2020, 20, 57. [Google Scholar] [CrossRef]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef]
- Öksüz, M.; Winter, L.; Pfannenberg, C.; Reischl, G.; Müssig, K.; Bares, R.; Dittmann, H. Peptide receptor radionuclide therapy of neuroendocrine tumors with (90)Y-DOTATOC: Is treatment response predictable by pre-therapeutic uptake of (68)Ga-DOTATOC? Diagn. Interv. Imaging 2014, 95, 289–300. [Google Scholar] [CrossRef]
- Gabriel, M.; Oberauer, A.; Dobrozemsky, G.; Decristoforo, C.; Putzer, D.; Kendler, D.; Uprimny, C.; Kovacs, P.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J. Nucl. Med. 2009, 50, 1427–1434. [Google Scholar] [CrossRef]
- Sharma, R.; Wang, W.M.; Yusuf, S.; Evans, J.; Ramaswami, R.; Wernig, F.; Frilling, A.; Mauri, F.; Nahhas, A.; Aboagye, E.; et al. 68Ga-DOTATATE PET/CT parameters predict response to peptide receptor radionuclide therapy in neuroendocrine tumours. Radiother. Oncol. 2019, 141, 108–115. [Google Scholar] [CrossRef]
- Fonti, R.; Panico, M.; Pellegrino, S.; Pulcrano, A.; Vastarella, L.A.; Torbati, A.H.M.; Giuliano, M.; Palmieri, G.; De Placido, S.; Del Vecchio, S. Heterogeneity of SSTR2 Expression Assessed by 68Ga-DOTATOC PET/CT Using Coefficient of Variation in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2022, 63, 1509–1514. [Google Scholar] [CrossRef]
- Tirosh, A.; Papadakis, G.Z.; Millo, C.; Hammoud, D.; Sadowski, S.M.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Yang, L.; Nockel, P.; et al. Prognostic Utility of Total 68Ga-DOTATATE-Avid Tumor Volume in Patients with Neuroendocrine Tumors. Gastroenterology 2018, 154, 998–1008.e1. [Google Scholar] [CrossRef]
- Toriihara, A.; Baratto, L.; Nobashi, T.; Park, S.; Hatami, N.; Davidzon, G.; Kunz, P.L.; Iagaru, A. Prognostic value of somatostatin receptor expressing tumor volume calculated from 68Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2244–2251. [Google Scholar] [CrossRef]
- Staal, F.C.R.; Aalbersberg, E.A.; van der Velden, D.; Wilthagen, E.A.; Tesselaar, M.E.T.; Beets-Tan, R.G.H.; Maas, M. GEP-NET radiomics: A systematic review and radiomics quality score assessment. Eur. Radiol. 2022, 32, 7278–7294. [Google Scholar] [CrossRef]
- Blazevic, A.; Starmans, M.P.A.; Brabander, T.; Dwarkasing, R.S.; van Gils, R.A.H.; Hofland, J.; Franssen, G.J.H.; Feelders, R.A.; Niessen, W.J.; Klein, S.; et al. Predicting symptomatic mesenteric mass in small intestinal neuroendocrine tumors using radiomics. Endocr. Relat. Cancer. 2021, 28, 529–539. [Google Scholar] [CrossRef]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O.; et al. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget 2017, 8, 7039–7049. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Dekervel, J.; Van Cutsem, E.; Verslype, C.; Van Laere, K.; Bormans, G.; et al. [18F]AlF-NOTA-octreotide PET imaging: Biodistribution, dosimetry and first comparison with [68Ga]Ga-DOTATATE in neuroendocrine tumour patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, H.; Lindner, S.; Todica, A.; Cyran, C.C.; Tiling, R.; Auernhammer, C.J.; Spitzweg, C.; Boeck, S.; Unterrainer, M.; Gildehaus, F.J.; et al. Biodistribution and first clinical results of 18F-SiFAlin-TATE PET: A novel 18F-labeled somatostatin analog for imaging of neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Jackson, P.; Kong, G.; Ware, R.E.; Hofman, M.S.; Pattison, D.A.; Akhurst, T.A.; Drummond, E.; Roselt, P.; Callahan, J.; et al. 64Cu-SARTATE PET Imaging of Patients with Neuroendocrine Tumors Demonstrates High Tumor Uptake and Retention, Potentially Allowing Prospective Dosimetry for Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2019, 60, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Beiderwellen, K.J.; Poeppel, T.D.; Hartung-Knemeyer, V.; Buchbender, C.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Simultaneous 68Ga-DOTATOC PET/MRI in patients with gastroenteropancreatic neuroendocrine tumors: Initial results. Investig. Radiol. 2013, 48, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Pampaloni, M.H.; Nakakura, E.; VanBrocklin, H.; Slater, J.; Jivan, S.; Aparici, C.M.; Yee, J.; Bergsland, E. Simultaneous (68)Ga-DOTA-TOC PET/MRI with gadoxetate disodium in patients with neuroendocrine tumor. Abdom. Imaging 2015, 40, 1432–1440. [Google Scholar] [CrossRef]
