Methods for Evaluating the Efficacy of Medical Castration: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
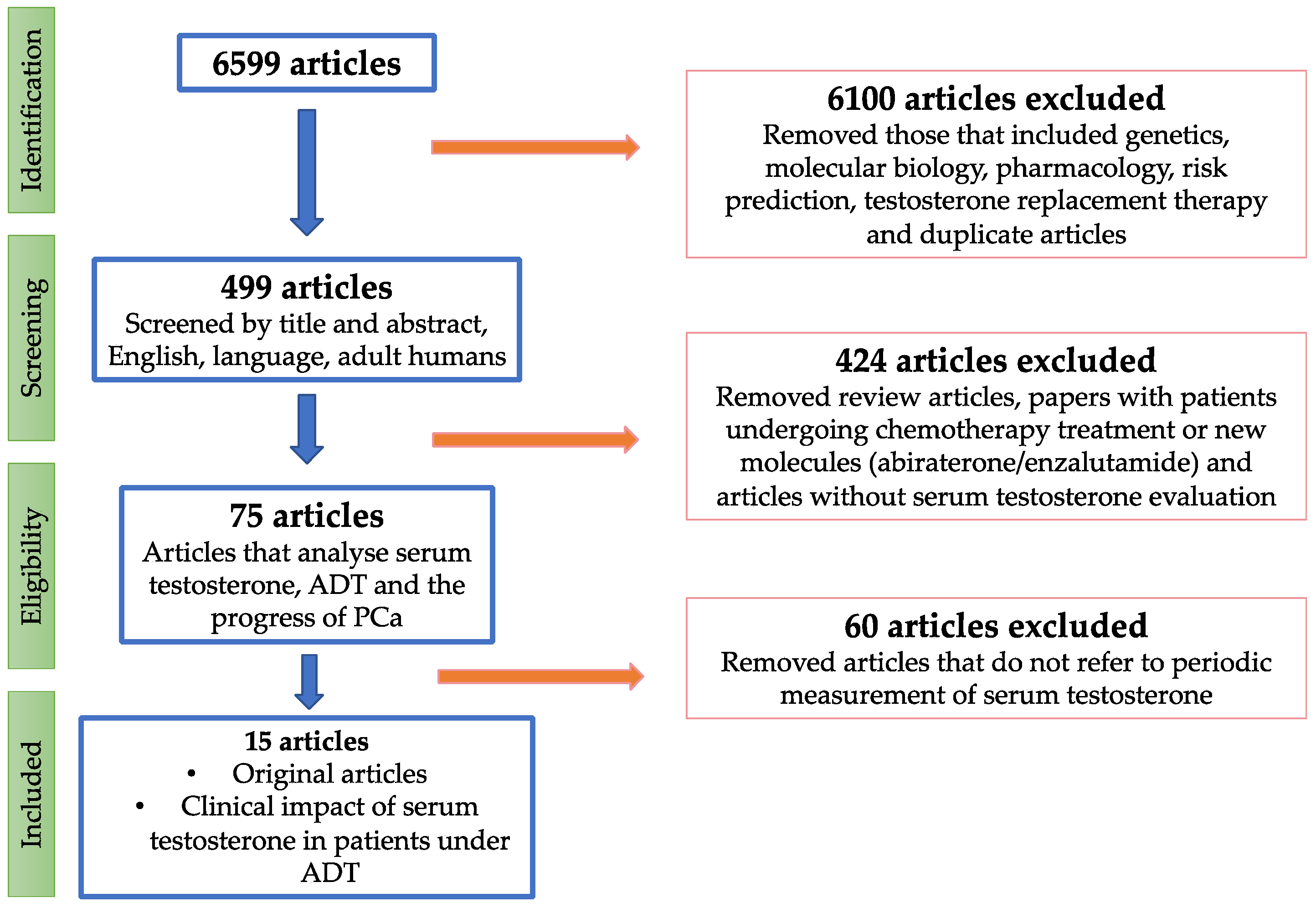
2. Evidence Acquisition
3. Evidence Synthesis
3.1. Serum Total Testosterone Measurement
3.1.1. Localised and Locally Advanced PCa
3.1.2. Metastatic PCa
3.1.3. Localised, Locally Advanced and Metastatic PCa
3.2. Serum-Free Testosterone Measurement
3.3. Serum LH Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. JNCI J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Frånlund, M.; Månsson, M.; Godtman, R.A.; Aus, G.; Holmberg, E.; Kollberg, K.S.; Lodding, P.; Pihl, C.G.; Stranne, J.; Lilja, H.; et al. Results from 22 Years of Followup in the Göteborg Randomized Population-Based Prostate Cancer Screening Trial. J. Urol. 2022, 208, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Culp, M.B.B.; Ma, J.; Islami, F.; Fedewa, S.A. Prostate Cancer Incidence 5 Years after Us Preventive Services Task Force Recommendations against Screening. J. Natl. Cancer Inst. 2021, 113, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Fallara, G.; Belladelli, F.; Robesti, D.; Raggi, D.; Nocera, L.; Marandino, L.; Galsky, M.D.; Montorsi, F.; Malavaud, B.; Ploussard, G.; et al. Androgen Annihilation versus Advanced Androgen Blockage as First Line Treatment for Metastatic Castration Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2022, 179, 103801. [Google Scholar] [CrossRef]
- Kamran, S.C.; Zietman, A.L. Radiation Treatment in Prostate Cancer: Covering the Waterfront. BJU Int. 2021, 128, 398–407. [Google Scholar] [CrossRef]
- Menges, D.; Yebyo, H.G.; Sivec-Muniz, S.; Haile, S.R.; Barbier, M.C.; Tomonaga, Y.; Schwenkglenks, M.; Puhan, M.A. Treatments for Metastatic Hormone-sensitive Prostate Cancer: Systematic Review, Network Meta-analysis, and Benefit-harm assessment. Eur. Urol. Oncol. 2022, 6, 605–616. [Google Scholar] [CrossRef]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Peeling, W.B. Phase III studies to compare goserelin (zoladex) with orchiectomy and with diethylstilbestrol in treatment of prostatic carcinoma. Urology 1989, 33, 45–52. [Google Scholar] [CrossRef]
- Byar, D.P.; Corle, D.K. Hormone Therapy for Prostate Cancer: Results of the Veterans Administration Cooperative Urological Research Group Studies. NCI Monogr. 1988, 7, 165–170. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and End Points of Clinical Trials for Patients with Progressive Prostate Cancer and Castrate Levels of Testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.J.; Souza, A.D.; Matadeen, J.; Croos, P. Ciba Corning ACS: 180 Testosterone Assay Evaluated. Clin. Chem. 1996, 42, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Oefelein, M.G.; Feng, A.; Scolieri, M.J.; Ricchiutti, D.; Resnick, M.I. Reassessment of the definition of castrate levels of testosterone: Implications for clinical decision making. Urology 2000, 56, 1021–1024. [Google Scholar] [CrossRef]
