Molecular Characterization and Treatment Approaches for Pediatric H3 K27-Altered Diffuse Midline Glioma: Integrated Systematic Review of Individual Clinical Trial Participant Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
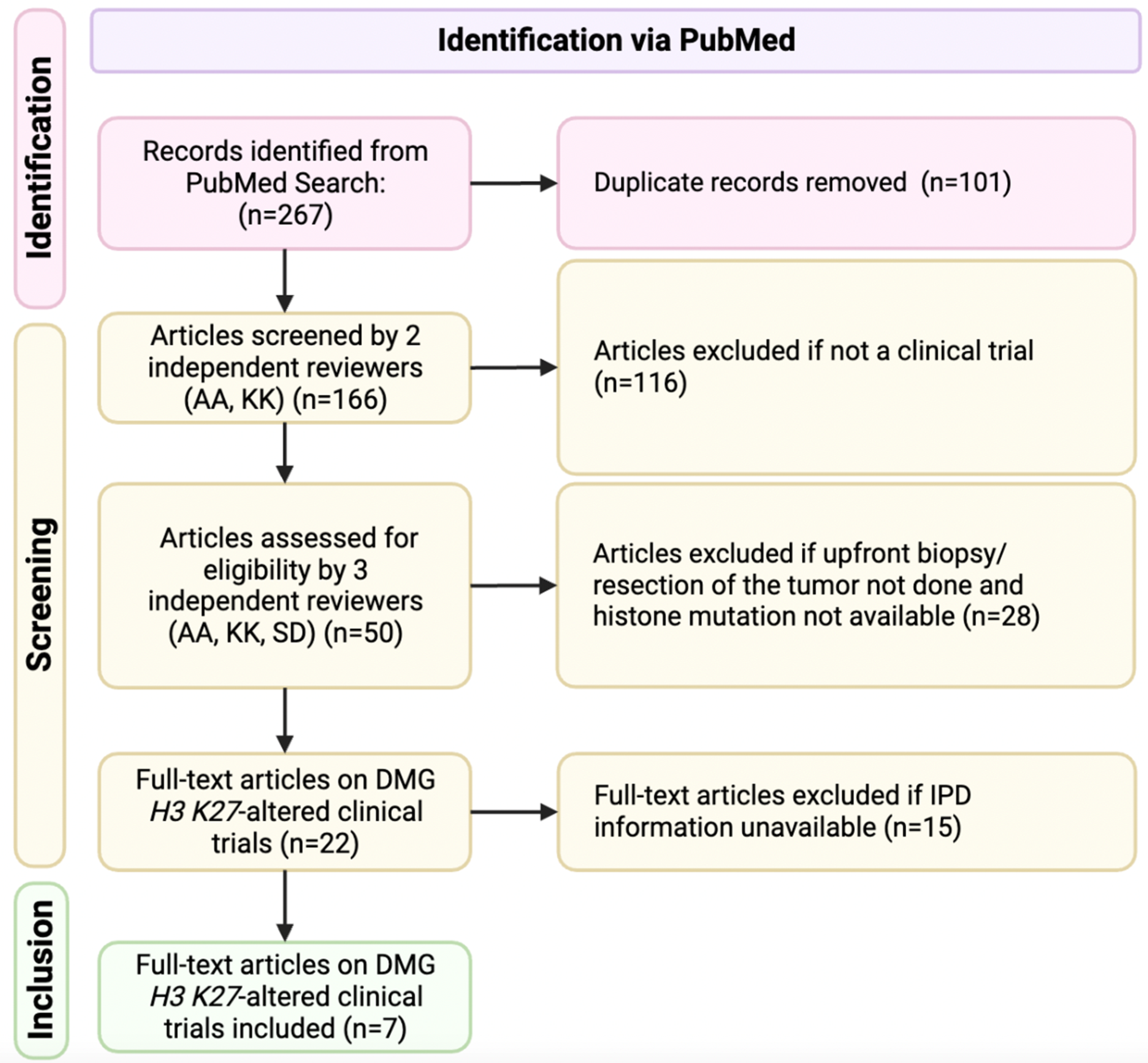
2.1. Study Selection Criteria
2.2. Quality Evaluation of Clinical Trials
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Survival Differences
3.3. Genetic Alterations
3.4. Treatment and Tumor Location
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Feng, L.L.; Ji, P.G.; Liu, J.H.; Guo, S.C.; Zhai, Y.L.; Sankey, E.W.; Wang, Y.; Xue, Y.R.; Wang, N.; et al. Clinical Features and Molecular Markers on Diffuse Midline Gliomas with H3K27M Mutations: A 43 Cases Retrospective Cohort Study. Front. Oncol. 2020, 10, 602553. [Google Scholar] [CrossRef]
- Zheng, L.; Gong, J.; Yu, T.; Zou, Y.; Zhang, M.; Nie, L.; Chen, X.; Yue, Q.; Liu, Y.; Mao, Q.; et al. Diffuse Midline Gliomas with Histone H3 K27M Mutation in Adults and Children: A Retrospective Series of 164 Cases. Am. J. Surg. Pathol. 2022, 46, 863–871. [Google Scholar] [CrossRef]
- Karremann, M.; Gielen, G.H.; Hoffmann, M.; Wiese, M.; Colditz, N.; Warmuth-Metz, M.; Bison, B.; Claviez, A.; van Vuurden, D.G.; von Bueren, A.O.; et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018, 20, 123–131. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Del Baldo, G.; Carai, A.; Abbas, R.; Cacchione, A.; Vinci, M.; Di Ruscio, V.; Colafati, G.S.; Rossi, S.; Diomedi Camassei, F.; Maestro, N.; et al. Targeted therapy for pediatric diffuse intrinsic pontine glioma: A single-center experience. Ther. Adv. Med. Oncol. 2022, 14, 17588359221113693. [Google Scholar] [CrossRef] [PubMed]
- DeWire, M.; Fuller, C.; Hummel, T.R.; Chow, L.M.L.; Salloum, R.; de Blank, P.; Pater, L.; Lawson, S.; Zhu, X.; Dexheimer, P.; et al. A phase I/II study of ribociclib following radiation therapy in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). J. Neurooncol. 2020, 149, 511–522. [Google Scholar] [CrossRef]
- El-Khouly, F.E.; Veldhuijzen van Zanten, S.E.M.; Jansen, M.H.A.; Bakker, D.P.; Sanchez Aliaga, E.; Hendrikse, N.H.; Vandertop, W.P.; van Vuurden, D.G.; Kaspers, G.J.L. A phase I/II study of bevacizumab, irinotecan and erlotinib in children with progressive diffuse intrinsic pontine glioma. J. Neurooncol. 2021, 153, 263–271. [Google Scholar] [CrossRef]
- Gallego Perez-Larraya, J.; Garcia-Moure, M.; Labiano, S.; Patino-Garcia, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef]