- Klain, M.; Gaudieri, V.; Petretta, M.; Zampella, E.; Storto, G.; Nappi, C.; Buonerba, C.; Crocetto, F.; Gallicchio, R.; Volpe, F.; et al. Combined bone scintigraphy and fluorocholine PET/computed tomography predicts response to radium-223 therapy in patients with prostate cancer. Futur. Sci. OA 2021, 7, FSO719. [Google Scholar] [CrossRef]
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in targeted alpha-particle therapeutic applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar] [CrossRef]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef] [PubMed]

| Authors | Patients (n) | Primary Tumor Location | Endpoints | Sensitivity |
|---|---|---|---|---|
| Gabriel et al. [40] | 84 | 20 (24%) pancreas 30 (36%) GI tract 34 (41%) other sites | Identification of primary tumor and metastatic disease Comparison with SPECT and CT | 97% |
| Ambrosini et al. [43] | 223 | 64 (29%) pancreas 55 (25%) GI tract 104 (47%) other sites | Identification of bone metastases Comparison with CT | 100% |
| Frilling et al. [41] | 52 | 27 (52%) pancreas 19 (37%) GI tract 5 (10%) other sites | Identification of primary tumor and metastatic disease Comparison with CT and MRI | 100% |
| Prasad et al. [42] | 59 | 16 (27%) pancreas 16 (27%) GI tract 27 (46%) other sites | Identification of undiagnosed primary tumor | 59% |
| Putzer et al. [44] | 51 | 11 (22%) pancreas 24 (47%) GI tract 16 (31%) other sites | Identification of bone metastases Comparison with CT and bone scintigraphy | 97% |
| Authors | Patients (n) | Study Population | Cycles/Intervals (n/weeks) | Activity (GBq) | Endpoints | Median Follow-Up (Months) | PFS (Months) |
|---|---|---|---|---|---|---|---|
| Kwekkeboom et al. [71] | 504 | Suspected or histologically proven GEP-NETs | 4/6–10 | 27.8–29.6 (Cumulative) | Safety, OR, OS | 19 | 32 |
| Strosberg et al. [76] | 229 | Histologically proven advanced midgut-NETs | 4/8 | 7.4 | Safety, OR, PFS | 14 | 30 |
| Strosberg et al. [77] | 231 | Histologically proven advanced midgut-NETs | 4/8 | 7.4 | OS | 76 | 30 |
| Sansovini et al. [78] | 52 | Histologically proven pancreatic NETs | 5/6–8 | 17.8 or 25.5 (Cumulative) | Safety, OR, OS | 29 | 29 |
| Paganelli et al. [79] | 43 | Histologically proven GI-NETs | 6–8 | 18.4 or 25.7 (Cumulative) | Safety, OR, PFS | 118 | 59.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscopo, L.; Zampella, E.; Pellegrino, S.; Volpe, F.; Nappi, C.; Gaudieri, V.; Fonti, R.; Vecchio, S.D.; Cuocolo, A.; Klain, M. Diagnosis, Management and Theragnostic Approach of Gastro-Entero-Pancreatic Neuroendocrine Neoplasms. Cancers 2023, 15, 3483. https://doi.org/10.3390/cancers15133483
Piscopo L, Zampella E, Pellegrino S, Volpe F, Nappi C, Gaudieri V, Fonti R, Vecchio SD, Cuocolo A, Klain M. Diagnosis, Management and Theragnostic Approach of Gastro-Entero-Pancreatic Neuroendocrine Neoplasms. Cancers. 2023; 15(13):3483. https://doi.org/10.3390/cancers15133483
Chicago/Turabian StylePiscopo, Leandra, Emilia Zampella, Sara Pellegrino, Fabio Volpe, Carmela Nappi, Valeria Gaudieri, Rosa Fonti, Silvana Del Vecchio, Alberto Cuocolo, and Michele Klain. 2023. "Diagnosis, Management and Theragnostic Approach of Gastro-Entero-Pancreatic Neuroendocrine Neoplasms" Cancers 15, no. 13: 3483. https://doi.org/10.3390/cancers15133483
APA StylePiscopo, L., Zampella, E., Pellegrino, S., Volpe, F., Nappi, C., Gaudieri, V., Fonti, R., Vecchio, S. D., Cuocolo, A., & Klain, M. (2023). Diagnosis, Management and Theragnostic Approach of Gastro-Entero-Pancreatic Neuroendocrine Neoplasms. Cancers, 15(13), 3483. https://doi.org/10.3390/cancers15133483








