An Investigation of Social Status among Adolescents and Young Adults Who Have Been Diagnosed with Cancer in Canada
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Recruitment
2.3. Procedure
2.4. Measures
2.4.1. Social Status
2.4.2. Psychosocial Well-Being
2.4.3. Demographic and Clinical Data
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Sample
3.2. Aim 1: Social Status in AYAs Diagnosed with Cancer and a Comparison Sample
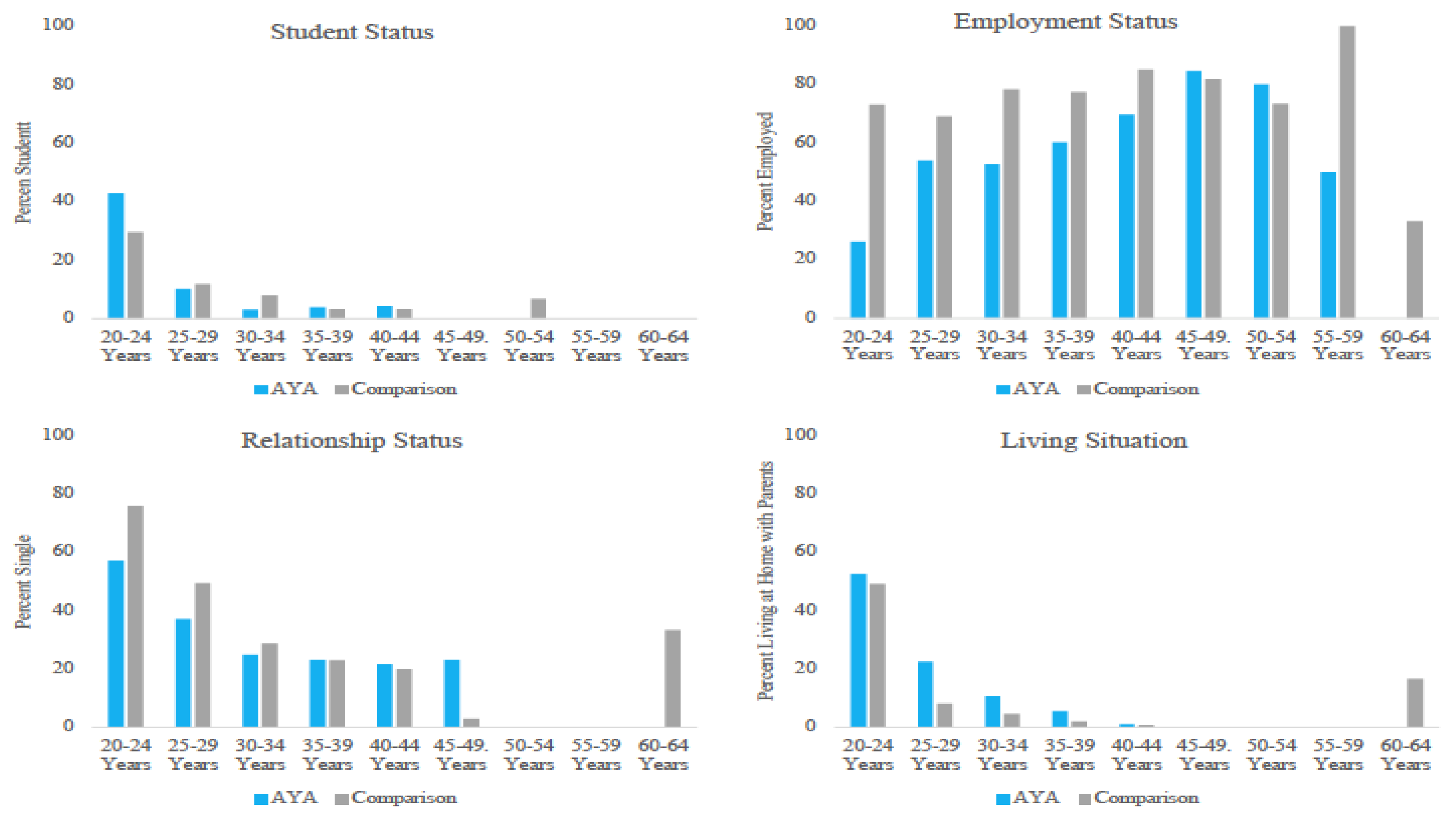
3.2.1. Education Status
3.2.2. Employment Status
3.2.3. Relationship Status
3.2.4. Living Situation
3.3. Aim 2: Change in Education and Employment Status
3.4. Aim 3: Predictors of Social Status
3.4.1. Employment Status
3.4.2. Relationship Status
3.4.3. Living Situation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canadian Cancer Society; Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Society Statistics 2017; Canadian Cancer Society: Toronto, ON, Canada, 2017. [Google Scholar]
- Harju, E.; Roser, K.; Dehler, S.; Michel, G. Health-related quality of life in adolescent and young adult cancer survivors. Support. Care Cancer 2018, 26, 3099–3110. [Google Scholar] [CrossRef]
- Canadian Partnership Against Cancer. Canadian Framework for the Care and Support of Adolescents and Young Adults with Cancer; Canadian Partnership Against Cancer: Toronto, ON, Canada, 2019. [Google Scholar]
- Schulte, F.S.M.; Chalifour, K.; Eaton, G.; Garland, S.N. Quality of life among survivors of adolescent and young adult cancer in Canada: A Young Adults With Cancer in Their Prime (YACPRIME) study. Cancer 2021, 127, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Devine, K.A.; Christen, S.; Mulder, R.L.; Brown, M.C.; Ingerski, L.M.; Mader, L.; Potter, E.J.; Sleurs, C.; Viola, A.S.; Waern, S.; et al. Recommendations for the surveillance of education and employment outcomes in survivors of childhood, adolescent, and young adult cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Cancer 2022, 128, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Schulte, F.; Kunin-Batson, A.S.; Olson-Bullis, B.A.; Banerjee, P.; Hocking, M.C.; Janzen, L.; Kahalley, L.S.; Wroot, H.; Forbes, C.; Krull, K.R. Social attainment in survivors of pediatric central nervous system tumors: A systematic review and meta-analysis from the Children’s Oncology Group. J. Cancer Surviv. 2019, 13, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.H.M.; van der Meer, D.J.; van Eenbergen, M.; Manten-Horst, E.; van der Graaf, W.T.A.; Husson, O. Short- and long-term impact of cancer on employment and financial outcomes of adolescents and young adults (AYAs): A large population-based case-control registry study in the Netherlands. ESMO Open 2022, 7, 100521. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, A.C.; Yi, J.; Wright, J.; Warner, E.L.; Smith, K.R. Marriage and divorce among young adult cancer survivors. J. Cancer Surviv. 2012, 6, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Moke, D.J.; Oberley, M.J.; Bhojwani, D.; Parekh, C.; Orgel, E. Association of clinical trial enrollment and survival using contemporary therapy for pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2018, 65, e26788. [Google Scholar] [CrossRef]