- Gojo, J.; Pavelka, Z.; Zapletalova, D.; Schmook, M.T.; Mayr, L.; Madlener, S.; Kyr, M.; Vejmelkova, K.; Smrcka, M.; Czech, T.; et al. Personalized Treatment of H3K27M-Mutant Pediatric Diffuse Gliomas Provides Improved Therapeutic Opportunities. Front. Oncol. 2019, 9, 1436. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Jain, P.; Liang, W.S.; Kilburn, L.; Kline, C.; Gupta, N.; Panditharatna, E.; Magge, S.N.; Zhang, B.; Zhu, Y.; et al. A pilot precision medicine trial for children with diffuse intrinsic pontine glioma-PNOC003: A report from the Pacific Pediatric Neuro-Oncology Consortium. Int. J. Cancer 2019, 145, 1889–1901. [Google Scholar] [CrossRef]
- Rodriguez, D.; Calmon, R.; Aliaga, E.S.; Warren, D.; Warmuth-Metz, M.; Jones, C.; Mackay, A.; Varlet, P.; Le Deley, M.C.; Hargrave, D.; et al. MRI and Molecular Characterization of Pediatric High-Grade Midline Thalamic Gliomas: The HERBY Phase II Trial. Radiology 2022, 304, 174–182. [Google Scholar] [CrossRef]
- Di Ruscio, V.; Del Baldo, G.; Fabozzi, F.; Vinci, M.; Cacchione, A.; de Billy, E.; Megaro, G.; Carai, A.; Mastronuzzi, A. Pediatric Diffuse Midline Gliomas: An Unfinished Puzzle. Diagnostics 2022, 12, 2064. [Google Scholar] [CrossRef]
- Jovanovich, N.; Habib, A.; Head, J.; Hameed, F.; Agnihotri, S.; Zinn, P.O. Pediatric diffuse midline glioma: Understanding the mechanisms and assessing the next generation of personalized therapeutics. Neurooncol. Adv. 2023, 5, vdad040. [Google Scholar] [CrossRef]
- Castel, D.; Philippe, C.; Calmon, R.; Le Dret, L.; Truffaux, N.; Boddaert, N.; Pagès, M.; Taylor, K.R.; Saulnier, P.; Lacroix, L.; et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015, 130, 815–827. [Google Scholar] [CrossRef]
- Buczkowicz, P.; Hoeman, C.; Rakopoulos, P.; Pajovic, S.; Letourneau, L.; Dzamba, M.; Morrison, A.; Lewis, P.; Bouffet, E.; Bartels, U.; et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 2014, 46, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Haag, D.; Mack, N.; Benites Goncalves da Silva, P.; Statz, B.; Clark, J.; Tanabe, K.; Sharma, T.; Jager, N.; Jones, D.T.W.; Kawauchi, D.; et al. H3.3-K27M drives neural stem cell-specific gliomagenesis in a human iPSC-derived model. Cancer Cell 2021, 39, 407–422. [Google Scholar] [CrossRef]
- Solomon, D.A.; Wood, M.D.; Tihan, T.; Bollen, A.W.; Gupta, N.; Phillips, J.J.; Perry, A. Diffuse Midline Gliomas with Histone H3-K27M Mutation: A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathol. 2016, 26, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Le, H.T.; Jea, A.; McNall-Knapp, R.; Dunn, I.F. Risk stratification of H3 K27M-mutant diffuse midline gliomas based on anatomical locations: An integrated systematic review of individual participant data. J. Neurosurg. Pediatr. 2022, 30, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Yuen, B.T.; Knoepfler, P.S. Histone H3.3 mutations: A variant path to cancer. Cancer Cell 2013, 24, 567–574. [Google Scholar] [CrossRef]
- Buccoliero, A.M.; Giunti, L.; Moscardi, S.; Castiglione, F.; Provenzano, A.; Sardi, I.; Scagnet, M.; Genitori, L.; Caporalini, C. Pediatric High Grade Glioma Classification Criteria and Molecular Features of a Case Series. Genes 2022, 13, 624. [Google Scholar] [CrossRef]
