The Role of Staging Laparoscopy for Gastric Cancer Patients: Current Evidence and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
2. Indications for Staging Laparoscopy (SL)
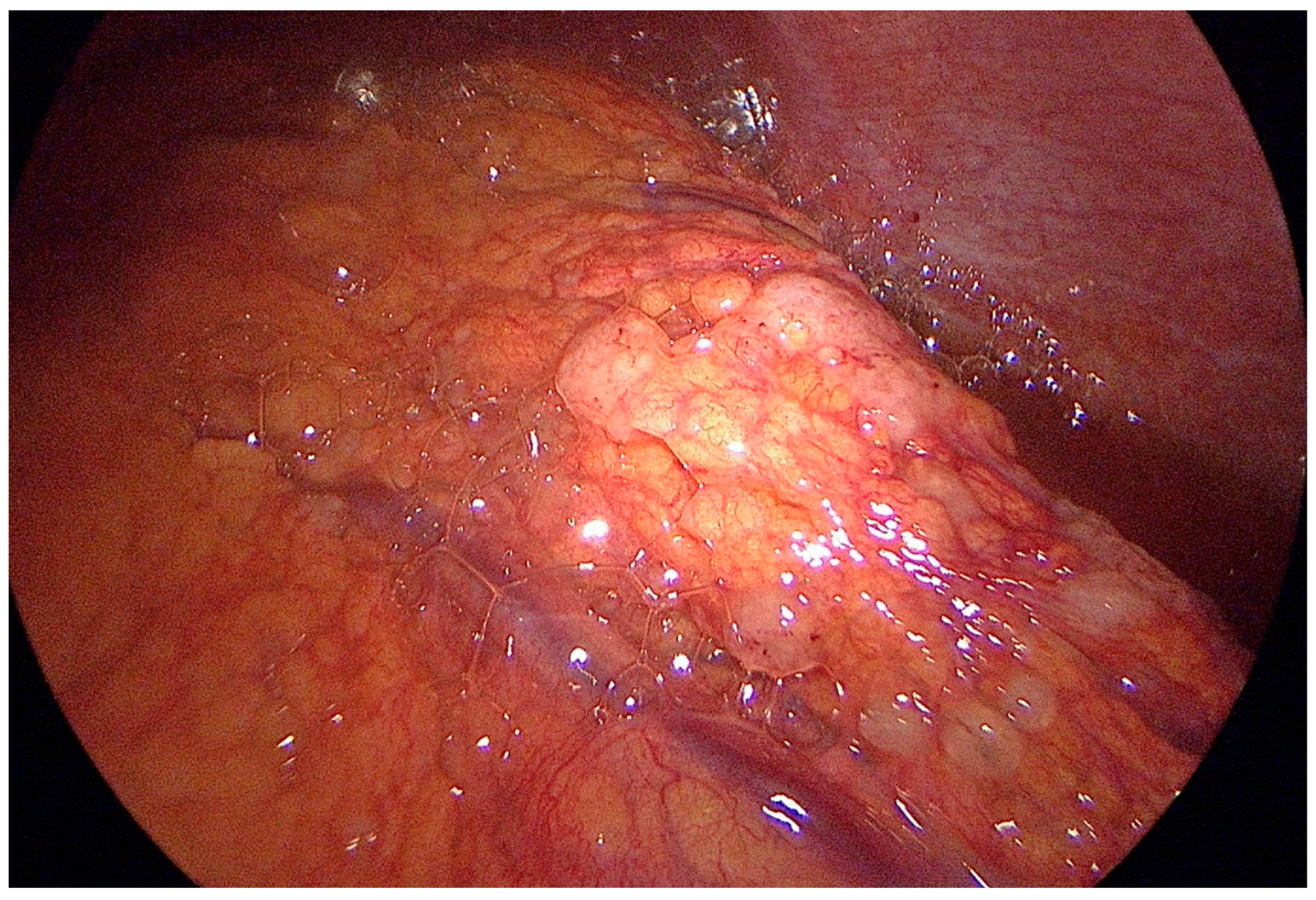
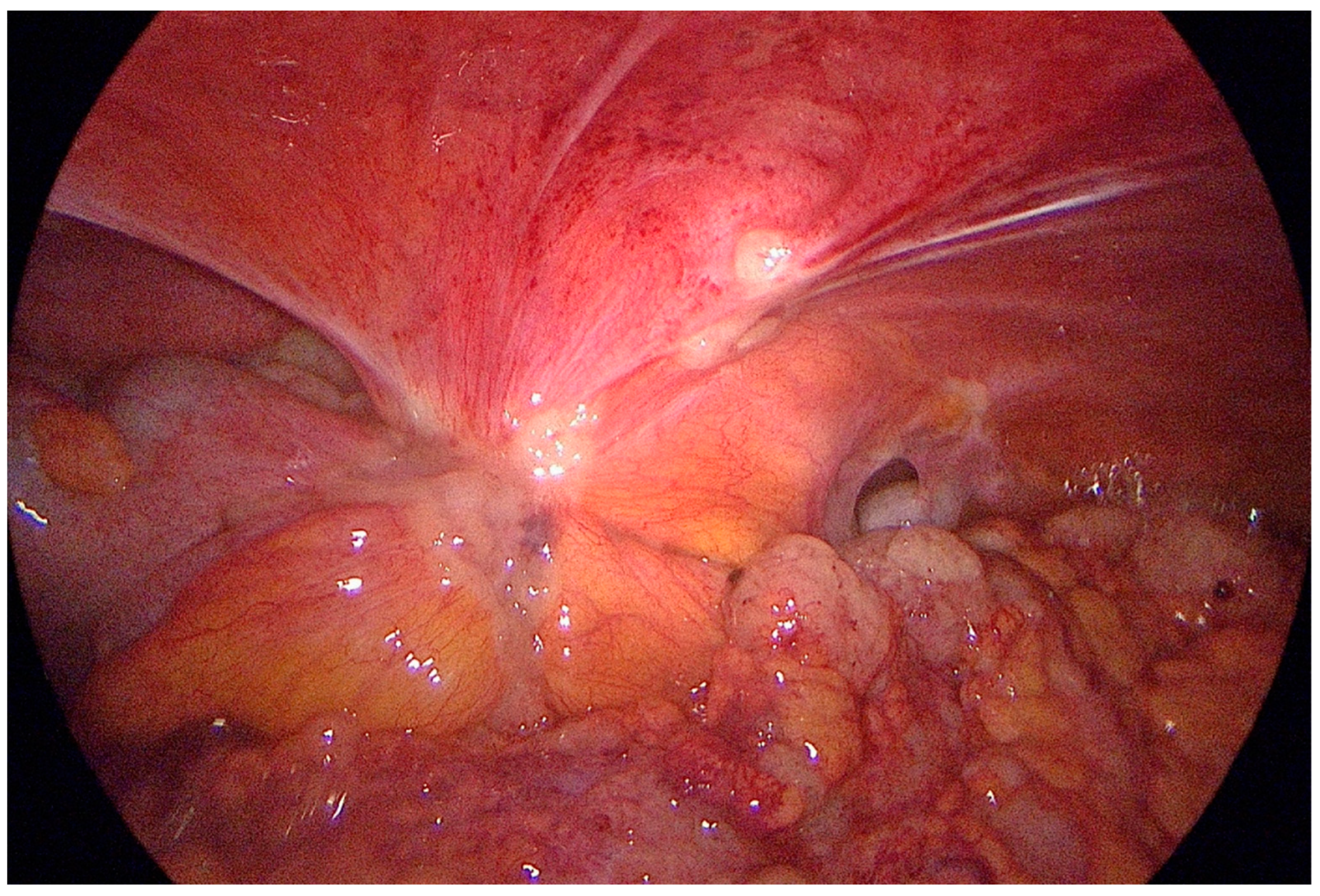
3. Technique of Staging Laparoscopy with Peritoneal Cytology (PC)
4. Performance and Clinical Significance of SL with PC
5. Will There Still Be Room for SL in the Era of Artificial Intelligence?
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; D’Ignazio, A.; Cammillini, F.; Angotti, R.; Messina, M.; Marrelli, D.; Roviello, F. Comparison between 7th and 8th edition of AJCC TNM staging system for gastric cancer: Old problems and new perspectives. Transl. Gastroenterol. Hepatol. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Shandall, A.; Johnson, C. Laparoscopy or scanning in oesophageal and gastric carcinoma? Br. J. Surg. 1985, 72, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.; Bancewicz, J.; Ingram, G. Assessment of gastric cancer by laparoscopy. Br. Med. J. (Clin. Res. Ed.) 1984, 288, 1577. [Google Scholar] [CrossRef]
- Groh, E.M.; Gupta, S.; Brown, Z.J.; Enewold, L.; Gamble, L.A.; Hernandez, J.M.; Davis, J.L. Staging Laparoscopy is Underutilized in the Management of Gastric Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.; Schena, C.A.; Laterza, V.; Quero, G.; Fiorillo, C.; Strippoli, A.; Pozzo, C.; Papa, V.; Alfieri, S. The Role of Surgery in the Management of Gastric Cancer: State of the Art. Cancers 2022, 14, 5542. [Google Scholar] [CrossRef]