- Morote, J.; Orsola, A.; Planas, J.; Trilla, E.; Raventós, C.X.; Cecchini, L.; Catalán, R. Redefining Clinically Significant Castration Levels in Patients with Prostate Cancer Receiving Continuous Androgen Deprivation Therapy. J. Urol. 2007, 178, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; O’Callaghan, C.; Ding, K.; Toren, P.; Dearnaley, D.; Higano, C.S.; Horwitz, E.; Malone, S.; Goldenberg, L.; Gospodarowicz, M.; et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: A secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J. Clin. Oncol. 2015, 33, 1151–1156. [Google Scholar] [CrossRef]
- Taieb, J.; Mathian, B.; Oise Millot, F.; Patricot, M.-C.; Mathieu, E.; Queyrel, N.; Lacroix, I.; Somma-Delpero, C.; Boudou, P. Testosterone Measured by 10 Immunoassays and by Isotope-Dilution Gas Chromatography-Mass Spectrometry in Sera from 116 Men, Women, and Children. Clin. Chem. 2003, 49, 1381–1395. [Google Scholar] [CrossRef]
- Wang, C.; Catlin, D.H.; Demers, L.M.; Starcevic, B.; Swerdloff, R.S. Measurement of Total Serum Testosterone in Adult Men: Comparison of Current Laboratory Methods Versus Liquid Chromatography-Tandem Mass Spectrometry. J. Clin. Endocrinol. Metab. 2004, 89, 534–543. [Google Scholar] [CrossRef]
- Regis, L.; Planas, J.; Carles, J.; Maldonado, X.; Comas, I.; Ferrer, R.; Morote, J. Free Testosterone During Androgen Deprivation Therapy Predicts Castration-Resistant Progression Better Than Total Testosterone. Prostate 2017, 77, 114–120. [Google Scholar] [CrossRef]
- Morote, J.; Comas, I.; Ferrer, R.; Planas, J.; Celma, A.; Regis, L. Accuracy of Serum Luteinizing Hormone and Serum Testosterone Measurements to Assess the Efficacy of Medical Castration in Prostate Cancer Patients. J. Biomed. Sci. 2017, 24, 81. [Google Scholar] [CrossRef]
- Morote, J.; Comas, I.; Ferrer, R.; Regis, L.; Celma, A.; Santamaría, A.; Planas, J.; Trilla, E. Serum Luteinizing Hormone Testing Can Identify Optimal Medical Castration. Eur. Urol. Open Sci. 2020, 19, 24–26. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (Prisma-p) 2015: Elaboration and Explanation. BMJ 2015, 349, 7647. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Symp. 2006, 2006, 359–363. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Perachino, M.; Cavalli, V.; Bravi, F. Testosterone Levels in Patients with Metastatic Prostate Cancer Treated with Luteinizing Hormone-Releasing Hormone Therapy: Prognostic Significance? BJU Int. 2010, 105, 648–651. [Google Scholar] [CrossRef]
