Unraveling the Significance of EPH/Ephrin Signaling in Liver Cancer: Insights into Tumor Progression and Therapeutic Implications
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Epidemiology of Liver Cancer
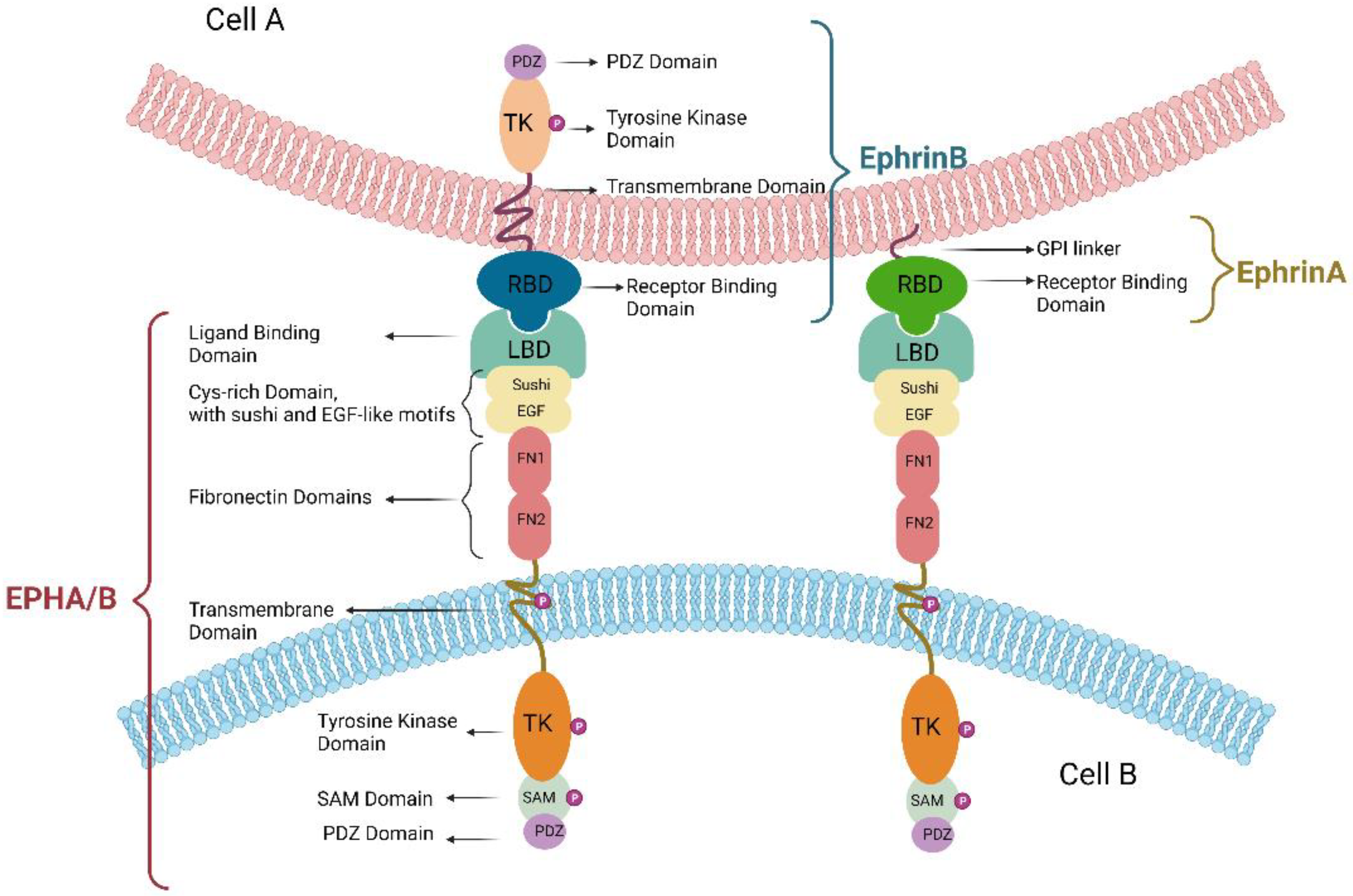
1.2. The EPH/Ephrin Signaling System
1.3. The EPH/Ephrin Molecular Structure
2. The EPH/Ephrin Signaling in HCC—Preclinical Data
2.1. The Role of EPH/Ephrin Signaling in HCC Proliferation and Metastasis
2.2. The EPH/Ephrin Signaling in Viral Hepatitis-Related HCC
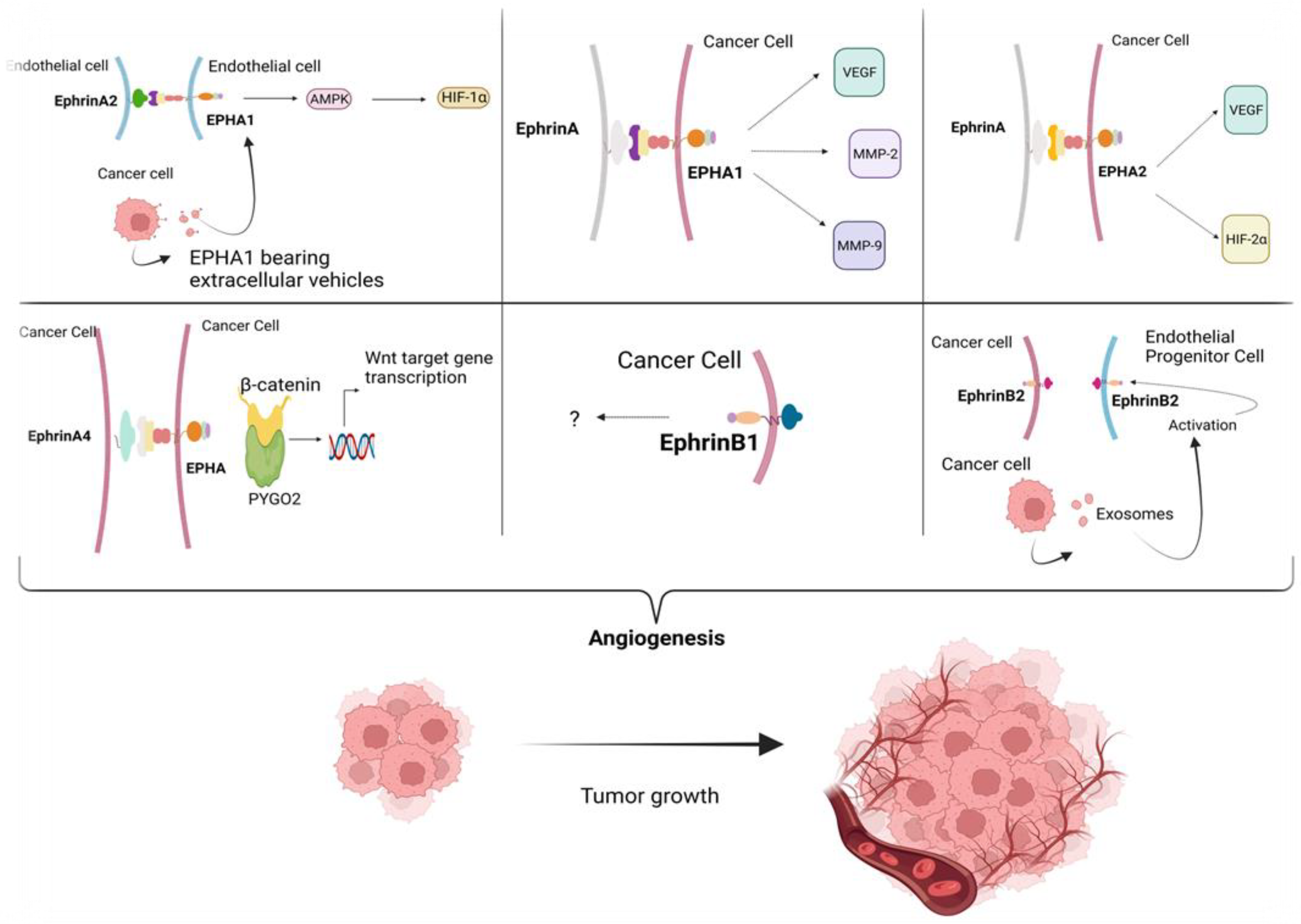
2.3. The Role of EPH/Ephrin Signaling in HCC Angiogenesis
2.4. The Role of EPH/Ephrin Signaling in Hypoxic HCC Tumor Microenvironment (TME)
2.5. The Role of EPH/Ephrin Signaling in Epigenetic Regulation of HCC
3. The EPH/Ephrin Signaling in HCC—Clinical Importance
3.1. The Role of EPH/Ephrin Signaling as Biomarkers
3.2. The Interconnection between AFP and EPH/Ephrin Signaling in HCC
3.3. HCC Prognostic Models Taking into Consideration the EPH/Ephrin System
4. The Role of EPH/Ephrin Signaling in CCA
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hytiroglou, P.; Bioulac-Sage, P.; Theise, N.D.; Sempoux, C. Etiology, Pathogenesis, Diagnosis, and Practical Implications of Hepatocellular Neoplasms. Cancers 2022, 14, 3670. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, Y.; Yuan, H.; Fang, Q.; Cai, N.; Suo, C.; Jin, L.; Zhang, T.; Chen, X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J. Hepatol. 2019, 70, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Foerster, F.; Gairing, S.J.; Müller, L.; Galle, P.R. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. J. Hepatol. 2022, 76, 446–457. [Google Scholar] [CrossRef]
- Tapper, E.B.; Parikh, N.D. Diagnosis and Management of Cirrhosis and Its Complications. JAMA 2023, 329, 1589. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.A.; Colombo, M. Treatment for Viral Hepatitis as Secondary Prevention for Hepatocellular Carcinoma. Cells 2021, 10, 3091. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Chrysavgis, L.; Vachliotis, I.D.; Chartampilas, E.; Cholongitas, E. Nonalcoholic fatty liver disease and hepatocellular carcinoma:Insights in epidemiology, pathogenesis, imaging, prevention and therapy. Semin. Cancer Biol. 2023, 93, 20–35. [Google Scholar] [CrossRef]
- Rizvi, S.; Gores, G.J. Pathogenesis, Diagnosis, and Management of Cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef]
- Gupta, A.; Kurzrock, R.; Adashek, J.J. Evolution of the Targeted Therapy Landscape for Cholangiocarcinoma: Is Cholangiocarcinoma the ‘NSCLC’ of GI Oncology? Cancers 2023, 15, 1578. [Google Scholar] [CrossRef]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Dedes, N.; Gkolemi, N.; Machairas, N.; Theocharis, S. The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment. Int. J. Mol. Sci. 2023, 24, 3015. [Google Scholar] [CrossRef]
- Psilopatis, I.; Karniadakis, I.; Danos, K.S.; Vrettou, K.; Michaelidou, K.; Mavridis, K.; Agelaki, S.; Theocharis, S. May EPH/Ephrin Targeting Revolutionize Lung Cancer Treatment? Int. J. Mol. Sci. 2023, 24, 93. [Google Scholar] [CrossRef]
- Psilopatis, I.; Souferi-Chronopoulou, E.; Vrettou, K.; Troungos, C.; Theocharis, S. EPH/Ephrin-Targeting Treatment in Breast Cancer: A New Chapter in Breast Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 15275. [Google Scholar] [CrossRef]
- Hadjimichael, A.C.; Pergaris, A.; Kaspiris, A.; Foukas, A.F.; Kokkali, S.; Tsourouflis, G.; Theocharis, S. The EPH/Ephrin System in Bone and Soft Tissue Sarcomas’ Pathogenesis and Therapy: New Advancements and a Literature Review. Int. J. Mol. Sci. 2022, 23, 5171. [Google Scholar] [CrossRef] [PubMed]
- Pergaris, A.; Danas, E.; Gajdzis, P.; Levidou, G.; Gajdzis, M.; Cassoux, N.; Gardrat, S.; Donizy, P.; Korkolopoulou, P.; Kavantzas, N.; et al. EPHA2, EPHA4, and EPHA6 Expression in Uveal Melanomas: Searching for the Culprits of Neoplasia. Diagnostics 2022, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Goutas, D.; Pergaris, A.; Goutas, N.; Theocharis, S. Utilizing Exosomal-EPHs/Ephrins as Biomarkers and as a Potential Platform for Targeted Delivery of Therapeutic Exosomes. Int. J. Mol. Sci. 2022, 23, 3551. [Google Scholar] [CrossRef]
- Psilopatis, I.; Pergaris, A.; Vrettou, K.; Tsourouflis, G.; Theocharis, S. The EPH/Ephrin System in Gynecological Cancers: Focusing on the Roots of Carcinogenesis for Better Patient Management. Int. J. Mol. Sci. 2022, 23, 3249. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Petrogiannopoulos, L.; Pergaris, A.; Theocharis, S. The EPH/Ephrin System in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 2761. [Google Scholar] [CrossRef]