- Salati, M.; Di Emidio, K.; Tarantino, V.; Cascinu, S. Second-line treatments: Moving towards an opportunity to improve survival in advanced gastric cancer? ESMO Open 2017, 2, e000206. [Google Scholar] [CrossRef]
- Coutzac, C.; Pernot, S.; Chaput, N.; Zaanan, A. Immunotherapy in advanced gastric cancer, is it the future? Crit. Rev. Oncol. Hematol. 2019, 133, 25–32. [Google Scholar] [CrossRef]
- Rosa, F.; Alfieri, S.; Tortorelli, A.P.; Fiorillo, C.; Costamagna, G.; Doglietto, G.B. Trends in clinical features, postoperative outcomes, and long-term survival for gastric cancer: A Western experience with 1,278 patients over 30 years. World J. Surg. Oncol. 2014, 12, 217. [Google Scholar] [CrossRef]
- Burbidge, S.; Mahady, K.; Naik, K. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin. Radiol. 2013, 68, 251–255. [Google Scholar] [CrossRef]
- Ramos, R.F.; Scalon, F.M.; Scalon, M.M.; Dias, D.I. Staging laparoscopy in gastric cancer to detect peritoneal metastases: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- SAGES. Guidelines for Diagnostic Laparoscopy. Reviewed and Approved in April 2010. Available online: https://www.sages.org/publications/guidelines/guidelines-for-diagnostic-laparoscopy/ (accessed on 1 May 2023).