- Aristizabal, P.; Winestone, L.E.; Umaretiya, P.; Bona, K. Disparities in Pediatric Oncology: The 21st Century Opportunity to Improve Outcomes for Children and Adolescents with Cancer. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e315–e326. [Google Scholar] [CrossRef]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef]
- Beland, Y. Canadian Community Health Survey—Methodological overview. Health Rep. 2002, 13, 9–14. [Google Scholar]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Tedeschi, R.G.; Calhoun, L.G. The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. J. Trauma Stress 1996, 9, 455–471. [Google Scholar] [CrossRef]
- Sherbourne, C.D.; Stewart, A.L. The MOS social support survey. Soc. Sci. Med. 1991, 32, 705–714. [Google Scholar] [CrossRef]
- Waller, L. Fostering a Sense of Belonging in the Workplace: Enhancing Well-Being and a Positive and Coherent Sense of Self. In The Palgrave Handbook of Workplace Well-Being; Dhiman, S., Ed.; Palgrave Macmillan: London, UK, 2020. [Google Scholar]
- Mahon, K.N.; Garland, S.N.; Eaton, G.; Chalifour, K.; Lane, B.E.; Fowler, K.; Gambin, L.; Clair, L. The financial impact of cancer on Canadian young adults. J. Cancer Surviv. 2021, 17, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.I.; Razis, E.D. CNS Tumors in Adolescents and Young Adults: The Need for a Holistic Specialized Approach. JCO Oncol. Pract. 2020, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Arpawong, T.E.; Oland, A.; Milam, J.E.; Ruccione, K.; Meeske, K.A. Post-traumatic growth among an ethnically diverse sample of adolescent and young adult cancer survivors. Psychooncology 2013, 22, 2235–2244. [Google Scholar] [CrossRef]
- Phillips, F.; Jones, B.L. Understanding the lived experience of Latino adolescent and young adult survivors of childhood cancer. J. Cancer Surviv. 2014, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.H.; Gabeau, D.; Pinnix, C.C.; Deville, C., Jr.; Gibbs, I.C.; Winkfield, K.M. Why Racial Justice Matters in Radiation Oncology. Adv. Radiat. Oncol. 2020, 5, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.A.; Ashford, J.M.; Wright, E.; Xu, J.; Zhang, H.; Merchant, T.E.; Conklin, H.M. The impact of socioeconomic status (SES) on cognitive outcomes following radiotherapy for pediatric brain tumors: A prospective, longitudinal trial. Neuro Oncol. 2021, 23, 1173–1182. [Google Scholar] [CrossRef]
- Tsang, D.S.; Schulte, F. Beyond the brain: Socioeconomic status and race in pediatric brain tumor survivorship. Neuro Oncol. 2021, 23, 1050–1051. [Google Scholar] [CrossRef]