- Nikas, I.; Giaginis, C.; Petrouska, K.; Alexandrou, P.; Michail, A.; Sarantis, P.; Tsourouflis, G.; Danas, E.; Pergaris, A.; Politis, P.K.; et al. EPHA2, EPHA4, and EPHA7 Expression in Triple-Negative Breast Cancer. Diagnostics 2022, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Masaoutis, C.; Georgantzoglou, N.; Sarantis, P.; Theochari, I.; Tsoukalas, N.; Bobos, M.; Alexandrou, P.; Pergaris, A.; Rontogianni, D.; Theocharis, S. Ephrin Receptors (Ephs) Expression in Thymic Epithelial Tumors: Prognostic Implications and Future Therapeutic Approaches. Diagnostics 2021, 11, 2265. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Maru, Y.; Hagiwara, K.; Nishida, J.; Takaku, F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science 1987, 238, 1717–1720. [Google Scholar] [CrossRef]
- Arora, S.; Scott, A.M.; Janes, P.W. Eph Receptors in Cancer. Biomedicines 2023, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Kania, A.; Klein, R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell. Biol. 2016, 17, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Sun, J.; Lee, M.; Hwang, Y.S.; Daar, I.O. Wnt4 and ephrinB2 instruct apical constriction via Dishevelled and non-canonical signaling. Nat. Commun. 2023, 14, 337. [Google Scholar] [CrossRef]
- Boyd, A.W.; Bartlett, P.F.; Lackmann, M. Therapeutic targeting of EPH receptors and their ligands. Nat. Rev. Drug. Discov. 2014, 13, 39–62. [Google Scholar] [CrossRef]
- Wilkinson, D.G. Regulation of cell differentiation by Eph receptor and ephrin signaling. Cell. Adhes. Migr. 2014, 8, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, S.; Turner, C.J.; Adams, R.H. Regulation of Angiogenesis by Eph–Ephrin Interactions. Trends Cardiovasc. Med. 2007, 17, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Otaki, N. Bone cell interactions through Eph/ephrin. Cell Adhes. Migr. 2012, 6, 148–156. [Google Scholar] [CrossRef]
- Chatzizacharias, N.A.; Giaginis, C.T.; Agapitos, E.; Theocharis, S.E. The role of ephrins’ receptors and ephrins’ ligands in normal placental development and disease. Expert. Opin. Ther. Targets 2014, 18, 269–275. [Google Scholar] [CrossRef]
- Pergaris, A.; Danas, E.; Goutas, D.; Sykaras, A.G.; Soranidis, A.; Theocharis, S. Molecular Sciences the Clinical Impact of the EPH/Ephrin System in Cancer: Unwinding the Thread. Int. J. Mol. Sci. 2021, 22, 8412. [Google Scholar] [CrossRef] [PubMed]
- Barquilla, A.; Pasquale, E.B. Eph receptors and ephrins: Therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 467–487. [Google Scholar] [CrossRef] [PubMed]
- Rahim, A.; Yoon, Y.; Dimovasili, C.; Shao, Z.; Huang, Q.; Zhang, E.; Kezunovic, N.; Chen, L.; Schaffner, A.; Huntley, G.W.; et al. Presenilin1 familial Alzheimer disease mutants inactivate EFNB1-and BDNF-dependent neuroprotection against excitotoxicity by affecting neuroprotective complexes of N-methyl-d-Aspartate receptor. Brain Commun. 2020, 2, fcaa100. [Google Scholar] [CrossRef] [PubMed]
- de Boer, E.C.W.; van Gils, J.M.; van Gils, M.J. Ephrin-Eph signaling usage by a variety of viruses. Pharmacol. Res. 2020, 159, 105038. [Google Scholar] [CrossRef]
- Anderton, M.; van der Meulen, E.; Blumenthal, M.J.; Schäfer, G. The role of the eph receptor family in tumorigenesis. Cancers 2021, 13, 206. [Google Scholar] [CrossRef]
- Haramis, A.-P.G.; Perrakis, A. Selectivity and promiscuity in Eph receptors. Structure 2006, 14, 169–171. [Google Scholar] [CrossRef]
- Wang, H.; Hou, W.; Perera, A.; Bettler, C.; Beach, J.R.; Ding, X.; Li, J.; Denning, M.F.; Dhanarajan, A.; Cotler, S.J.; et al. Targeting EphA2 suppresses hepatocellular carcinoma initiation and progression by dual inhibition of JAK1/STAT3 and AKT signaling. Cell. Rep. 2021, 34, 108765. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, W. EPHA2, a promising therapeutic target for hepatocellular carcinoma. Mol. Cell. Oncol. 2021, 8, 1910009. [Google Scholar] [CrossRef]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef]
- Jin, R.; Lin, H.; Li, G.; Xu, J.; Shi, L.; Chang, C.; Cai, X. TR4 nuclear receptor suppresses HCC cell invasion via downregulating the EphA2 expression article. Cell. Death Dis. 2018, 9, 283. [Google Scholar] [CrossRef]