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Coburn, N.; Cosby, R.; Klein, L.; Knight, G.; Malthaner, R.; Mamazza, J.; Mercer, C.D.; Ringash, J. Staging and surgical approaches in gastric cancer: A clinical practice guideline. Curr. Oncol. 2017, 24, 324–331. [Google Scholar] [CrossRef]
- De Manzoni, G.; Marrelli, D.; Baiocchi, G.L.; Morgagni, P.; Saragoni, L.; Degiuli, M.; Donini, A.; Fumagalli, U.; Mazzei, M.A.; Pacelli, F.; et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer 2017, 20, 20–30. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hartmann, J.T.; Probst, S.; Schmalenberg, H.; Hollerbach, S.; Hofheinz, R.; Rethwisch, V.; Seipelt, G.; Homann, N.; Wilhelm, G.; et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 2008, 26, 1435–1442. [Google Scholar] [CrossRef]
- Harmon, R.L.; Sugarbaker, P.H. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int. Semin. Surg. Oncol. 2005, 2, 3. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Management of peritoneal-surface malignancy: The surgeon’s role. Langenbecks Arch. Surg. 1999, 384, 576–587. [Google Scholar] [CrossRef]
- Terashima, M.; Iwasaki, Y.; Mizusawa, J.; Katayama, H.; Nakamura, K.; Katai, H.; Yoshikawa, T.; Ito, Y.; Kaji, M.; Kimura, Y.; et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer 2019, 22, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Tsuburaya, A.; Mizusawa, J.; Tanaka, Y.; Fukushima, N.; Nashimoto, A.; Sasako, M.; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br. J. Surg. 2014, 101, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, N.; Blum, M.; Chiang, Y.J.; Estrella, J.S.; Roy-Chowdhuri, S.; Fournier, K.; Mansfield, P.; Ajani, J.A.; Badgwell, B.D. Yield of Staging Laparoscopy and Lavage Cytology for Radiologically Occult Peritoneal Carcinomatosis of Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 4332–4337. [Google Scholar] [CrossRef] [PubMed]
- Solaini, L.; Bencivenga, M.; D’Ignazio, A.; Milone, M.; Marino, E.; De Pascale, S.; Rosa, F.; Sacco, M.; Fumagalli Romario, U.; Graziosi, L.; et al. Which gastric cancer patients could benefit from staging laparoscopy? A GIRCG multicenter cohort study. Eur. J. Surg. Oncol. 2022, 48, 1778–1784. [Google Scholar] [CrossRef]
- Tsuchida, K.; Yoshikawa, T.; Tsuburaya, A.; Cho, H.; Kobayashi, O. Indications for staging laparoscopy in clinical T4M0 gastric cancer. World J. Surg. 2011, 35, 2703–2709. [Google Scholar] [CrossRef]
- Fukagawa, T. Role of staging laparoscopy for gastric cancer patients. Ann. Gastroenterol. Surg. 2019, 3, 496–505. [Google Scholar] [CrossRef]
- Hu, Y.F.; Deng, Z.W.; Liu, H.; Mou, T.Y.; Chen, T.; Lu, X.; Wang, D.; Yu, J.; Li, G.X. Staging laparoscopy improves treatment decision-making for advanced gastric cancer. World J. Gastroenterol. 2016, 22, 1859–1868. [Google Scholar] [CrossRef]
- Irino, T.; Sano, T.; Hiki, N.; Ohashi, M.; Nunobe, S.; Kumagai, K.; Ida, S.; Yamaguchi, T. Diagnostic staging laparoscopy in gastric cancer: A prospective cohort at a cancer institute in Japan. Surg. Endosc. 2018, 32, 268–275. [Google Scholar] [CrossRef]
- Liu, K.; Chen, X.Z.; Zhang, W.H.; Zhang, D.Y.; Luo, Y.; Yu, Y.; Yang, K.; Yang, S.J.; Chen, X.L.; Sun, L.F.; et al. “Four-Step Procedure” of laparoscopic exploration for gastric cancer in West China Hospital: A retrospective observational analysis from a high-volume institution in China. Surg. Endosc. 2019, 33, 1674–1682. [Google Scholar] [CrossRef]
- Possik, R.A.; Franco, E.L.; Pires, D.R.; Wohnrath, D.R.; Ferreira, E.B. Sensitivity, specificity, and predictive value of laparoscopy for the staging of gastric cancer and for the detection of liver metastases. Cancer 1986, 58, 1–6. [Google Scholar] [CrossRef]