- Pickles, T.; Hamm, J.; Morris, W.J.; Schreiber, W.E.; Tyldesley, S. Incomplete Testosterone Suppression with Luteinizing Hormone-Releasing Hormone Agonists: Does It Happen and Does It Matter? BJU Int. 2012, 110, E500–E507. [Google Scholar] [CrossRef]
- Dason, S.; Allard, C.B.; Tong, J.; Shayegan, B. Defining a New Testosterone Threshold for Medical Castration: Results from a Prospective Cohort Series. J. Can. Urol. Assoc. 2013, 7, E263. [Google Scholar] [CrossRef]
- Bertaglia, V.; Tucci, M.; Fiori, C.; Aroasio, E.; Poggio, M.; Buttigliero, C.; Grande, S.; Saini, A.; Porpiglia, F.; Berruti, A. Effects of Serum Testosterone Levels after 6 Months of Androgen Deprivation Therapy on the Outcome of Patients with Prostate Cancer. Clin. Genitourin. Cancer 2013, 11, 325–330. [Google Scholar] [CrossRef]
- Yasuda, Y.; Fujii, Y.; Yuasa, T.; Yamamoto, S.; Yonese, J.; Fukui, I. Do Testosterone Levels Have Prognostic Significance in Patients with Metastatic Prostate Cancer Treated with Combined Androgen Blockade? Int. J. Urol. 2015, 22, 132–133. [Google Scholar] [CrossRef]
- Kamada, S.; Sakamoto, S.; Ando, K.; Muroi, A.; Fuse, M.; Kawamura, K.; Imamoto, T.; Suzuki, H.; Nagata, M.; Nihei, N.; et al. Nadir Testosterone after Long-Term Followup Predicts Prognosis in Patients with Prostate Cancer Treated with Combined Androgen Blockade. J. Urol. 2015, 194, 1264–1270. [Google Scholar] [CrossRef]
- Shiota, M.; Fujimoto, N.; Yokomizo, A.; Takeuchi, A.; Kashiwagi, E.; Dejima, T.; Kiyoshima, K.; Inokuchi, J.; Tatsugami, K.; Eto, M. The Prognostic Impact of Serum Testosterone during Androgen-Deprivation Therapy in Patients with Metastatic Prostate Cancer and the SRD5A2 Polymorphism. Prostate Cancer Prostatic Dis. 2016, 19, 191–196. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, B.; Ye, D.W. Serum Testosterone Level Predicts the Effective Time of Androgen Deprivation Therapy in Metastatic Prostate Cancer Patients. Asian J. Androl. 2017, 19, 178–183. [Google Scholar] [PubMed]