- Niu, X.; Sun, H.; Qiu, F.; Liu, J.; Yang, T.; Han, W. miR-10b-5p Suppresses the Proliferation and Invasion of Primary Hepatic Carcinoma Cells by Downregulating EphA2. Biomed Res. Int. 2021, 2021, 1382061. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, Y.; Sun, H.; Pan, Y.; Wu, M.; Zhang, J. Deregulation of miR-520d-3p promotes hepatocellular carcinoma development via lncRNA MIAT regulation and EPHA2 signaling activation. Biomed. Pharmacother. 2019, 109, 1630–1639. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Zhou, M.; Shi, H.; Yu, Z.; Zhu, Y.; Yu, F. EphA1 receptor silencing by small interfering RNA has antiangiogenic and antitumor efficacy in hepatocellular carcinoma. Oncol. Rep. 2010, 23, 563–570. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, C.; Chen, X.; Tao, C.; Cheng, H.; Lu, X. Suppression of tumor cell proliferation and migration by human umbilical cord mesenchymal stem cells: A possible role for apoptosis and Wnt signaling. Oncol. Lett. 2018, 15, 8536–8544. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Ping, F.; Liu, H.; Sun, J.; Wang, Y.; Shen, A.; Ding, J.; Geng, M. Identification and Therapeutic Intervention of Coactivated Anaplastic Lymphoma Kinase, Fibroblast Growth Factor Receptor 2, and Ephrin Type-A Receptor 5 Kinases in Hepatocellular Carcinoma. Hepatology 2019, 69, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Zhao, J.-S.; Li, J.-J.; Wang, T.; Cheng, S.-Q.; Yuan, Y.; Wang, F.; Wang, X.-F.; Xie, D. Liver cancer: EphrinA2 promotes tumorigenicity through Rac1/Akt/NF-κB signaling pathway. Hepatology 2010, 51, 535–544. [Google Scholar] [CrossRef]
- Yu, H.; Shi, G. Cisplatin chemotherapy-induced miRNA-210 signaling inhibits hepatocellular carcinoma cell growth. Transl. Cancer Res. 2019, 8, 626. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; He, F.; Wang, X.M. Abnormal nuclear expression of pygopus-2 in human primary hepatocellular carcinoma correlates with a poor prognosis. Histopathology 2015, 67, 176–184. [Google Scholar] [CrossRef]
- Yuan, W.; Zhao, H.; Zhou, A.; Wang, S. Interference of EFNA4 suppresses cell proliferation, invasion and angiogenesis in hepatocellular carcinoma by downregulating PYGO2. Cancer Biol. Ther. 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Lin, J.; Zeng, C.; Zhang, J.; Song, Z.; Qi, N.; Liu, X.; Zhang, Z.; Li, A.; Chen, F. EFNA4 promotes cell proliferation and tumor metastasis in hepatocellular carcinoma through a PIK3R2/GSK3β/β-catenin positive feedback loop. Mol. Ther. Nucleic Acids 2021, 25, 328–341. [Google Scholar] [CrossRef]
- Wang, T.H.; Yeh, C.T.; Ho, J.Y.; Ng, K.F.; Chen, T.C. OncomiR miR-96 and miR-182 promote cell proliferation and invasion through targeting ephrinA5 in hepatocellular carcinoma. Mol. Carcinog. 2016, 55, 366–375. [Google Scholar] [CrossRef]
- Dai, B.; Shi, X.; Ma, N.; Ma, W.; Zhang, Y.; Yang, T.; Zhang, J.; He, L. HMQ-T-B10 induces human liver cell apoptosis by competitively targeting EphrinB2 and regulating its pathway. J. Cell. Mol. Med. 2018, 22, 5231–5243. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhang, Y.; Hu, N.; Shan, C.; Zhang, S.; Zhang, W.; Zhang, X.; Ye, L. miRNA-520b and miR-520e sensitize breast cancer cells to complement attack via directly targeting 3′UTR of CD46. Cancer Biol. Ther. 2010, 10, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Zhang, H.; Wang, X.; Li, S.; Zhang, L.; Deng, T.; Zhou, L.; Liu, R.; Wang, X.; Bai, M.; et al. miR-370 regulates cell proliferation and migration by targeting EGFR in gastric cancer. Oncol. Rep. 2017, 38, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Abdel-Hafiz, H.; Suhail, M.; Al-Mars, A.; Zakaria, M.K.; Fatima, K.; Ahmad, S.; Azhar, E.; Chaudhary, A.; Qadri, I. Hepatitis B virus, HBx mutants and their role in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 10238–10248. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-H.; Liu, W.-D.; Zhang, Z.-Y.; Tang, L.-H.; Li, D.; Tian, Z.-J.; Lin, S.-W.; Li, Y.-J. Influence of miR-520e-mediated MAPK signalling pathway on HBV replication and regulation of hepatocellular carcinoma cells via targeting EphA2. J. Viral Hepat. 2019, 26, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, C.C.; El-Saghire, H.; Pochet, N.; Schuster, C.; Baumert, T.F. High-throughput approaches to unravel hepatitis C virus-host interactions. Virus Res. 2016, 18, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lupberger, J.; Zeisel, M.B.; Xiao, F.; Thumann, C.; Fofana, I.; Zona, L.; Davis, C.; Mee, C.; Turek, M.; Gorke, S.; et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med. 2011, 17, 589–595. [Google Scholar] [CrossRef]
- Huang, P.; Liu, M.; Zang, F.; Yao, Y.; Yue, M.; Wang, J.; Fan, H.; Zhuo, L.; Wu, J.; Xia, X.; et al. The development of hepatocellular carcinoma in HCV-infected patients treated with DAA: A comprehensive analysis. Carcinogenesis 2018, 39, 1497–1505. [Google Scholar] [CrossRef]
- Nagaoki, Y.; Imamura, M.; Nishida, Y.; Daijo, K.; Teraoka, Y.; Honda, F.; Nakamura, Y.; Morio, K.; Fujino, H.; Nakahara, T.; et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: Comparison with interferon-based therapy. J. Med. Virol. 2019, 91, 650–658. [Google Scholar] [CrossRef]