- Kriplani, A.K.; Kapur, B.M. Laparoscopy for pre-operative staging and assessment of operability in gastric carcinoma. Gastrointest Endosc. 1991, 37, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Lowy, A.M.; Mansfield, P.F.; Leach, S.D.; Ajani, J. Laparoscopic staging for gastric cancer. Surgery 1996, 119, 611–614. [Google Scholar] [CrossRef] [PubMed]
- D’Ugo, D.; Persiani, R.; Pende, V. Impact of diagnostic laparoscopy on the management of gastric cancer: Prospective study of 120 consecutive patients with primary gastric adenocarcinoma (Br J Surg 2002; 89: 471-5). Br. J. Surg. 2002, 89, 1325–1326, author reply 1326. [Google Scholar] [CrossRef] [PubMed]
- Burke, E.C.; Karpeh, M.S.; Conlon, K.C.; Brennan, M.F. Laparoscopy in the management of gastric adenocarcinoma. Ann. Surg. 1997, 225, 262–267. [Google Scholar] [CrossRef]
- Sarela, A.I.; Lefkowitz, R.; Brennan, M.F.; Karpeh, M.S. Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am. J. Surg. 2006, 191, 134–138. [Google Scholar] [CrossRef]
- Leake, P.A.; Cardoso, R.; Seevaratnam, R.; Lourenco, L.; Helyer, L.; Mahar, A.; Law, C.; Coburn, N.G. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer 2012, 15 (Suppl. S1), S38–S47. [Google Scholar] [CrossRef]
- Gertsen, E.C.; Brenkman, H.J.F.; van Hillegersberg, R.; van Sandick, J.W.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; van Lanschot, J.J.B.; Lagarde, S.M.; et al. 18F-Fludeoxyglucose-Positron Emission Tomography/Computed Tomography and Laparoscopy for Staging of Locally Advanced Gastric Cancer: A Multicenter Prospective Dutch Cohort Study (PLASTIC). JAMA Surg. 2021, 156, e215340. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef]
- Portilla, A.G.; Shigeki, K.; Dario, B.; Marcello, D. The intraoperative staging systems in the management of peritoneal surface malignancy. J. Surg. Oncol. 2008, 98, 228–231. [Google Scholar] [CrossRef]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef]
- Badgwell, B.; Ikoma, N.; Murphy, M.B.; Wang, X.; Estrella, J.; Roy-Chowdhuri, S.; Das, P.; Minsky, B.D.; Lano, E.; Song, S.; et al. A Phase II Trial of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Perfusion with Chemotherapy for Patients with Gastric Cancer and Carcinomatosis or Positive Cytology. Ann. Surg. Oncol. 2021, 28, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Huang, C.Q.; Suo, T.; Mei, L.J.; Yang, G.L.; Cheng, F.L.; Zhou, Y.F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Bonnot, P.E.; Mercier, F.; Laplace, N.; Villeneuve, L.; Passot, G.; Bakrin, N.; Kepenekian, V.; Glehen, O. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur. J. Surg. Oncol. 2021, 47, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Schena, C.A.; El Halabieh, M.A.; Abatini, C.; Vita, E.; Strippoli, A.; Inzani, F.; Rodolfino, E.; Romano, B.; Pacelli, F.; et al. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): A bidirectional approach for gastric cancer peritoneal metastasis. Surg. Oncol. 2020, 34, 270–275. [Google Scholar] [CrossRef]
- Eveno, C.; Jouvin, I.; Pocard, M. PIPAC EstoK 01: Pressurized IntraPeritoneal Aerosol Chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: A randomized and multicenter phase II study. Pleura Peritoneum 2018, 3, 20180116. [Google Scholar] [CrossRef]
- Manzanedo, I.; Pereira, F.; Perez-Viejo, E.; Serrano, A. Gastric Cancer with Peritoneal Metastases: Current Status and Prospects for Treatment. Cancers 2023, 15, 1777. [Google Scholar] [CrossRef]
- Marano, L.; Marrelli, D.; Sammartino, P.; Biacchi, D.; Graziosi, L.; Marino, E.; Coccolini, F.; Fugazzola, P.; Valle, M.; Federici, O.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Synchronous Peritoneal Metastases: Multicenter Study of ‘Italian Peritoneal Surface Malignancies Oncoteam-S.I.C.O.’. Ann. Surg. Oncol. 2021, 28, 9060–9070. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef]