- Warner, E.L.; Kent, E.E.; Trevino, K.M.; Parsons, H.M.; Zebrack, B.J.; Kirchhoff, A.C. Social well-being among adolescents and young adults with cancer: A systematic review. Cancer 2016, 122, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Canadian Partnership Against Cancer. Approaches for Addressing Mental Health & Return to Work Needs of Cancer Survivors: An Environmental Scan; Canadian Partnership Against Cancer: Toronto, ON, Canada, 2019. [Google Scholar]

| AYA (n = 622) | Comparison (n = 1688) | p | |
|---|---|---|---|
| n (%) | n (%) | ||
| Characteristic | |||
| Sex | 1.00 | ||
| Male | 84 (13.5) | 221 (13.1) | |
| Female | 537 (86.3) | 1467 (86.9) | |
| Age at Time of Study | 1.00 | ||
| 20–29 years | 131 (21.1) | 363 (21.5) | |
| 30–39 years | 377 (60.6) | 1019 (60.3) | |
| 40–49 years | 105 (16.9) | 282 (16.7) | |
| 50–64 years | 9 (1.4) | 24 (1.4) | |
| Income (CAD) | |||
| <CAD 40,000 | 135 (21.7) | 389 (23.0) | |
| CAD 40,000–80,000 | 164 (26.4) | 485 (28.7) | |
| >CAD 80,000 | 273 (43.9) | 814 (48.2) | |
| Missing | 50 (8) | 0 (0) | |
| Province of Residence | |||
| Alberta | 101 (16.2) | 279 (16.5) | |
| British Columbia | 90 (14.5) | 249 (14.8) | |
| Manitoba | 37 (5.9) | 98 (5.8) | |
| New Brunswick | 11 (1.8) | 33 (2.0) | |
| Newfoundland and Labrador | 66 (10.6) | 163 (9.7) | |
| Northwest Territories | 1 (0.2) | 3 (0.2) | |
| Nova Scotia | 34 (5.5) | 101 (6.0) | |
| Nunavut | 0 (0) | 0 (0) | |
| Ontario | 195 (31.4) | 561 (33.2) | |
| Prince Edward Island | 5 (0.8) | 12 (0.7) | |
| Quebec | 65 (10.5) | 139 (8.2) | |
| Saskatchewan | 15 (2.4) | (45 (2.7) | |
| Yukon | 2 (0.3) | 5 (0.3) | |
| Race/Ethnicity | |||
| White | 543 (87.3) | 1489 (88.2) | |
| Non-White | 79 (12.7) | 150 (8.9) | |
| Asian | 21 (3.4) | ||
| Multi-racial | 25 (4.0) | ||
| Aboriginal/First Nations | 13 (2.1) | ||
| Other | 20 (3.2) | ||
| Diagnosis | |||
| Breast | 170 (27.3) | ||
| Female Genitourinary | 60 (9.6) | ||
| Male Genitourinary | 9 (1.4) | ||
| Thyroid | 45 (7.2) | ||
| Blood | 173 (27.8) | ||
| Head and Neck | 46 (7.4) | ||
| Gastrointestinal | 59 (9.5) | ||
| Skin | 18 (2.9) | ||
| Other | 34 (5.5) | ||
| Multiple Types | 8 (1.3) | ||
| Diagnosis Stage | |||
| Stage 1 | 85 (13.7) | ||
| Stage 2 | 170 (27.3) | ||
| Stage 3 | 145 (23.3) | ||
| Stage 4 | 87 (14.0) | ||
| Don’t Know/Not Applicable | 135 (21.7) | ||
| Treatment | |||
| Surgery | 261 (42.0) | ||
| Chemotherapy | 267 (42.9) | ||
| Radiation | 183 (29.4) | ||
| Age at Diagnosis, years (mean, SD) | 29.05 (5.99) | ||
| % on Treatment | 184 (29.6) | ||
| Time Since Treatment, years (mean, SD) | 4.45 (5.42) | ||
| Education (n = 613) | 0.93 | ||
| Part-time Student | 7 (1.1) | 43 (2.5) | |
| Full-time Student | 37 (5.9) | 87 (5.2) | |
| Other | 536 (86.2) | 1557 (92.2) | |
| Employment (n = 613) | <0.001 | ||
| Working Part-time | 95 (15.3) | 190 (11.3) | |
| Working Full-time | 256 (41.2) | 1113 (65.9) | |
| Other | 271 (43.6) | 385 (22.8) | |
| Relationship (n = 613) | <0.09 | ||
| Single | 171 (27.5) | 526 (31.2) | |
| Married/Common-Law | 415 (66.7) | 1020 (60.4) | |
| Widowed/Separated/Divorced | 30 (4.8) | 138 (8.2) | |
| Other | 4 (0.2) | ||
| Living Situation (n = 613) | <0.001 | ||