- Werbrouck, C.; Evangelista, C.C.S.; Lobón-Iglesias, M.J.; Barret, E.; Le Teuff, G.; Merlevede, J.; Brusini, R.; Kergrohen, T.; Mondini, M.; Bolle, S.; et al. TP53 Pathway Alterations Drive Radioresistance in Diffuse Intrinsic Pontine Gliomas (DIPG). Clin. Cancer Res. 2019, 25, 6788–6800. [Google Scholar] [CrossRef]
- Pedersen, H.; Schmiegelow, K.; Hamerlik, P. Radio-Resistance and DNA Repair in Pediatric Diffuse Midline Gliomas. Cancers 2020, 12, 2813. [Google Scholar] [CrossRef]
- Kazarian, E.; Marks, A.; Cui, J.; Darbinyan, A.; Tong, E.; Mueller, S.; Cha, S.; Aboian, M.S. Topographic correlates of driver mutations and endogenous gene expression in pediatric diffuse midline gliomas and hemispheric high-grade gliomas. Sci. Rep. 2021, 11, 14377. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Jain, P.; Resnick, A.C. Shared ACVR1 mutations in FOP and DIPG: Opportunities and challenges in extending biological and clinical implications across rare diseases. Bone 2018, 109, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Fontebasso, A.M.; Papillon-Cavanagh, S.; Schwartzentruber, J.; Nikbakht, H.; Gerges, N.; Fiset, P.O.; Bechet, D.; Faury, D.; De Jay, N.; Ramkissoon, L.A.; et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat. Genet. 2014, 46, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Song, S.W.; Kim, Y.H.; Cho, Y.H.; Hong, S.H.; Kim, J.H.; Ra, Y.S.; Chong, S. Clinical Features and Prognosis of Diffuse Midline Glioma: A Series of 24 Cases. Brain Tumor Res. Treat. 2022, 10, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Kramm, C.M.; Butenhoff, S.; Rausche, U.; Warmuth-Metz, M.; Kortmann, R.D.; Pietsch, T.; Gnekow, A.; Jorch, N.; Janssen, G.; Berthold, F.; et al. Thalamic high-grade gliomas in children: A distinct clinical subset? Neuro Oncol. 2011, 13, 680–689. [Google Scholar] [CrossRef]
- Liu, H.; Qin, X.; Zhao, L.; Zhao, G.; Wang, Y. Epidemiology and Survival of Patients with Brainstem Gliomas: A Population-Based Study Using the SEER Database. Front. Oncol. 2021, 11, 692097. [Google Scholar] [CrossRef]
- Dono, A.; Takayasu, T.; Ballester, L.Y.; Esquenazi, Y. Adult diffuse midline gliomas: Clinical, radiological, and genetic characteristics. J. Clin. Neurosci. 2020, 82, 1–8. [Google Scholar] [CrossRef]
- Hu, J.; Western, S.; Kesari, S. Brainstem Glioma in Adults. Front. Oncol. 2016, 6, 180. [Google Scholar] [CrossRef]