- Gilly, F.N.; Cotte, E.; Brigand, C.; Monneuse, O.; Beaujard, A.C.; Freyer, G.; Glehen, O. Quantitative prognostic indices in peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2006, 32, 597–601. [Google Scholar] [CrossRef]
- Chia, C.S.; You, B.; Decullier, E.; Vaudoyer, D.; Lorimier, G.; Abboud, K.; Bereder, J.M.; Arvieux, C.; Boschetti, G.; Glehen, O.; et al. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann. Surg. Oncol. 2016, 23, 1971–1979. [Google Scholar] [CrossRef]
- Manzanedo, I.; Pereira, F.; Rihuete Caro, C.; Perez-Viejo, E.; Serrano, A.; Gutierrez Calvo, A.; Regueira, F.M.; Casado-Adam, A.; Cascales-Campos, P.A.; Arteaga, X.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Carcinomatosis: Multicenter Study of Spanish Group of Peritoneal Oncologic Surgery (GECOP). Ann. Surg. Oncol. 2019, 26, 2615–2621. [Google Scholar] [CrossRef]
- Rau, B.; Brandl, A.; Piso, P.; Pelz, J.; Busch, P.; Demtroder, C.; Schule, S.; Schlitt, H.J.; Roitman, M.; Tepel, J.; et al. Peritoneal metastasis in gastric cancer: Results from the German database. Gastric Cancer 2020, 23, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, E.K.; Pisters, P.W. Staging and preoperative evaluation of upper gastrointestinal malignancies. Semin. Oncol. 2004, 31, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Bentrem, D.; Wilton, A.; Mazumdar, M.; Brennan, M.; Coit, D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann. Surg. Oncol. 2005, 12, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Mezhir, J.J.; Shah, M.A.; Jacks, L.M.; Brennan, M.F.; Coit, D.G.; Strong, V.E. Positive peritoneal cytology in patients with gastric cancer: Natural history and outcome of 291 patients. Ann. Surg. Oncol. 2010, 17, 3173–3180. [Google Scholar] [CrossRef]
- De Andrade, J.P.; Mezhir, J.J. The critical role of peritoneal cytology in the staging of gastric cancer: An evidence-based review. J. Surg. Oncol. 2014, 110, 291–297. [Google Scholar] [CrossRef]
- Ciesla, S.; Lisiecki, R.; Lawnicka, A.; Kudlinski, B.; Ostrowska, P.; Davi, A.; Veroux, M.; Murawa, D. Clinical Significance of Peritoneal Fluid Examination for Free Cancer Cells in Patients Qualified for Surgery for Gastric Cancer. Front. Surg. 2021, 8, 685868. [Google Scholar] [CrossRef]
- Leake, P.A.; Cardoso, R.; Seevaratnam, R.; Lourenco, L.; Helyer, L.; Mahar, A.; Rowsell, C.; Coburn, N.G. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer 2012, 15 (Suppl. S1), S27–S37. [Google Scholar] [CrossRef]
- Karanicolas, P.J.; Elkin, E.B.; Jacks, L.M.; Atoria, C.L.; Strong, V.E.; Brennan, M.F.; Coit, D.G. Staging laparoscopy in the management of gastric cancer: A population-based analysis. J. Am. Coll. Surg. 2011, 213, 644–651, 651 e641. [Google Scholar] [CrossRef]
- Muntean, V.; Mihailov, A.; Iancu, C.; Toganel, R.; Fabian, O.; Domsa, I.; Muntean, M.V. Staging laparoscopy in gastric cancer. Accuracy and impact on therapy. J. Gastrointest. Liver Dis. 2009, 18, 189–195. [Google Scholar]
- Schnelldorfer, T.; Ware, M.P.; Liu, L.P.; Sarr, M.G.; Birkett, D.H.; Ruthazer, R. Can We Accurately Identify Peritoneal Metastases Based on Their Appearance? An Assessment of the Current Practice of Intraoperative Gastrointestinal Cancer Staging. Ann. Surg. Oncol. 2019, 26, 1795–1804. [Google Scholar] [CrossRef]
- Ribeiro, U., Jr.; Gama-Rodrigues, J.J.; Safatle-Ribeiro, A.V.; Bitelman, B.; Ibrahim, R.E.; Ferreira, M.B.; Laudanna, A.A.; Pinotti, H.W. Prognostic significance of intraperitoneal free cancer cells obtained by laparoscopic peritoneal lavage in patients with gastric cancer. J. Gastrointest. Surg. 1998, 2, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, S.; Mohammed, M.F. Multienergy Computed Tomography: A New Horizon in Computed Tomographic Imaging. Radiol. Clin. N. Am. 2018, 56, xv–xvi. [Google Scholar] [CrossRef]