- Saviano, A.; Habersetzer, F.; Lupberger, J.; Simo-Noumbissie, P.; Schuster, C.; Doffoël, M.; Schmidt-Mutter, C.; Baumert, T.F. Safety and Antiviral Activity of EGFR Inhibition by Erlotinib in Chronic Hepatitis C Patients: A Phase Ib Randomized Controlled Trial. Clin. Transl. Gastroenterol. 2022, 13, e00492. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Tsagkaris, C.; Papadakis, M.; Papazoglou, A.S.; Moysidis, D.V.; Zografos, C.G.; Theocharis, S. Angiogenesis in gastrointestinal stromal tumors: From bench to bedside. World J. Gastrointest. Oncol. 2022, 14, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi-Parsian, A.; Griffin, R.J.; Kore, R.A.; Todorova, V.K.; Makhoul, I. Tumor-endothelial cell interaction in an experimental model of human hepatocellular carcinoma. Exp. Cell. Res. 2018, 372, 16–24. [Google Scholar] [CrossRef]
- Cuypers, A.; Truong, A.C.K.; Becker, L.M.; Saavedra-García, P.; Carmeliet, P. Tumor vessel co-option: The past & the future. Front. Oncol. 2022, 12, 1–20. [Google Scholar] [CrossRef]
- Iida, H.; Honda, M.; Kawai, H.F.; Yamashita, T.; Shirota, Y.; Wang, B.-C.; Miao, H.; Kaneko, S. Ephrin-A1 expression contributes to the malignant characteristics of {alpha}-fetoprotein producing hepatocellular carcinoma. Gut 2005, 54, 843–851. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, J.; Zhou, W.; Huang, X.-F.; Chen, Q.; Wang, W.; Zhai, L.; Li, S.; Tang, Z. Sorafenib blocks the activation of the HIF-2α/VEGFA/EphA2 pathway, and inhibits the rapid growth of residual liver cancer following high-intensity focused ultrasound therapy in vivo. Pathol. Res. Pract. 2021, 220, 153270. [Google Scholar] [CrossRef]
- Sawai, Y.; Tamura, S.; Fukui, K.; Ito, N.; Imanaka, K.; Saeki, A.; Sakuda, S.; Kiso, S.; Matsuzawa, Y. Expression of ephrin-B1 in hepatocellular carcinoma: Possible involvement in neovascularization. J. Hepatol. 2003, 39, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, J. Hypoxia inducible factor in hepatocellular carcinoma: A therapeutic target. World J. Gastroenterol. 2015, 21, 12171–12178. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Z.; Xie, G.-R.; Chen, D. Hypoxia and hepatocellular carcinoma: The therapeutic target for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2007, 22, 1178–1182. [Google Scholar] [CrossRef]
- Box, A.H.; Demetrick, D.J. Cell cycle kinase inhibitor expression and hypoxia-induced cell cycle arrest in human cancer cell lines. Carcinogenesis 2004, 25, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Gwak, G.-Y.; Yoon, J.-H.; Kim, K.M.; Lee, H.-S.; Chung, J.W.; Gores, G.J. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J. Hepatol. 2005, 42, 358–364. [Google Scholar] [CrossRef]
- Dai, C.-X.; Gao, Q.; Qiu, S.-J.; Ju, M.-J.; Cai, M.-Y.; Xu, Y.-F.; Zhou, J.; Zhang, B.-H.; Fan, J. Hypoxia-inducible factor-1 alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC Cancer 2009, 9, 418. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, X.-P.; Song, K.; Shang, Z.-J. Ephrin-A1 Is Up-Regulated by Hypoxia in Cancer Cells and Promotes Angiogenesis of HUVECs through a Coordinated Cross-Talk with eNOS. PLoS ONE 2013, 8, e74464. [Google Scholar] [CrossRef]
- Heiss, C.; Rodriguez-Mateos, A.; Kelm, M. Central Role of eNOS in the Maintenance of Endothelial Homeostasis. Antioxid. Redox Signal. 2015, 22, 1230–1242. [Google Scholar] [CrossRef]
- Wada, H.; Yamamoto, H.; Kim, C.; Uemura, M.; Akita, H.; Tomimaru, Y.; Hama, N.; Kawamoto, K.; Kobayashi, S.; Eguchi, H.; et al. Association between ephrin-A1 mRNA expression and poor prognosis after hepatectomy to treat hepatocellular carcinoma. Int. J. Oncol. 2014, 45, 1051–1058. [Google Scholar] [CrossRef]
- Husain, A.; Chiu, Y.-T.; Sze, K.M.-F.; Ho, D.W.-H.; Tsui, Y.-M.; Suarez, E.M.S.; Zhang, V.X.; Chan, L.-K.; Lee, E.; Lee, J.M.-F.; et al. Ephrin-A3/EphA2 axis regulates cellular metabolic plasticity to enhance cancer stemness in hypoxic hepatocellular carcinoma. J. Hepatol. 2022, 77, 383–396. [Google Scholar] [CrossRef]
- Zheng, S.-S.; Chen, X.-H.; Yin, X.; Zhang, B.-H. Prognostic significance of HIF-1α expression in hepatocellular carcinoma: A meta-analysis. PLoS ONE 2013, 8, e65753. [Google Scholar] [CrossRef]
- Ito, K.; Suda, T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell. Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef]
- Chakrabarty, R.P.; Chandel, N.S. Mitochondria as Signaling Organelles Control Mammalian Stem Cell Fate. Cell. Stem Cell. 2021, 28, 394–408. [Google Scholar] [CrossRef]
- Shen, W.; Xi, H.; Zhang, K.; Cui, J.; Li, J.; Wang, N.; Wei, B.; Chen, L. Prognostic role of EphA2 in various human carcinomas: A meta-analysis of 23 related studies. Growth Factors 2014, 32, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Dariya, B.; Kasa, P.; Peela, S.; El-Rayes, B.F. Epigenetics in hepatocellular carcinoma. Semin. Cancer Biol. 2022, 86, 622–632. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in Cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef]