- Tombal, B.; Cornel, E.B.; Persad, R.; Stari, A.; Gómez Veiga, F.; Schulman, C. Clinical Outcomes and Testosterone Levels Following Continuous Androgen Deprivation in Patients with Relapsing or Locally Advanced Prostate Cancer: A Post Hoc Analysis of the ICELAND Study. J. Urol. 2017, 198, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Sayyid, R.K.; Sayyid, A.K.; Klaassen, Z.; Fadaak, K.; Goldberg, H.; Chandrasekar, T.; Ahmad, A.; Leao, R.; Perlis, N.; Chadwick, K.; et al. Testosterone Responders to Continuous Androgen Deprivation Therapy Show Considerable Variations in Testosterone Levels on Followup: Implications for Clinical Practice. J. Urol. 2018, 199, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Sakamoto, S.; Minhui, X.; Tamura, T.; Otsuka, K.; Sato, K.; Maimaiti, M.; Kamada, S.; Takei, A.; Fuse, M.; et al. Testosterone reduction of ≥480 ng/dL predicts favorable prognosis of japanese men with advanced prostate cancer treated with androgen-deprivation therapy. Clin. Genitourin. Cancer 2017, 15, e1107–e1115. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, G.; Hurmuz, P.; Yuce, D.; Akyol, F. Prognostic Significance of Castrate Testosterone Levels for Patients with Intermediate and High Risk Prostate Cancer. World J. Clin. Oncol. 2019, 10, 283–292. [Google Scholar] [CrossRef]
- Tremblay, S.; Summers-Trasiewicz, L.; Pouliot, F.; Crook, J.M.; Ding, K.; Klotz, L.; Toren, P. Interpreting Testosterone and Concomitant Prostate Specific Antigen Values during Androgen Deprivation Therapy for Recurrent Prostate Cancer. J. Urol. 2021, 206, 1166–1176. [Google Scholar] [CrossRef]
- Shore, N.D.; Saad, F.; Cookson, M.S.; George, D.J.; Saltzstein, D.R.; Tutrone, R.; Akaza, H.; Bossi, A.; Van Veenhuyzen, D.F.; Selby, B.; et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N. Engl. J. Med. 2020, 382, 2187–2196. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART II. J. Urol. 2021, 205, 22–29. [Google Scholar] [CrossRef]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Rosner, W.; Auchus, R.J.; Azziz, R.; Sluss, P.M.; Raff, H. Position Statement: Utility, Limitations, and Pitfalls in Measuring Testosterone: An Endocrine Society Position Statement. J. Clin. Endocrinol. Metab. 2007, 92, 405–413. [Google Scholar] [CrossRef]
- Morote, J.; Regis, L.; Celma, A.; Planas, J. Determinación de la testosterona sérica durante la supresión androgénica en pacientes con cáncer de próstata: Una revisión sistemática. Actas. Urol. Esp. 2016, 40, 477–484. [Google Scholar] [CrossRef]
- Morote, J.; Comas, I.; Planas, J.; Celma, A.; Ferrer, R.; Regis, L. Behavior of chemiluminescent assays to measure serum testosterone during androgen deprivation therapy. Int. J. Urol. 2016, 23, 957–958. [Google Scholar] [CrossRef]