- Enomoto, T.; Aoki, M.; Hamasaki, M.; Abe, H.; Nonaka, M.; Inoue, T.; Nabeshima, K. Midline Glioma in Adults: Clinicopathological, Genetic, and Epigenetic Analysis. Neurol. Med.-Chir. 2020, 60, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, C.; Liu, K.X.; Haas-Kogan, D.A.; Warren, K.E. Reirradiation practices for children with diffuse intrinsic pontine glioma. Neurooncol. Pract. 2021, 8, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Chavaz, L.; Janssens, G.O.; Bolle, S.; Mandeville, H.; Ramos-Albiac, M.; Van Beek, K.; Benghiat, H.; Hoeben, B.; Morales La Madrid, A.; Seidel, C.; et al. Neurological Symptom Improvement After Re-Irradiation in Patients With Diffuse Intrinsic Pontine Glioma: A Retrospective Analysis of the SIOP-E-HGG/DIPG Project. Front. Oncol. 2022, 12, 926196. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Howman, A.; Wheatley, K.; Wherton, D.; Boota, N.; Pizer, B.; Fisher, D.; Kearns, P.; Picton, S.; Saran, F.; et al. Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy--results of a United Kingdom phase II trial (CNS 2007 04). Eur. J. Cancer 2013, 49, 3856–3862. [Google Scholar] [CrossRef]
- Cohen, K.J.; Heideman, R.L.; Zhou, T.; Holmes, E.J.; Lavey, R.S.; Bouffet, E.; Pollack, I.F. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: A report from the Children’s Oncology Group. Neuro Oncol. 2011, 13, 410–416. [Google Scholar] [CrossRef]
- Evans, M.; Gill, R.; Bull, K.S. Does a Bevacizumab-based regime have a role in the treatment of children with diffuse intrinsic pontine glioma? A systematic review. Neurooncol. Adv. 2022, 4, vdac100. [Google Scholar] [CrossRef]
- Jing, L.; Qian, Z.; Gao, Q.; Sun, R.; Zhen, Z.; Wang, G.; Yang, X.; Li, H.; Guo, T.; Zhang, W. Diffuse midline glioma treated with epigenetic agent-based immunotherapy. Signal Transduct. Target. Ther. 2023, 8, 23. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Hoffman, S.E.; Kappel, A.D.; Valdes, P.A.; Essayed, W.; Klinger, N.V.; Kang, K.D.; Totsch, S.K.; Olsen, H.E.; Schlappi, C.W.; et al. Immunotherapy approaches for the treatment of diffuse midline gliomas. Oncoimmunology 2022, 11, 2124058. [Google Scholar] [CrossRef]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, C.; Li, S.; Wang, J.; Zhang, H. Immune Microenvironment and Immunotherapies for Diffuse Intrinsic Pontine Glioma. Cancers 2023, 15, 602. [Google Scholar] [CrossRef] [PubMed]

| Inclusion | Exclusion |
|---|---|
| (1) patients >18yo |
| (2) non-clinical trial publications |
| (3) published in languages different than English |
| (4) published prior to 2018 |
| (5) upfront tumor biopsy/resection not done |
| (6) histone mutation status not available |
| (7) IPD unavailable |
| Authors & Year | Country | NOS (No. of Stars) | ||
|---|---|---|---|---|
| Selection | Comparability | Outcome | ||
| Del Baldo et al., 2022 [5] | Italy | 4 | 0 | 3 |
| DeWire et al., 2020 [6] | USA | 4 | 0 | 3 |
| El-Khouly et al., 2021 [7] | Netherlands | 4 | 0 | 2 |
| Pérez-Larraya et al., 2022 [8] | Spain | 4 | 0 | 2 |
| Gojo et al., 2020 [9] | Austria | 4 | 0 | 3 |
| Mueller et al., 2019 [10] | USA | 4 | 0 | 3 |
| Rodriguez et al., 2022 [11] | Italy | 4 | 0 | 3 |