- Li, R.; Zhang, D.; Han, Y.; Chen, K.; Guo, W.; Chen, Y.; Wang, S. Neddylation of EphB1 Regulates Its Activity and Associates with Liver Fibrosis. Int. J. Mol. Sci. 2023, 24, 3415. [Google Scholar] [CrossRef] [PubMed]
- Haber, P.K.; Castet, F.; Torres-Martin, M.; Andreu-Oller, C.; Puigvehí, M.; Miho, M.; Radu, P.; Dufour, J.-F.; Verslype, C.; Zimpel, C.; et al. Molecular Markers of Response to Anti-PD1 Therapy in Advanced Hepatocellular Carcinoma. Gastroenterology 2022, 164, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Ikai, I.; Arii, S.; Kojiro, M.; Ichida, T.; Makuuchi, M.; Matsuyama, Y.; Nakanuma, Y.; Okita, K.; Omata, M.; Takayasu, K.; et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004, 101, 796–802. [Google Scholar] [CrossRef]
- Toso, C.; Dupuis-Lozeron, E.; Majno, P.; Berney, T.; Kneteman, N.M.; Perneger, T.; Morel, P.; Mentha, G.; Combescure, C. A model for dropout assessment of candidates with or without hepatocellular carcinoma on a common liver transplant waiting list. Hepatology 2012, 56, 149–156. [Google Scholar] [CrossRef]
- Takayasu, K.; Arii, S.; Kudo, M.; Ichida, T.; Matsui, O.; Izumi, N.; Matsuyama, Y.; Sakamoto, M.; Nakashima, O.; Ku, Y.; et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J. Hepatol. 2012, 56, 886–892. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kubota, N.; Crouchet, E.; Koneru, B.; Marquez, C.A.; Jajoriya, A.K.; Panda, G.; Qian, T.; Zhu, S.; Goossens, N.; et al. Molecular signatures of long-term hepatocellular carcinoma risk in nonalcoholic fatty liver disease. Sci. Transl. Med. 2022, 14, eabo4474. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-D.; Lee, M.-J.; Yu, G.-R.; Kim, I.-H.; Yu, H.-C.; Song, E.-Y.; Kim, D.-G. EFNA1 ligand and its receptor EphA2: Potential biomarkers for hepatocellular carcinoma. Int. J. Cancer 2010, 126, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Dong, C.; Zhang, J.; Fu, S.; Lv, Y.; Wu, J. A comprehensive prognostic and immunological analysis of ephrin family genes in hepatocellular carcinoma. Front. Mol. Biosci. 2022, 9, 943384. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Liu, D.; Rong, D.; Zhang, S. Hypoxic Characteristic in the Immunosuppressive Microenvironment of Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 611058. [Google Scholar] [CrossRef]
- Yin, L.; Cai, Z.; Zhu, B.; Xu, C. Identification of key pathways and genes in the dynamic progression of HCC based on WGCNA. Genes 2018, 9, 92. [Google Scholar] [CrossRef]
- Villanueva, A.; Portela, A.; Sayols, S.; Battiston, C.; Hoshida, Y.; Méndez-González, J.; Imbeaud, S.; Letouzé, E.; Hernandez-Gea, V.; Cornella, H.; et al. DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology 2015, 61, 1945–1956. [Google Scholar] [CrossRef]
- Tampaki, M.; Papatheodoridis, G.V.; Cholongitas, E. Management of Hepatocellular Carcinoma in Decompensated Cirrhotic Patients: A Comprehensive Overview. Cancers 2023, 15, 1310. [Google Scholar] [CrossRef]
- Papatheodoridi, M.; Tampaki, M.; Lok, A.S.; Papatheodoridis, G.V. Risk of HBV reactivation during therapies for HCC: A systematic review. Hepatology 2022, 75, 1257–1274. [Google Scholar] [CrossRef]
- Papatheodoridi, M.; Su, T.; Hadziyannis, E.; Liao, C.; Orfanidou, A.; Yang, H.; Zachou, K.; Liu, C.; Kourikou, A.; Gatselis, N.; et al. Hepatocellular carcinoma after treatment cessation in non-cirrhotic HBeAg-negative chronic hepatitis B: A multicentre cohort study. Liver Int. 2022, 42, 541–550. [Google Scholar] [CrossRef]
- Machairas, N.; Tsilimigras, D.I.; Pawlik, T.M. Current Landscape of Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma. Cancers 2022, 14, 2018. [Google Scholar] [CrossRef]
- Machairas, N.; Papaconstantinou, D.; Dorovinis, P.; Tsilimigras, D.I.; Keramida, M.D.; Kykalos, S.; Schizas, D.; Pawlik, T.M. Meta-Analysis of Repeat Hepatectomy versus Radiofrequency Ablation for Recurrence of Hepatocellular Carcinoma. Cancers 2022, 14, 5398. [Google Scholar] [CrossRef] [PubMed]
- Machairas, N.; Tsilimigras, D.I.; Pawlik, T.M. State-of-the-art surgery for hepatocellular carcinoma. Langenbeck’s Arch. Surg. 2021, 406, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, G.C.; Malago, M.; Machairas, N.; Fouzas, I.; Paul, A. AGMA Score: A Novel Prognostic Score for Patients Undergoing Liver Transplant for Hepatocellular Carcinoma. Transplant. Proc. 2019, 51, 1923–1925. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.S.P.; Severson, E.; Haberberger, J.; Duncan, D.L.; Hemmerich, A.; Edgerly, C.; Ferguson, N.L.; Frampton, G.; Owens, C.; Williams, E.; et al. Landscape of Biomarkers in Non-small Cell Lung Cancer Using Comprehensive Genomic Profiling and PD-L1 Immunohistochemistry. Pathol. Oncol. Res. 2021, 27, 592997. [Google Scholar] [CrossRef]