- Morote, J.; Comas, I.; Planas, J.; Maldonado, X.; Celma, A.; Placer, J.; Ferrer, R.; Carles, J.; Regis, L. Serum testosterone levels in prostate cancer patients undergoing luteinizing hormone-releasing hormone agonist therapy. Clin. Genitourin. Cancer 2018, 16, e491–e496. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 4, 352–360. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 12, 1132–1142. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 32, 2974–2986. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 1, 13–24. [Google Scholar] [CrossRef]
- Attard, G.; Murphy, L.; Clarke, N.W.; Cross, W.; Jones, R.J.; Parker, C.C.; Gillessen, S.; Cook, A.; Brawley, C.; Amos, C.L.; et al. Abiraterone Acetate and Prednisolone with or without Enzalutamide for High-Risk Non-Metastatic Prostate Cancer: A Meta-Analysis of Primary Results from Two Randomised Controlled Phase 3 Trials of the STAMPEDE Platform Protocol. Lancet 2022, 399, 447–460. [Google Scholar] [CrossRef]
- De La Cerda, J.; Dunshee, C.; Gervasi, L.; Sieber, P.; Belkoff, L.; Tutrone, R.; Lu, S.; Gatoulis, S.C.; Brown, B.; Migoya, E.; et al. A Phase I Clinical Trial Evaluating the Safety and Dosing of Relugolix with Novel Hormonal Therapy for the Treatment of Advanced Prostate Cancer. Target. Oncol. 2023, 18, 383–390. [Google Scholar] [CrossRef]
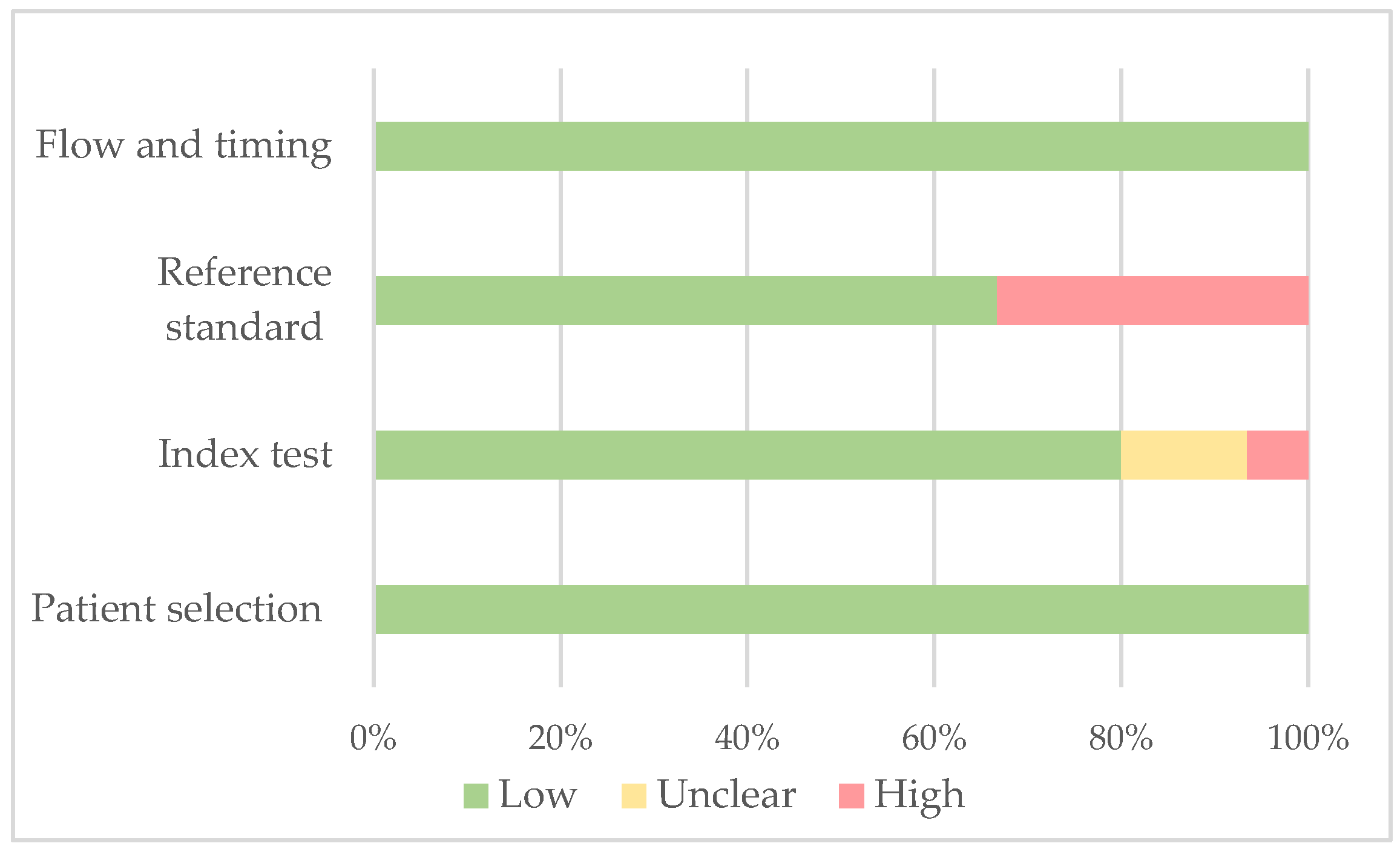
| Patient Selection | Patient Selection | Index Test | Index Test | Reference | Flow and Timing | |
|---|---|---|---|---|---|---|
| Author, Year | Selection Criteria Clearly Described | Clinical Data Available | Castration Threshold Pre-Specified | Serum Testosterone Measurement Method Specified | Reference Standard | Adequate Follow-Up |
| Morote et al., 2007 [14] | + | + | + | + | + | + |
| Perachino et al., 2009 [24] | + | + | ? | + | − | + |
| Pickles et al., 2012 [25] | + | + | + | + | + | + |
| Dason et al., 2013 [26] | + | + | + | + | + | + |
| Bertaglia et al., 2013 [27] | + | + | + | + | + | + |
| Yasuda et al., 2015 [28] | + | + | ? | + | − | + |
| Klotz et al., 2015 [15] | + | + | + | − | + | + |
| Kamada et al., 2015 [29] | + | + | + | + | + | + |
| Shiota et al., 2016 [30] | + | + | ? | + | − | + |
| Wang et al., 2017 [31] | + | + | ? | + | + | + |
| Tombal et al., 2017 [32] | + | + | + | + | − | + |
| Sayyid et al., 2017 [33] | + | + | + | + | + | + |
| Yamamoto et al., 2017 [34] | + | + | + | + | + | + |
| Ozyigit et al., 2019 [35] | + | + | + | + | + | + |
| Tremblay et al., 2021 [36] | + | + | + | − | − | + |
| Author, Year | nº Patients | Clinical Stage | Treatment Received | Follow-Up Method | Time of Determination (Months) | Measurement Method |
|---|---|---|---|---|---|---|
| Morote et al., 2007 [14] | 73 | 50 LA; 23 BR | 45 LH-RH; 28 MAB | ST | 6, 12, 18 | CLIA |