| Articles | Country | Trial Overview/Intervention | Total Number Participants | Participants Included in Analysis |
|---|---|---|---|---|
| Del Baldo et al., 2022 [5] | Italy | Targeted therapies combined with standard of care for pediatric DMG. | 25 | 25 |
| DeWire et al., 2020 [6] | USA | Addition of ribociclib following standard-of-care radiotherapy for DMG. | 10 | 10 |
| El-Khouly et al., 2021 [7] | Netherlands | Combined treatment with bevacizumab, irinotecan and erlotinib for DMG. | 9 | 4 |
| Gojo et al., 2020 [9] | Austria | Feasibility and outcomes of including personalized treatment via molecular tumor analysis with focal irradiation and backbone therapy for DMG. | 18 | 17 |
| Mueller et al., 2019 [10] | USA | Evaluated whether whole-exome sequencing and RNA sequencing of paired normal and tumor tissues could be incorporated into personalized treatment of DMG. | 17 | 13 |
| Pérez-Larraya et al., 2022 [8] | Spain | Dose-escalation study of DNX-2401, an oncolytic adenovirus, followed by radiotherapy for DMG. | 12 | 10 |
| Rodriguez et al., 2022 [11] | Italy | Bevacizumab in combination with temozolomide and radiotherapy in pediatric patients with high-grade glioma. | 121 | 29 |
| Parameters | Number of Patients | Overall Survival | p-Value | ||
|---|---|---|---|---|---|
| Mean | STD | ||||
| Age | <5 | 16 | 20.50 | 9.72 | 0.0163 * |
| 5–10 | 52 | 14.54 | 6.07 | ||
| 10+ | 40 | 14.99 | 7.69 | ||
| Sex | Female | 57 | 15.61 | 6.84 | 0.9845 |
| Male | 51 | 15.58 | 8.30 | ||
| Anatomical location | pons | 77 | 16.30 | 7.41 | 0.1229 |
| thalamus | 31 | 13.83 | 7.63 | ||
| Histone Mutation | H3.1 (HIST1H3B/C) | 27 | 19.62 | 7.67 | 0.0011 * |
| H3.3 (H3F3A) | 18 | 14.25 | 7.02 | ||
| TP53 | N | 23 | 21.41 | 8.38 | <0.0001 * |
| Y | 51 | 13.71 | 6.40 | ||
| ACVR | N | 59 | 14.89 | 7.16 | 0.0081 * |
| Y | 15 | 20.85 | 9.03 | ||
| ATRX | N | 54 | 16.26 | 7.94 | 0.7698 |
| Y | 20 | 15.66 | 7.91 | ||
| PIK3CA | N | 63 | 15.59 | 7.29 | 0.1824 |
| Y | 11 | 19.04 | 10.63 | ||
| mTOR | N | 63 | 15.52 | 7.95 | 0.1275 |
| Y | 11 | 19.45 | 6.84 | ||
| BRAF | N | 71 | 15.87 | 7.72 | 0.2311 |
| Y | 3 | 21.47 | 11.59 | ||
| PDGFRA | N | 60 | 16.87 | 8.22 | 0.0838 |
| Y | 14 | 12.82 | 5.27 | ||
| FGFR3 | N | 70 | 16.19 | 8.04 | 0.6855 |
| Y | 4 | 14.53 | 4.56 | ||
| Personalized treatment approach | N | 59 | 14.33 | 6.57 | 0.0548 |
| Y | 49 | 17.12 | 8.35 | ||
| Nimotuzumab/vinorelbine | N | 74 | 14.86 | 7.36 | 0.1368 |
| Y | 34 | 17.19 | 7.74 | ||
| Temozolomide | N | 49 | 14.68 | 7.76 | 0.2539 |
| Y | 59 | 16.35 | 7.30 | ||
| Bevacizumab | N | 61 | 14.89 | 8.08 | 0.2683 |
| Y | 47 | 16.51 | 6.71 | ||
| Panobinostat | N | 101 | 15.65 | 7.64 | 0.7551 |
| Y | 7 | 14.73 | 6.08 | ||
| Immunotherapy | N | 37 | 14.17 | 7.77 | 0.1576 |
| Y | 71 | 16.33 | 7.34 | ||
| Other chemotherapy | N | 66 | 14.88 | 6.90 | 0.2163 |