- Silverman, I.M.; Hollebecque, A.; Friboulet, L.; Owens, S.; Newton, R.C.; Zhen, H.; Féliz, L.; Zecchetto, C.; Melisi, D.; Burn, T.C. Clinicogenomic Analysis of FGFR2 -Rearranged Cholangiocarcinoma Identifies Correlates of Response and Mechanisms of Resistance to Pemigatinib. Cancer Discov. 2021, 11, 326–339. [Google Scholar] [CrossRef]
- Naganuma, A.; Sakuda, T.; Murakami, T.; Aihara, K.; Watanuki, Y.; Suzuki, Y.; Shibasaki, E.; Masuda, T.; Uehara, S.; Yasuoka, H.; et al. Microsatellite Instability-high Intrahepatic Cholangiocarcinoma with Portal Vein Tumor Thrombosis Successfully Treated with Pembrolizumab. Intern. Med. 2020, 59, 2261–2267. [Google Scholar] [CrossRef]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Morizane, C.; Valle, J.W.; Karasic, T.B.; Abrams, T.A.; Kelley, R.K.; Cassier, P.; Furuse, J.; et al. Abstract CT010: Primary results of phase 2 FOENIX-CCA2: The irreversible FGFR1-4 inhibitor futibatinib in intrahepatic cholangiocarcinoma (iCCA) with FGFR2 fusions/rearrangements. Cancer Res. 2021, 81, CT010. [Google Scholar] [CrossRef]
- Lowery, M.A.; Burris, H.A., 3rd; Janku, F.; Shroff, R.T.; Cleary, J.M.; Azad, N.S.; Goyal, L.; Maher, E.A.; Gore, L.; Hollebecque, A.; et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: A phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Federman, N.; McDermott, R. Larotrectinib, a highly selective tropomyosin receptor kinase (TRK) inhibitor for the treatment of TRK fusion cancer. Expert. Rev. Clin. Pharmacol. 2019, 12, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Adashek, J.J.; Menta, A.K.; Reddy, N.K.; Desai, A.P.; Roszik, J.; Subbiah, V. Tissue-Agnostic Activity of BRAF plus MEK Inhibitor in BRAF V600–Mutant Tumors. Mol. Cancer Ther. 2022, 21, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, M.; Sacco, A.; Forgione, L.; Normanno, N. Genomic alterations in cholangiocarcinoma: Clinical significance and relevance to therapy. Explor. Target. Anti-Tumor Ther. 2022, 3, 200–223. [Google Scholar] [CrossRef]
- Cui, X.-D.; Lee, M.-J.; Kim, J.-H.; Hao, P.-P.; Liu, L.; Yu, G.-R.; Kim, D.-G. Activation of mammalian target of rapamycin complex 1 (mTORC1) and Raf/Pyk2 by growth factor-mediated Eph receptor 2 (EphA2) is required for cholangiocarcinoma growth and metastasis. Hepatology 2013, 57, 2248–2260. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, J.; Zhang, Y.; Gao, X.; Wang, Z.; Yang, J.; Yan, S.; Zhu, Y.; Zhang, Z.; Xu, D.; et al. Mutated EPHA2 is a target for combating lymphatic metastasis in intrahepatic cholangiocarcinoma. Int. J. Cancer 2019, 144, 2440–2452. [Google Scholar] [CrossRef]
- Suksawat, M.; Techasen, A.; Namwat, N.; Yongvanit, P.; Khuntikeo, N.; Titapun, A.; Koonmee, S.; Loilome, W. Upregulation of endothelial nitric oxide synthase (eNOS) and its upstream regulators in Opisthorchis viverrini associated cholangiocarcinoma and its clinical significance. Parasitol. Int. 2017, 66, 486–493. [Google Scholar] [CrossRef]
- Khansaard, W.; Techasen, A.; Namwat, N.; Yongvanit, P.; Khuntikeo, N.; Puapairoj, A.; Loilome, W. Increased EphB2 expression predicts cholangiocarcinoma metastasis. Tumor Biol. 2014, 35, 10031–10041. [Google Scholar] [CrossRef]
- Rimassa, L.; Finn, R.S.; Sangro, B. Combination immunotherapy for hepatocellular carcinoma. J. Hepatol. 2023, 2023, 143747. [Google Scholar] [CrossRef]
- Kim, C.G.; Kim, C.; Yoon, S.E.; Kim, K.H.; Choi, S.J.; Kang, B.; Kim, H.R.; Park, S.-H.; Shin, E.-C.; Kim, Y.-Y.; et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J. Hepatol. 2021, 74, 350–359. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Arvanitakis, K.; Stergiou, I.E.; Lekakis, V.; Davakis, S.; Christodoulou, M.-I.; Germanidis, G.; Theocharis, S. The Role of TLR4 in the Immunotherapy of Hepatocellular Carcinoma: Can We Teach an Old Dog New Tricks? Cancers 2023, 15, 2795. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Dedes, N.; Kouroumalis, E.; Theocharis, S. The Role of the NLRP3 Inflammasome in HCC Carcinogenesis and Treatment: Harnessing Innate Immunity. Cancers 2022, 14, 3150. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Ferraro, D.; Carbone, G.; Frampton, A.E.; Vennarecci, G.; Kykalos, S.; Schizas, D.; Theocharis, S.; Machairas, N. The Emerging Role of Metformin in the Treatment of Hepatocellular Carcinoma: Is There Any Value in Repurposing Metformin for HCC Immunotherapy? Cancers 2023, 15, 3161. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Anang, N.