| Perachino et al., 2009 [24] | 129 | M | LH-RH | ST | every 3 | CLIA |
| Pickles et al., 2012 [25] | 2196 | L and LA | RT and LH-RH | ST | serial measurements (mean 2 months) | CLIA |
| Dason et al., 2013 [26] | 32 | 14 M; 5 LA; 13 BR | LH-RH and LHRH-ant | ST | every 3 | CLIA |
| Bertaglia et al., 2013 [27] | 153 | 51 M; 99 BR | LH-RH | ST | at 6 | CLIA |
| Yasuda et al., 2015 [28] | 69 | M | MAB | ST | every 3–6 | CLIA |
| Klotz et al., 2015 [15] | 626 | BR | LH-RH agonist | ST | every 2 (1 y) | non reported |
| Kamada et al., 2015 [29] | 225 | 70 L; 51 LA; 104 M | MAB | ST | every 3 | CLIA |
| Shiota et al., 2016 [30] | 96 | M | 9 LH-RH, 87 LH-RH + surgical castration | ST | 2 times (1–5) | CLIA |
| Wang et al., 2017 [31] | 206 | M | LH-RH | ST | 1,3,6 | CLIA |
| Tombal et al., 2017 [32] | 361 | LA + BR | LH-RH | ST | every 6 | CLIA |
| Sayyid et al., 2017 [33] | 950 | L + LA + BR + M | LH-RH | ST | every 1–4 | CLIA |
| Yamamoto et al., 2017 [34] | 222 | LA + M | LH-RH | ST | non reported | CLIA |
| Ozyigit et al., 2019 [35] | 173 | L | RT and LH-RH | ST | every 3 (2 y); every 4 (3 and 4 y); every 6 (thereafter) | CLIA |
| Tremblay et al., 2021 [36] | 678 | BR | LH-RH | ST | every 2 (2 y) | non reported |
| Author, Year | PSA ng/mL | Threshold Value | Event | Aim | Summary |
|---|---|---|---|---|---|
| Morote et al., 2007 [14] | 81.2 (mean) | <32 ng/dL | CR | CRFS | 32 ng/dL was the minimum level of ST with an impact on CRFS. If micro elevations > 50 ng/dL, using bicalutamide improved CRFS. |
| Pickles et al., 2012 [25] | - | <20 ng/dL | ST ↑ | CRFS | Microelevations > 30–50 ng/dL predict 58% CRFS at five years. If there are no microdeletions, the CRFS rate increases to 78%. |
| Klotz et al., 2015 [15] | - | <20 ng/dL | CR | CRFS, CSS | Nadir ST < 20 ng/dL showed higher CRFS and CSS rates. Microelevations >50 ng/dL are linked to lower rates of CRFS and CSS. |
| Tombal et al., 2017 [32] | - | X | CR | CRFS, CSS | No differences in CSS and CRFS progression among the ≤20 ng/dL, >20 to ≤50 ng/dL, and >50 ng/dL testosterone-level subgroups. |
| Ozyigit et al., 2019 [35] | 14 (median) | <20 ng/dL | BR | BRFS | Both <20 ng/mL and <50 ng/mL ST levels are valid for predicting BRFS. However, <20 ng/dL have significantly better BRFS compared to <50 ng/mL |
| Tremblay et al., 2021 [36] | - | X | CR | CRFS, CSS, OS | ST > 20 ng/dL in the 1st year of ADT was observed to result in increases with rises >50 ng/dL. The number of ST breakthroughs had no association with CRPC, CSS or OS. |
| Author, Year | PSA ng/mL | Threshold Value | Event | Aim | Summary |