- Volpe, S.; Mastroleo, F.; Krengli, M.; Jereczek-Fossa, B.A. Quo vadis Radiomics? Bibliometric analysis of 10-year Radiomics journey. Eur. Radiol. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Shin, S.S.; Heo, S.H.; Choi, Y.D.; Lim, H.S.; Park, Y.K.; Park, C.H.; Jeong, Y.Y.; Kang, H.K. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur. Radiol. 2012, 22, 654–662. [Google Scholar] [CrossRef]
- Makino, T.; Fujiwara, Y.; Takiguchi, S.; Tsuboyama, T.; Kim, T.; Nushijima, Y.; Yamasaki, M.; Miyata, H.; Nakajima, K.; Mori, M.; et al. Preoperative T staging of gastric cancer by multi-detector row computed tomography. Surgery 2011, 149, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Ba-Ssalamah, A.; Prokop, M.; Uffmann, M.; Pokieser, P.; Teleky, B.; Lechner, G. Dedicated multidetector CT of the stomach: Spectrum of diseases. Radiographics 2003, 23, 625–644. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, A.Y.; Oh, S.T.; Kim, J.S.; Kim, K.W.; Kim, P.N.; Lee, M.G.; Ha, H.K. Gastric cancer staging at multi-detector row CT gastrography: Comparison of transverse and volumetric CT scanning. Radiology 2005, 236, 879–885. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Kretzschmar, A.; Bichev, D.; Deist, T.; Hinke, A.; Breithaupt, K.; Dogan, Y.; Gebauer, B.; Schumacher, G.; Reichardt, P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur. J. Cancer 2011, 47, 2306–2314. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, S.I.; Lim, D.H.; Park, K.W.; Oh, S.Y.; Kwon, H.C.; Hwang, I.G.; Lee, S.C.; Nam, E.; Shin, D.B.; et al. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 2012, 30, 1513–1518. [Google Scholar] [CrossRef]
- Ford, H.E.; Marshall, A.; Bridgewater, J.A.; Janowitz, T.; Coxon, F.Y.; Wadsley, J.; Mansoor, W.; Fyfe, D.; Madhusudan, S.; Middleton, G.W.; et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014, 15, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Borggreve, A.S.; Goense, L.; Brenkman, H.J.F.; Mook, S.; Meijer, G.J.; Wessels, F.J.; Verheij, M.; Jansen, E.P.M.; van Hillegersberg, R.; van Rossum, P.S.N.; et al. Imaging strategies in the management of gastric cancer: Current role and future potential of MRI. Br. J. Radiol. 2019, 92, 20181044. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Clemente, A.; Spinelli, D.; Ierardi, A.M.; Marasco, G.; Farina, D.; Brocchi, S.; Ravaioli, M.; Pettinari, I.; Cescon, M.; et al. Gastric Cancer Staging: Is It Time for Magnetic Resonance Imaging? Cancers 2020, 12, 1402. [Google Scholar] [CrossRef]
- Chung, J.J.; Semelka, R.C.; Martin, D.R.; Marcos, H.B. Colon diseases: MR evaluation using combined T2-weighted single-shot echo train spin-echo and gadolinium-enhanced spoiled gradient-echo sequences. J. Magn. Reson. Imaging 2000, 12, 297–305. [Google Scholar] [CrossRef]
- Guo, H.L.; He, L.; Zhu, Y.C.; Wu, K.; Yuan, F. Comparison between multi-slice spiral CT and magnetic resonance imaging in the diagnosis of peritoneal metastasis in primary ovarian carcinoma. OncoTargets Ther. 2018, 11, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Seevaratnam, R.; Cardoso, R.; McGregor, C.; Lourenco, L.; Mahar, A.; Sutradhar, R.; Law, C.; Paszat, L.; Coburn, N. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer 2012, 15 (Suppl. S1), S3–S18. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, D.H.; Guo, L.; Hu, C.H.; Fang, X.; Meng, Q.; Ping, X.X.; Lu, Z.W. The utility of MRI for pre-operative T and N staging of gastric carcinoma: A systematic review and meta-analysis. Br. J. Radiol. 2015, 88, 20140552. [Google Scholar] [CrossRef]
- Laghi, A.; Bellini, D.; Rengo, M.; Accarpio, F.; Caruso, D.; Biacchi, D.; Di Giorgio, A.; Sammartino, P. Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: Systematic review and meta-analysis. Radiol. Med. 2017, 122, 1–15. [Google Scholar] [CrossRef]