| Living with Parents | 74 (11.9) | 112 (6.6) | |
| Living Alone | 99 (15.9) | 314 (18.6) | |
| Living Alone with Children | 31 (5) | 175 (10.4) | |
| Living with Others | 175 (28.1) | 291 (17.2) | |
| Living with Others and Children | 194 (31.2) | 696 (41.2) | |
| Other | 49 (8.0) | 95 (5.6) |
| Employment Status | Relationship Status | Living Situation | ||||
|---|---|---|---|---|---|---|
| Variable | Adjusted Odds Ratio (AOR) | 95% CI | Adjusted Odds Ratio (AOR) | 95% CI | Adjusted Odds Ratio (AOR) | 95% CI |
| Sex | ||||||
| Male | reference | reference | reference | |||
| Female | 1.37 | 0.67–2.81 | 0.94 | 0.40–2.20 | 1.27 | 0.36–4.51 |
| Current Age | 1.07 | 1.02–1.12 | 0.89 | 0.84–0.94 | 0.75 | 0.68–0.84 |
| Race/Ethnicity | ||||||
| White | reference | reference | reference | |||
| Other | 1.41 | 0.66–3.02 | 0.99 | 0.41–2.36 | 3.19 | 1.02–9.97 |
| Cancer Diagnosis | ||||||
| Blood | 1.54 | 0.81–2.93 | 1.59 | 0.78–3.22 | 1.55 | 0.54–4.45 |
| Other | reference | reference | reference | |||
| Chemotherapy | ||||||
| Yes | 0.68 | 0.37–1.27 | 1.78 | 0.83–3.81 | 2.49 | 0.65–9.95 |
| No | reference | reference | reference | |||
| Metastatic Status | ||||||
| Yes | reference | reference | reference | |||
| No | 3.23 | 1.08–9.62 | 1.03 | 0.32–3.35 | - | - |
| Physical Component | 1.07 | 1.04–1.10 | 0.98 | 0.95–1.01 | 1.01 | 0.96–1.06 |
| Mental Component | 1.06 | 1.03–1.09 | 1.04 | 1.01–1.07 | 1.03 | 0.99–1.08 |
| Post-traumatic Growth | 1.00 | 0.98–1.01 | 1.01 | 1.00–1.03 | 0.97 | 0.95–0.99 |
| Social Support | 0.89 | 0.65–1.21 | 0.27 | 0.18–0.41 | 1.23 | 0.70–2.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulte, F.S.M.; Hou, S.H.J.; Bender, J.L.; Tulk, J.; Wurz, A.; Petrella, A.; Sabiston, C.M.; D’Agostino, N.; Chalifour, K.; Eaton, G.; et al. An Investigation of Social Status among Adolescents and Young Adults Who Have Been Diagnosed with Cancer in Canada. Cancers 2023, 15, 3436. https://doi.org/10.3390/cancers15133436
Schulte FSM, Hou SHJ, Bender JL, Tulk J, Wurz A, Petrella A, Sabiston CM, D’Agostino N, Chalifour K, Eaton G, et al. An Investigation of Social Status among Adolescents and Young Adults Who Have Been Diagnosed with Cancer in Canada. Cancers. 2023; 15(13):3436. https://doi.org/10.3390/cancers15133436
Chicago/Turabian StyleSchulte, Fiona S. M., Sharon H. J. Hou, Jacqueline L. Bender, Joshua Tulk, Amanda Wurz, Anika Petrella, Catherine M. Sabiston, Norma D’Agostino, Karine Chalifour, Geoff Eaton, and et al. 2023. "An Investigation of Social Status among Adolescents and Young Adults Who Have Been Diagnosed with Cancer in Canada" Cancers 15, no. 13: 3436. https://doi.org/10.3390/cancers15133436
APA StyleSchulte, F. S. M., Hou, S. H. J., Bender, J. L., Tulk, J., Wurz, A., Petrella, A., Sabiston, C. M., D’Agostino, N., Chalifour, K., Eaton, G., & Garland, S. N. (2023). An Investigation of Social Status among Adolescents and Young Adults Who Have Been Diagnosed with Cancer in Canada. Cancers, 15(13), 3436. https://doi.org/10.3390/cancers15133436