-A.A.S.; Sharma, R.; Andrews, M.C.; Reuben, A.; Levine, J.H.; Cogdill, A.P.; Mancuso, J.J.; Wargo, J.A.; Pe’er, D.; et al. Combination anti–CTLA-4 plus anti–PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc. Natl. Acad. Sci. USA 2019, 116, 22699–22709. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, A.; Zanuso, V.; Manfredi, G.F.; Murphy, R.; Pinato, D.J.; Rimassa, L. Immunotherapy in hepatocellular carcinoma: How will it reshape treatment sequencing? Ther. Adv. Med. Oncol. 2023, 15, 175883592211480. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chan, S.L.; Chon, H.J. Could We Predict the Response of Immune Checkpoint Inhibitor Treatment in Hepatocellular Carcinoma? Cancers 2022, 14, 3213. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Gadaleta-Caldarola, G.; Brandi, G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: Current management and future challenges. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 1245–1251. [Google Scholar] [CrossRef]
- Song, X.; Kelley, R.K.; Khan, A.A.; Standifer, N.; Zhou, D.; Lim, K.; Krishna, R.; Liu, L.; Wang, K.; McCoon, P.; et al. Exposure-Response Analyses of Tremelimumab Monotherapy or in Combination with Durvalumab in Patients with Unresectable Hepatocellular Carcinoma. Clin. Cancer Res. 2023, 29, 754–763. [Google Scholar] [CrossRef]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.; Klümpen, H.; Malka, D.; Primrose, J.; Rimassa, L.; Stenzinger, A.; Valle, J.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Manthopoulou, E.; Ramai, D.; Dhar, J.; Samanta, J.; Ioannou, A.; Lusina, E.; Sacco, R.; Facciorusso, A. Cholangiocarcinoma in the Era of Immunotherapy. Vaccines 2023, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Parakh, S.; Lee, F.T.; Tebbutt, N.C.; Ameratunga, M.; Lee, S.T.; O’keefe, G.J.; Gong, S.J.; Vanrenen, C.; Caine, J.; et al. A phase 1 safety and bioimaging trial of antibody DS-8895a against EphA2 in patients with advanced or metastatic EphA2 positive cancers. Investig. New. Drugs 2022, 40, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Satoh, T.; Iwasa, S.; Yamaguchi, K.; Muro, K.; Komatsu, Y.; Nishina, T.; Esaki, T.; Hasegawa, J.; Kakurai, Y.; et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: A first-in-human phase i dose escalation and dose expansion study in patients with advanced solid tumors. J. Immunother. Cancer 2019, 7, 219. [Google Scholar] [CrossRef]
- Wagner, M.J.; Mitra, R.; McArthur, M.J.; Baze, W.; Barnhart, K.; Wu, S.Y.; Rodriguez-Aguayo, C.; Zhang, X.; Coleman, R.L.; Lopez-Berestein, G.; et al. Preclinical Mammalian Safety Studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA). Mol. Cancer Ther. 2017, 16, 1114–1123. [Google Scholar] [CrossRef]
- Huang, Z.R.; Tipparaju, S.K.; Kirpotin, D.B.; Pien, C.; Kornaga, T.; Noble, C.O.; Koshkaryev, A.; Tran, J.; Kamoun, W.S.; Drummond, D.C. Formulation optimization of an ephrin A2 targeted immunoliposome encapsulating reversibly modified taxane prodrugs. J. Control. Release 2019, 310, 47–57. [Google Scholar] [CrossRef]
- Papazoglou, S.; Tsagkaris, C.; Moysidis, D.V.; Papadakos, S.; Galkin, O.Y.; Orel, V.E.; Syvak, L.A. Nanotherapy based on magneto-mechanochemical modulation of tumor redox state. WIREs Nanomed. Nanobiotechnol. 2022, 15, e1868. [Google Scholar] [CrossRef]

| Author; Year | Epigenetic Mechanism | EPH/Ephrin Target | Mechanisms | Outcomes | Ref. |
|---|---|---|---|---|---|
| Niu; 2021 | miR-10b-5p | EPHA2 | miR-10b-5p expression is downregulated. | miR-10b-5p plays a role in reducing cell proliferation and promoting apoptosis in HCC by regulating EPHA2. miR-10b-5p could be a promising clinical target for HCC treatment. | [42] |
| EPHA2 expression is upregulated. | |||||
| miR-10b-5p or knocking down EphA2: decreased cellular proliferation, facilitated apoptosis, increased expression of Bax and Caspase-3 and decreased Bcl-2. | |||||
| Xiang; 2019 | miR-520d-3p | EPHA2 | miR-520d-3p expression was significantly lower in HCC tissues and cells compared to tumor-adjacent tissues and normal liver cells (L02) and was associated with poor OS. | MIAT is a suppressor of miR-520d-3p and identifies EPHA2 as a direct target of miR-520d-3p with possible therapeutic implications. | [43] |
| Long non-coding RNA myocardial infarction associated transcript (MIAT) was found to be upregulated in both HCC tissues and cell lines. | |||||
| EPHA2 was identified as a direct target of miR-520d-3p and it was confirmed that MIAT functions as a competitive endogenous RNA acting as a sponge for miR-520d-3p. | |||||
| Yu; 2019 | miRNA-210 | ephrinA3 | HCC patients who experienced tumor recurrences after chemotherapy exhibited high levels of miR-210 expression. | Targeting the miR-210-induced ephrinA3 signaling could be a potential strategy to enhance the efficacy of cisplatin-based therapies in HCC. | [48] |