- Li, Z.Y.; Tang, L.; Li, Z.M.; Li, Y.L.; Fu, J.; Zhang, Y.; Li, X.T.; Ying, X.J.; Ji, J.F. Four-Point Computed Tomography Scores for Evaluation of Occult Peritoneal Metastasis in Patients with Gastric Cancer: A Region-to-Region Comparison with Staging Laparoscopy. Ann. Surg. Oncol. 2020, 27, 1103–1109. [Google Scholar] [CrossRef]
- Cao, R.; Tang, L.; Fang, M.; Zhong, L.; Wang, S.; Gong, L.; Li, J.; Dong, D.; Tian, J. Artificial intelligence in gastric cancer: Applications and challenges. Gastroenterol. Rep. 2022, 10, goac064. [Google Scholar] [CrossRef]
- Zheng, Q.; Yang, L.; Zeng, B.; Li, J.; Guo, K.; Liang, Y.; Liao, G. Artificial intelligence performance in detecting tumor metastasis from medical radiology imaging: A systematic review and meta-analysis. EClinicalMedicine 2021, 31, 100669. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qi, L.; Feng, Q.X.; Liu, C.; Sun, S.W.; Zhang, J.; Yang, G.; Ge, Y.Q.; Zhang, Y.D.; Liu, X.S. Machine Learning-Based Computational Models Derived From Large-Scale Radiographic-Radiomic Images Can Help Predict Adverse Histopathological Status of Gastric Cancer. Clin. Transl. Gastroenterol. 2019, 10, e00079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Yu, Y.; Liu, J.J.; Jiang, L.; Xue, H.D.; Lei, J.; Jin, Z.; Yu, J.C. Prediction of the Depth of Tumor Invasion in Gastric Cancer: Potential Role of CT Radiomics. Acad. Radiol. 2020, 27, 1077–1084. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Tian, C.; Song, H.; Fang, M.; Hu, C.; Zang, Y.; Cao, Y.; Dai, S.; Wang, F.; et al. Prognostic value of computed tomography radiomics features in patients with gastric cancer following curative resection. Eur. Radiol. 2019, 29, 3079–3089. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Chen, L.; Guan, W.; Guan, Y.; Ge, Y.; He, J.; Zhou, Z. Whole-lesion apparent diffusion coefficient histogram analysis: Significance in T and N staging of gastric cancers. BMC Cancer 2017, 17, 665. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, H.; Zhang, Y.; Chen, L.; Guan, W.; Guan, Y.; Ge, Y.; He, J.; Zhou, Z. Whole-volume apparent diffusion coefficient-based entropy parameters for assessment of gastric cancer aggressiveness. J. Magn. Reson. Imaging 2018, 47, 168–175. [Google Scholar] [CrossRef]
- Yardimci, A.H.; Sel, I.; Bektas, C.T.; Yarikkaya, E.; Dursun, N.; Bektas, H.; Afsar, C.U.; Gursu, R.U.; Yardimci, V.H.; Ertas, E.; et al. Computed tomography texture analysis in patients with gastric cancer: A quantitative imaging biomarker for preoperative evaluation before neoadjuvant chemotherapy treatment. Jpn. J. Radiol. 2020, 38, 553–560. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Xia, J.; Chen, L.; Guan, W.; Guan, Y.; Ge, Y.; He, J.; Zhou, Z. Predicting the nodal status in gastric cancers: The role of apparent diffusion coefficient histogram characteristic analysis. Magn. Reson. Imaging 2017, 42, 144–151. [Google Scholar] [CrossRef]
- Chen, W.; Wang, S.; Dong, D.; Gao, X.; Zhou, K.; Li, J.; Lv, B.; Li, H.; Wu, X.; Fang, M.; et al. Evaluation of Lymph Node Metastasis in Advanced Gastric Cancer Using Magnetic Resonance Imaging-Based Radiomics. Front. Oncol. 2019, 9, 1265. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Q.; Xu, L.; Wang, Z.; Su, K.; Liu, R.; Yen, E.A.; Liu, S.; Qin, J.; Rong, Y.; et al. Integrating tumor and nodal radiomics to predict lymph node metastasis in gastric cancer. Radiother. Oncol. 2020, 150, 89–96. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Chen, C.; Zhang, X.; Zha, X.; Lv, W.; Xie, J.; Huang, W.; Sun, Z.; Hu, Y.; et al. Radiomics Signature on Computed Tomography Imaging: Association with Lymph Node Metastasis in Patients with Gastric Cancer. Front. Oncol. 2019, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.X.; Liu, C.; Qi, L.; Sun, S.W.; Song, Y.; Yang, G.; Zhang, Y.D.; Liu, X.S. An Intelligent Clinical Decision Support System for Preoperative Prediction of Lymph Node Metastasis in Gastric Cancer. J. Am. Coll. Radiol. 