|---|---|---|---|---|---|
| Perachino et al., 2009 [24] | 185.8 (mean) | - | Death | CSS | Lower levels of ST at 6 m after ADT, higher CSS |
| Yasuda et al., 2015 [28] | 610 (mean) | - | CR | CRFS and OS | No prognostic impact in CRFS and OS of ST during MAB |
| Shiota et al., 2016 [30] | 181.8 (mean) | - | CR | CRFS and OS | The lowest quartile of serum testosterone levels during ADT was a significant predictor of better OS and CRFS. |
| Wang et al., 2017 [31] | 241 (median) | <25 ng/dL | CR | CRFS | ST ≤ 25 ng/dL after 1 m of ADT: best CRFS. ST 25 ng/dL after 1 m of ADT can distinguish patients who may benefit from adding docetaxel. |
| Author, Year | PSA ng/mL | Threshold Value | Event | Aim | Summary |
|---|---|---|---|---|---|
| Dason et al., 2013 [26] | 70.8 (mean) | <32 ng/dL | CR | CRFS | Microelevations >50 ng/mL excluded. ST at 9 m < 32 ng/dL, better CRFS. Mean ST during the 1st year of ADT < 32 ng/dL better CRFS. |
| Bertaglia et al., 2013 [27] | 21 (mean) | <30 ng/dL | CR | CRFS and OS | Differences only in M patients. ST at 6 m < 20 ng/dL higher OS. ST at 6 m < 20 vs. 20–50 vs. >50 ng/dL is related to CRFS. |
| Kamada et al., 2015 [29] | 42.6 (mean) | <20 ng/dL | CR | CRFS and OS | Nadir ST during follow-up <20 ng/dL higher OS but not improves CRFS. |
| Sayyid et al., 2017 [33] | 19.04 (median) | <20 ng/dL | CR | CRFS | Continuous ADT with initial ST < 20 ng/dL showed substantial long-term variations in ST, and lacked prognostic significance in CRPC progression. |
| Yamamoto et al., 2017 [34] | 86.03 (median) 571.5 (mean) | <20 ng/dL | CR | CRFS and OS | Testosterone reduction >480 ng/dL and ST < 20 ng/dL and are prognostic factors for primary ADT in advanced PCa. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, A.; Planas, J.; Trilla, E.; Morote, J. Methods for Evaluating the Efficacy of Medical Castration: A Systematic Review. Cancers 2023, 15, 3479. https://doi.org/10.3390/cancers15133479
Aguilar A, Planas J, Trilla E, Morote J. Methods for Evaluating the Efficacy of Medical Castration: A Systematic Review. Cancers. 2023; 15(13):3479. https://doi.org/10.3390/cancers15133479
Chicago/Turabian StyleAguilar, Adriana, Jacques Planas, Enrique Trilla, and Juan Morote. 2023. "Methods for Evaluating the Efficacy of Medical Castration: A Systematic Review" Cancers 15, no. 13: 3479. https://doi.org/10.3390/cancers15133479
APA StyleAguilar, A., Planas, J., Trilla, E., & Morote, J. (2023). Methods for Evaluating the Efficacy of Medical Castration: A Systematic Review. Cancers, 15(13), 3479. https://doi.org/10.3390/cancers15133479