| Y | 42 | 16.72 | 8.38 | ||
| Re-irradiation | N | 81 | 14.58 | 7.86 | 0.0047 * |
| Y | 27 | 18.62 | 5.52 | ||
| Radiation | N | 9 | 17.69 | 8.80 | 0.3843 |
| Y | 99 | 15.40 | 7.42 | ||
| Gene | Anatomical Location | Sex | |||||
|---|---|---|---|---|---|---|---|
| Pons | Thalamus | p-Value | Female | Male | p-Value | ||
| TP53 | N | 22 (42%) | 1 (5%) | 0.0018 * | 14 (36%) | 9 (26%) | 0.4519 |
| Y | 31 (58%) | 20 (95%) | 25 (64%) | 26 (74%) | |||
| ACVR | N | 38 (72%) | 21 (100%) | 0.004 * | 27 (69%) | 32 (91%) | 0.0218 * |
| Y | 15 (28%) | 0 (0%) | 12 (31%) | 3 (9%) | |||
| ATRX | N | 43 (81%) | 11 (52%) | 0.0194 * | 28 (72%) | 26 (74%) | 1.0000 |
| Y | 10 (19%) | 10 (48%) | 11 (28%) | 9 (26%) | |||
| PIK3CA | N | 46 (87%) | 17 (81%) | 0.4953 | 33 (85%) | 30 (86%) | 1.0000 |
| Y | 7 (13%) | 4 (19%) | 6 (15%) | 5 (14%) | |||
| mTOR | N | 43 (81%) | 20 (95%) | 0.1628 | 31 (79%) | 32 (91%) | 0.1980 |
| Y | 10 (19%) | 1 (5%) | 8 (21%) | 3 (9%) | |||
| BRAF | N | 51 (96%) | 20 (95%) | 1.0000 | 39 (100%) | 32 (91%) | 0.1010 |
| Y | 2 (4%) | 1 (5%) | 0 (0%) | 3 (9%) | |||
| PDGFRA | N | 43 (81%) | 17 (81%) | 1.0000 | 31 (79%) | 29 (83%) | 0.7732 |
| Y | 10 (19%) | 4 (19%) | 8 (21%) | 6 (17%) | |||
| FGFR3 | N | 51 (96%) | 19 (90%) | 0.3180 | 37 (95%) | 33 (94%) | 1.0000 |
| Y | 2 (4%) | 2 (10%) | 2 (5%) | 2 (6%) | |||
| Histone Mutation | H3.1 (HIST1H3B/C) | 23 (30%) | 4 (13%) | 0.0861 | 15 (26%) | 12 (24%) | 0.8254 |
| H3.3 (H3F3A) | 54 (70%) | 27 (87%) | 42 (74%) | 39 (76%) | |||
| Treatment | Anatomical Location | |||
|---|---|---|---|---|
| Pons | Thalamus | p-Value | ||
| Personalized treatment approach | N | 29 (49%) | 30 (51%) | <0.0001 * |
| Y | 48 (98%) | 1 (2%) | ||
| Nimotuzumab/vinorelbine | N | 43 (58%) | 31 (42%) | <0.0001 * |
| Y | 34 (100%) | 0 (0%) | ||
| Temozolomide | N | 48 (98%) | 1 (2%) | <0.0001 * |
| Y | 29 (49%) | 30 (51%) | ||
| Bevacizumab | N | 43 (70%) | 18 (30%) | 1.0000 |
| Y | 34 (72%) | 13 (28%) | ||
| Panobinostat | N | 70 (69%) | 31 (31%) | 0.1887 |
| Y | 7 (100%) | 0 (0%) | ||
| Immunotherapy | N | 19 (51%) | 18 (49%) | 0.0015 * |
| Y | 58 (82%) | 13 (18%) | ||
| Other chemotherapy | N | 36 (55%) | 30 (45%) | <0.0001 * |
| Y | 41 (98%) | 1 (2%) | ||
| Re-irradiation | N | 51 (63%) | 30 (37%) | 0.0005 * |
| Y | 26 (96%) | 1 (4%) | ||
| Radiation | N | 8 (89%) | 1 (11%) | 0.4416 |
| Y | 69 (70%) | 30 (30%) | ||
| Study Title | NCT Number | Therapeutic Intervention | Country |
|---|---|---|---|
| Stereotactic Biopsy Split-Course Radiation Therapy in Diffuse Midline Glioma, SPORT-DMG Study | NCT05077735 | Radiation: hypofractionated radiation therapy | USA |
| A Study of BXQ-350 in Children with Newly Diagnosed Diffuse Intrinsic Pontine Glioma (DIPG) or Diffuse Midline Glioma (DMG) | NCT04771897 | Drug: BXQ-350 | USA |