2019, 16, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Fang, M.J.; Tang, L.; Shan, X.H.; Gao, J.B.; Giganti, F.; Wang, R.P.; Chen, X.; Wang, X.X.; Palumbo, D.; et al. Deep learning radiomic nomogram can predict the number of lymph node metastasis in locally advanced gastric cancer: An international multicenter study. Ann. Oncol. 2020, 31, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Tang, L.; Li, Z.Y.; Fang, M.J.; Gao, J.B.; Shan, X.H.; Ying, X.J.; Sun, Y.S.; Fu, J.; Wang, X.X.; et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann. Oncol. 2019, 30, 431–438. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, D.; Chen, X.; He, D.; Yu, P.; Liu, B.; Wu, B.; Hu, J.; Song, B. Deep Convolutional Neural Network Based on Computed Tomography Images for the Preoperative Diagnosis of Occult Peritoneal Metastasis in Advanced Gastric Cancer. Front. Oncol. 2020, 10, 601869. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, X.; Wang, W.; Chen, C.; Yuan, Q.; Zhang, X.; Li, N.; Chen, H.; Yu, J.; Xie, Y.; et al. Noninvasive Prediction of Occult Peritoneal Metastasis in Gastric Cancer Using Deep Learning. JAMA Netw. Open 2021, 4, e2032269. [Google Scholar] [CrossRef]
- Bortot, B.; Mangogna, A.; Di Lorenzo, G.; Stabile, G.; Ricci, G.; Biffi, S. Image-guided cancer surgery: A narrative review on imaging modalities and emerging nanotechnology strategies. J. Nanobiotechnol. 2023, 21, 155. [Google Scholar] [CrossRef]
- Boussedra, S.; Benoit, L.; Koual, M.; Bentivegna, E.; Nguyen-Xuan, H.T.; Bats, A.S.; Azais, H. Fluorescence guided surgery to improve peritoneal cytoreduction in epithelial ovarian cancer: A systematic review of available data. Eur. J. Surg. Oncol. 2022, 48, 1217–1223. [Google Scholar] [CrossRef]

| Society | Recommendation |
|---|---|
| National Comprehensive Cancer Network (NCCN) [12] | Indicated in
|
| European Society for Medical Oncology (ESMO) [14] | Indicated in
|
| Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) [15] | Indicated in
|
| Japanese Gastric Cancer Association (JGCA) [16] | Weakly indicated in
|
| Italian Research Group for Gastric Cancer (GIRCG) [18] | Recommended in cases deemed to be at risk of peritoneal carcinomatosis not visible or doubtful at CT examination. Required in many randomized clinical trials of adjuvant and neoadjuvant therapy. PC, although limited by low sensitivity, is a useful completion of the final pathological staging. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schena, C.A.; Laterza, V.; De Sio, D.; Quero, G.; Fiorillo, C.; Gunawardena, G.; Strippoli, A.; Tondolo, V.; de’Angelis, N.; Alfieri, S.; et al. The Role of Staging Laparoscopy for Gastric Cancer Patients: Current Evidence and Future Perspectives. Cancers 2023, 15, 3425. https://doi.org/10.3390/cancers15133425
Schena CA, Laterza V, De Sio D, Quero G, Fiorillo C, Gunawardena G, Strippoli A, Tondolo V, de’Angelis N, Alfieri S, et al. The Role of Staging Laparoscopy for Gastric Cancer Patients: Current Evidence and Future Perspectives. Cancers. 2023; 15(13):3425. https://doi.org/10.3390/cancers15133425
Chicago/Turabian StyleSchena, Carlo Alberto, Vito Laterza, Davide De Sio, Giuseppe Quero, Claudio Fiorillo, Gayani Gunawardena, Antonia Strippoli, Vincenzo Tondolo, Nicola de’Angelis, Sergio Alfieri, and et al. 2023. "The Role of Staging Laparoscopy for Gastric Cancer Patients: Current Evidence and Future Perspectives" Cancers 15, no. 13: 3425. https://doi.org/10.3390/cancers15133425
APA StyleSchena, C. A., Laterza, V., De Sio, D., Quero, G., Fiorillo, C., Gunawardena, G., Strippoli, A., Tondolo, V., de’Angelis, N., Alfieri, S., & Rosa, F. (2023). The Role of Staging Laparoscopy for Gastric Cancer Patients: Current Evidence and Future Perspectives. Cancers, 15(13), 3425. https://doi.org/10.3390/cancers15133425