| Cisplatin treatment led to a decrease in miR-210 expression and an increase in ephrinA3 expression. | |||||
| Overexpression of miR-210 counteracted the effects of cisplatin and rescued HCC cell growth, while inhibition of miR-210 improved the chemosensitivity of HCC cells to cisplatin. | |||||
| Wang; 2016 | miR-96, miR-182 | ephrinA5 | miR-96 and miR-182 were upregulated in HCC compared to para-tumoral tissues. | miR-96 and miR-182 directly targeted ephrinA5 mRNA and suppressed its translation resulting in reduced HCC cell growth and migration. | [52] |
| miR-96 and miR-182 showed an inverse relationship with ephrinA5. | |||||
| miR-96 and miR-182 specifically bind to the 3′UTR region of ephrinA5 mRNA. | |||||
| Inhibition of miR-96 and miR-182 led to decreased proliferation and migration of HCC cells by negatively regulating ephrinA5 expression. | |||||
| Li; 2023 | Neddylation | EPHB1 | EPHB1 is neddylated by NEDD8 in HSC. | Neddylation of EPHB1 in HSCs: augmented in activated HSCs. These findings contribute to the understanding of the mechanisms underlying liver fibrosis and highlight EPHB1 as a potential target for therapeutic interventions. | [87] |
| EPHB1 neddylation was enhanced by TGF-β1 stimulation and inhibited by MLN4924. | |||||
| Neddylation was specific to EPHB1 and not in other tested EPHB family members. |
| Author; Year | Molecule | Method | Outcome | Ref. |
|---|---|---|---|---|
| Wang; 2021 | EPHA2 | IHC—Y588 phosphorylated EPHA2 (p-EPHA2)—153 HCC specimens and 63 non-tumor liver tissues—The Cancer Genome Atlas (TCGA). | Increased expression of p-EPHA2 and total EPHA2 correlated with poor prognosis. | [38] |
| EPHA2 signaling is correlated with a poor prognosis in HCC emphasizing its potential as a prognostic marker in this disease. | ||||
| Wang; 2019 | EPHA5 | Frozen tissue HCC patients. | Abnormal activation of ALK, FGFR2 and EPHA5 in a subset of HCC patients. | [46] |
| The concurrent activation of ALK, FGFR2 and EPHA5 could serve as a stratifying factor to identify a subgroup of HCC patients with an unfavorable prognosis. | ||||
| This subgroup may benefit from targeted therapeutic interventions, highlighting the potential for personalized treatment approaches. | ||||
| Hussain; 2022 | ephrinA3 | TCGA-LIHC, HKU-QMH cohorts. | Over two-fold overexpression of ephrinA3 | [80] |
| Increased expression of EFNA3 (>−4-fold) was associated with a more aggressive phenotype of HCC (venous invasion and more advanced TNM stage). | ||||
| Higher ephrinA3 expression had poorer OS in the TCGA-LIHC cohort. | ||||
| Feng; 2010 | ephrinA2 | 52 pairs of liver tissue: hcc vs. non-cancerous tissue. | EphrinA2 was lowest in normal liver tissues, relatively higher in primary HCCs and further elevated in portal vein tumor thrombus(PVT). | [47] |
| This observation suggests that ephrinA2 plays a role as prognostic biomarker for PVT. | ||||
| Lin; 2021 | ephrinA4 | IHC | EphrinA4 expression was significantly higher in liver tumor tissue compared to adjacent tissue. | [51] |
| A correlation between EFNA4 expression and AFP, as well as the risk of vascular invasion. | ||||
| Yuan; 2022 | ephrinA4 | GEPIA database | Upregulation of ephrinA4 expression in tumor samples of HCC patients compared to normal samples. | [50] |
| Correlated with the TNM stages of the patients. | ||||
| High level of ephrinA4 expression was positively associated with reduced OS and DFS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakos, S.P.; Stergiou, I.E.; Gkolemi, N.; Arvanitakis, K.; Theocharis, S. Unraveling the Significance of EPH/Ephrin Signaling in Liver Cancer: Insights into Tumor Progression and Therapeutic Implications. Cancers 2023, 15, 3434. https://doi.org/10.3390/cancers15133434
Papadakos SP, Stergiou IE, Gkolemi N, Arvanitakis K, Theocharis S. Unraveling the Significance of EPH/Ephrin Signaling in Liver Cancer: Insights into Tumor Progression and Therapeutic Implications. Cancers. 2023; 15(13):3434. https://doi.org/10.3390/cancers15133434
Chicago/Turabian StylePapadakos, Stavros P., Ioanna E. Stergiou, Nikolina Gkolemi, Konstantinos Arvanitakis, and Stamatios Theocharis. 2023. "Unraveling the Significance of EPH/Ephrin Signaling in Liver Cancer: Insights into Tumor Progression and Therapeutic Implications" Cancers 15, no. 13: 3434. https://doi.org/10.3390/cancers15133434
APA StylePapadakos, S. P., Stergiou, I. E., Gkolemi, N., Arvanitakis, K., & Theocharis, S. (2023). Unraveling the Significance of EPH/Ephrin Signaling in Liver Cancer: Insights into Tumor Progression and Therapeutic Implications. Cancers, 15(13), 3434. https://doi.org/10.3390/cancers15133434