| FUS Etoposide for DMG—A Feasibility Study | NCT05762419 | Drug: etoposide and device: focused ultrasound with neuro-navigator-controlled sonication | USA |
| rHSC-DIPGVax Plus Checkpoint Blockade for the Treatment of Newly Diagnosed DIPG and DMG | NCT04943848 | Biological: rHSC-DIPGVax; drug: balstilimab; and drug: zalifrelimab | USA |
| Biological Medicine for Diffuse Intrinsic Pontine Glioma (DIPG) Eradication 2.0 | NCT05476939 | Drug: everolimus; drug: ONC201; and radiation: radiotherapy | France |
| Combination Therapy for the Treatment of Diffuse Midline Gliomas | NCT05009992 | Drug: ONC201; radiation: radiation therapy; and drug: paxalisib | USA |
| A Study of the Drug Selinexor with Radiation Therapy in Patients with Newly-Diagnosed Diffuse Intrinsic Pontine (DIPG) Glioma and High-Grade Glioma (HGG) | NCT05099003 | Radiation: radiation therapy and drug: selinexor | USA |
| Loc3CAR: Locoregional Delivery of B7-H3-CAR T Cells for Pediatric Patients with Primary CNS Tumors | NCT05835687 | Drug: B7-H3-CAR T cells | USA |
| Phase I Study of Oral ONC206 in Recurrent and Rare Primary Central Nervous System Neoplasms | NCT04541082 | Drug: ONC206 | USA |
| Oral AMXT 1501 Dicaprate in Combination with IV DFMO | NCT05500508 | Drug: AMXT1501 and drug: DFMO | USA |
| ONC206 for Treatment of Newly Diagnosed, or Recurrent Diffuse Midline Gliomas, and Other Recurrent Malignant CNS Tumors (PNOC 023) | NCT04732065 | Drug: ONC206 and radiation: radiation therapy | USA and Switzerland |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damodharan, S.; Abbott, A.; Kellar, K.; Zhao, Q.; Dey, M. Molecular Characterization and Treatment Approaches for Pediatric H3 K27-Altered Diffuse Midline Glioma: Integrated Systematic Review of Individual Clinical Trial Participant Data. Cancers 2023, 15, 3478. https://doi.org/10.3390/cancers15133478
Damodharan S, Abbott A, Kellar K, Zhao Q, Dey M. Molecular Characterization and Treatment Approaches for Pediatric H3 K27-Altered Diffuse Midline Glioma: Integrated Systematic Review of Individual Clinical Trial Participant Data. Cancers. 2023; 15(13):3478. https://doi.org/10.3390/cancers15133478
Chicago/Turabian StyleDamodharan, Sudarshawn, Alexandra Abbott, Kaitlyn Kellar, Qianqian Zhao, and Mahua Dey. 2023. "Molecular Characterization and Treatment Approaches for Pediatric H3 K27-Altered Diffuse Midline Glioma: Integrated Systematic Review of Individual Clinical Trial Participant Data" Cancers 15, no. 13: 3478. https://doi.org/10.3390/cancers15133478
APA StyleDamodharan, S., Abbott, A., Kellar, K., Zhao, Q., & Dey, M. (2023). Molecular Characterization and Treatment Approaches for Pediatric H3 K27-Altered Diffuse Midline Glioma: Integrated Systematic Review of Individual Clinical Trial Participant Data. Cancers, 15(13), 3478. https://doi.org/10.3390/cancers15133478





